Radiation-Induced Hemorrhagic Cystitis in Prostate Cancer Survivors: The Hidden Toll
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Statistical Analyses
3. Results
3.1. Cohort Characteristics
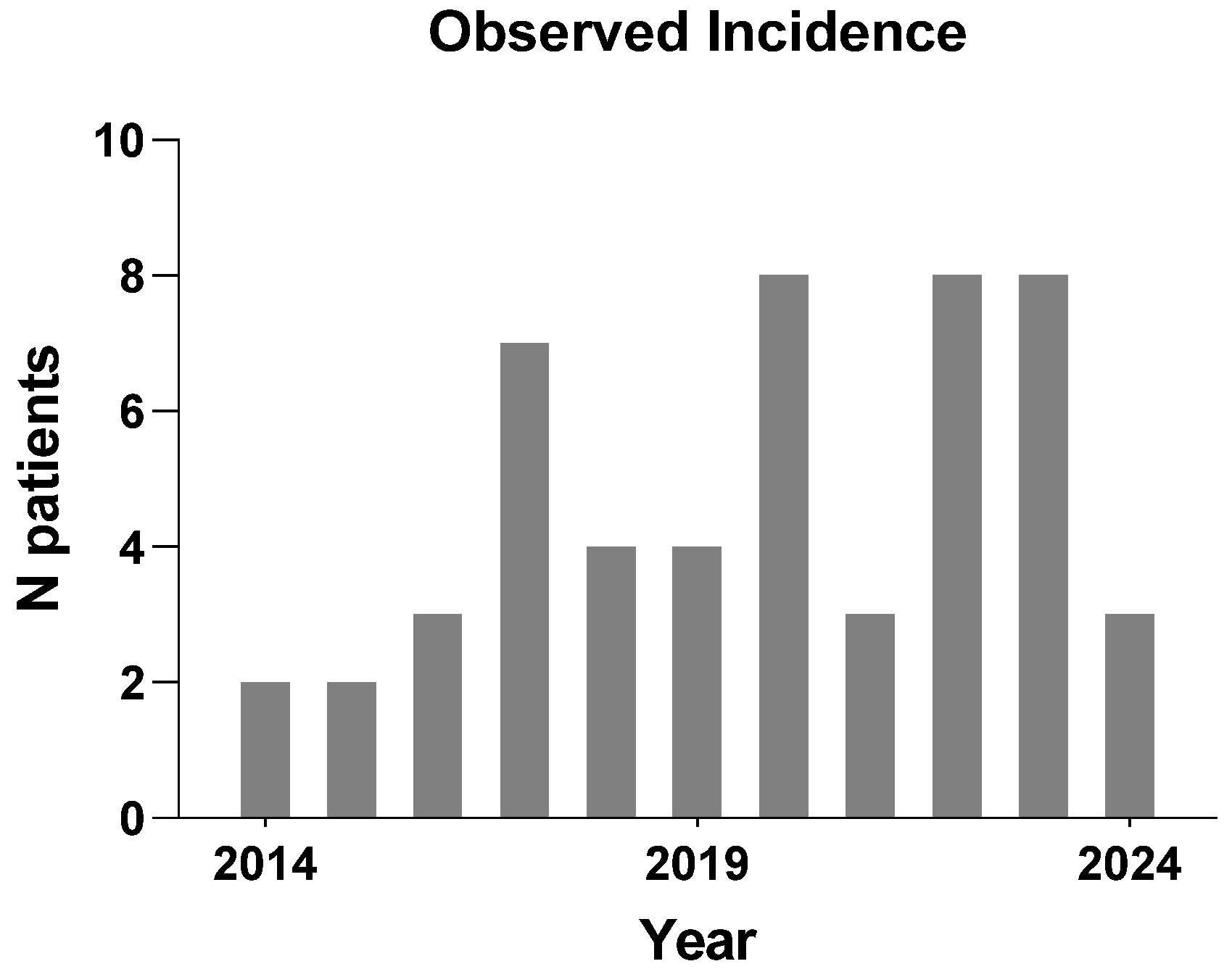
3.2. Estimated Incidence Rate
3.3. Outcome Predictors
3.3.1. Factors Associated with the Need for Multiple TUBF Procedures
3.3.2. Factors Associated with the Need for Multiple Admissions
3.4. Outcomes After Definitive Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Young, G.J.; Walsh, E.I.; Bryant, R.J.; et al. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2023, 388, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Barocas, D.A.; Alvarez, J.A.; Resnick, M.J.; Koyama, T.; Hoffman, K.E.; Tyson, M.D.; Conwill, R.; McCollum, D.; Cooperberg, M.R.; Goodman, M.; et al. Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 2017, 317, 1126–1140. [Google Scholar] [CrossRef]
- Chorbińska, J.; Krajewski, W.; Zdrojowy, R. Urological complications after radiation therapy—Nothing ventured, nothing gained: A Narrative Review. Transl. Cancer Res. 2021, 10, 1096. [Google Scholar] [CrossRef]
- Bologna, E.; Licari, L.C.; Franco, A.; Ditonno, F.; Manfredi, C.; De Nunzio, C.; Antonelli, A.; De Sio, M.; Coogan, C.; Vourganti, S.; et al. Incidence and Management of Radiation Cystitis After Pelvic Radiotherapy for Prostate Cancer: Analysis from a National Database. Urology 2024, 191, 86–92. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Sancho Pardo, G.; Mercadé Sanchez, A.; Balaña Lucena, J.; Pisano, F.; Cortez, J.C.; Territo, A.; Perez, J.H.; Sopeña, J.G.; Lopez, C.E.; et al. Radiation-induced haemorrhagic cystitis after prostate cancer radiotherapy: Factors associated to hospitalization and treatment strategies. Prostate Int. 2021, 9, 48–53. [Google Scholar] [CrossRef]
- David, R.; Buckby, A.; Kahokehr, A.A.; Lee, J.; Watson, D.I.; Leung, J.; O’callaghan, M.E. Long term genitourinary toxicity following curative intent intensity-modulated radiotherapy for prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2023, 26, 8–15. [Google Scholar] [CrossRef]
- Martin, S.E.; Begun, E.M.; Samir, E.; Azaiza, M.T.; Allegro, S.; Abdelhady, M. Incidence and Morbidity of Radiation-Induced Hemorrhagic Cystitis in Prostate Cancer. Urology 2019, 131, 190–195. [Google Scholar] [CrossRef]
- Browne, C.; Davis, N.F.; Mac Craith, E.; Lennon, G.M.; Mulvin, D.W.; Quinlan, D.M.; Quinlan, D.M.; Mc Vey, G.P.; Galvin, D.J. A Narrative Review on the Pathophysiology and Management for Radiation Cystitis. Adv. Urol. 2015, 2015, 346812. [Google Scholar] [CrossRef]
- Helissey, C.; Cavallero, S.; Brossard, C.; Dusaud, M.; Chargari, C.; François, S. Chronic Inflammation and Radiation-Induced Cystitis: Molecular Background and Therapeutic Perspectives. Cells 2021, 10, 21. [Google Scholar] [CrossRef]
- Ma, J.L.; Hennessey, D.B.; Newell, B.P.; Bolton, D.M.; Lawrentschuk, N. Radiotherapy-related complications presenting to a urology department: A more common problem than previously thought? BJU Int. 2018, 121 (Suppl. S3), 28–32. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE)|Protocol Development|CTEP. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 19 August 2024).
- Fajardo, L.F.; Berthrong, M. Radiation injury in surgical pathology. Part I. Am. J. Surg. Pathol. 1978, 2, 159–199. [Google Scholar] [CrossRef] [PubMed]
- Liem, X.; Saad, F.; Delouya, G. A Practical Approach to the Management of Radiation-Induced Hemorrhagic Cystitis. Drugs 2015, 75, 1471–1482. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Henderson, R.H.; Costa, J.A.; Hoppe, B.S.; Dagan, R.; Bryant, C.M.; Nichols, R.C.; Williams, C.R.; Harris, S.E.; Mendenhall, N.P. Hemorrhagic radiation cystitis. Am. J. Clin. Oncol. 2015, 38, 331–336. [Google Scholar] [CrossRef]
- Makino, K.; Sato, Y.; Takenaka, R.; Yamashita, H.; Akiyama, Y.; Yamada, Y.; Nakamura, M.; Kawai, T.; Yamada, D.; Suzuki, M.; et al. Cumulative Incidence and Clinical Risk Factors of Radiation Cystitis after Radiotherapy for Prostate Cancer. Urol. Int. 2023, 107, 440. [Google Scholar] [CrossRef]
- Akthar, A.S.; Liao, C.; Eggener, S.E.; Liauw, S.L. Patient-reported Outcomes and Late Toxicity After Postprostatectomy Intensity-modulated Radiation Therapy. Eur. Urol. 2019, 76, 686–692. [Google Scholar] [CrossRef]
- Oscarsson, N.; Müller, B.; Rosén, A.; Lodding, P.; Mölne, J.; Giglio, D.; Hjelle, K.M.; Vaagbø, G.; Hyldegaard, O.; Vangedal, M.; et al. Radiation-induced cystitis treated with hyperbaric oxygen therapy (RICH-ART): A randomised, controlled, phase 2–3 trial. Lancet Oncol. 2019, 20, 1602–1614. [Google Scholar] [CrossRef]
- Yang, T.K.; Wang, Y.J.; Li, H.J.; Yu, Y.F.; Huang, K.W.; Cheng, J.C.H. Efficacy and Safety of Hyperbaric Oxygen Therapy for Radiation-Induced Hemorrhagic Cystitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 4724. [Google Scholar] [CrossRef]
- Linder, B.J.; Tarrell, R.F.; Boorjian, S.A. Cystectomy for refractory hemorrhagic cystitis: Contemporary etiology, presentation and outcomes. J. Urol. 2014, 192, 1687–1692. [Google Scholar] [CrossRef]
- Tachibana, I.; Calaway, A.C.; Abedali, Z.; Szymanski, K.M.; Mellon, M.J.; Masterson, T.A.; Cary, C.; Kaimakliotis, H.Z.; Boris, R.S. Definitive surgical therapy for refractory radiation cystitis: Evaluating effectiveness, tolerability, and extent of surgical approach. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 789.e1–789.e7. [Google Scholar] [CrossRef]
- Hall, W.A.; Deshmukh, S.; Bruner, D.W.; Michalski, J.M.; Purdy, J.A.; Bosch, W.; Bahary, J.-P.; Patel, M.P.; Parliament, M.B.; Lock, M.I.; et al. Quality of Life Implications of Dose Escalated External Beam Radiation, Results of a Prospective Randomized Phase III Clinical Trial, RTOG 0126. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, W.; Luo, H.; Jin, F.; Wang, Y. The role of image-guided radiotherapy in prostate cancer: A systematic review and meta-analysis. Clin. Transl. Radiat. Oncol. 2023, 38, 81. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, V.; Guckenberger, M.; Haustermans, K.; Lagendijk, J.J.W.; Ménard, C.; Pötter, R.; Slotman, B.J.; Tanderup, K.; Thorwarth, D.; van Herk, M.; et al. Image guidance in radiation therapy for better cure of cancer. Mol. Oncol. 2020, 14, 1470–1491. [Google Scholar] [CrossRef]
- Bryant, C.M.; Henderson, R.H.; Nichols, R.C.; Mendenhall, W.M.; Hoppe, B.S.; Vargas, C.E.; Daniels, T.B.; Choo, C.R.; Parikh, R.R.; Giap, H.; et al. Consensus Statement on Proton Therapy for Prostate Cancer. Int. J. Part Ther. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Hasan, S.; Lazarev, S.; Garg, M.; Gozland, R.; Chang, J.; Hartsell, W.; Chen, J.; Tsai, H.; Vargas, C.; Simone, C.B.; et al. Proton therapy for high-risk prostate cancer: Results from the Proton Collaborative Group PCG 001-09 prospective registry trial. Prostate 2023, 83, 850–856. [Google Scholar] [CrossRef]
- Sosa, A.J.; Rooney, M.K.; Thames, H.D.; Sanders, J.W.; Swanson, D.M.; Choi, S.L.; Nguyen, Q.-N.; Mok, H.; Kuban, D.A.; Zhu, X.R.; et al. Proton therapy toxicity outcomes for localized prostate cancer: Long-term results at a comprehensive cancer center. Clin. Transl. Radiat. Oncol. 2024, 48, 100822. [Google Scholar] [CrossRef]

| Characteristics | Median/N (IQR/%) |
|---|---|
| Age (years) | 70 (65–73) |
| PSA (ng/mL) | 14.4 (8.3–24.1) |
| Gleason sum | |
| 6 | 4 (7.7%) |
| 7 | 28 (53.8%) |
| 8 | 8 (15.4%) |
| 9 | 7 (13.5%) |
| 10 | 3 (5.8%) |
| Neuroendocrine | 2 (2.8%) |
| Metastatic disease | 10 (19.2%) |
| BMI | 23.1 (20.4–25.5) |
| IHD | 29 (55.8%) |
| Hypertension | 39 (75%) |
| Diabetes mellitus | 22 (42.3%) |
| Age-adjusted CCI > 2 | 26 (50%) |
| ASA score | |
| 2 | 35 (67.3%) |
| 3 | 17 (32.7%) |
| On anticoagulation or antiplatelets | 27 (51.9%) |
| Primary RT | 41 (78.8%) |
| Salvage RT | 11 (21.2%) |
| Total dose received (Gy) | 74 (66–74) |
| Time to first TUBF (months) | 64 (33–97) |
| Hyperbaric Oxygen Therapy | 20 (38.5%) |
| Required blood transfusion | 28 (53.8%) |
| Required repeated (>2) transurethral fulguration | 11 (21.2%) |
| Required repeated (>2) hospitalization | 20 (38.5%) |
| Radical cystectomy | 3 (5.8%) |
| Patient Characteristics | Univariate p-Value | OR and 95% CI | Multivariate p-Value | OR and 95% CI |
|---|---|---|---|---|
| Age | 0.98 | 0.99 | ||
| Age-adjusted CCI | 0.69 | 0.56 | ||
| BMI | 0.67 | 0.51 | ||
| Hypertension | 0.15 | 0.29 | ||
| Diabetes | 0.48 | 0.81 | ||
| IHD | 0.05 | 5.17 (1.04–26.8) | - | confounder |
| Antiplatelets | 0.05 | 5.18 (1.08–26.8) | 0.05 | 2.8 (1.03–18.1) |
| Salvage RT | 0.73 | 0.49 | ||
| RT dose > 70 Gy | 0.8 | 0.99 | ||
| Time to first TUBF | 0.03 | 5.02 (1.12–22.6) | 0.02 | 1.8 (1.4–24.6) |
| Patient Characteristics | Univariate p-Value | OR and 95% CI | Multivariate p-Value | OR and 95% CI |
|---|---|---|---|---|
| Age | 0.02 | 0.46 (0.23–0.91) | 0.06 | |
| Age-adjusted CCI | 0.04 | 1.94 (1.03–3.68) | 0.30 | |
| BMI | 0.64 | 0.58 | ||
| Hypertension | 0.68 | 0.62 | ||
| Diabetes | 0.82 | 0.45 | ||
| IHD | 0.35 | 0.70 | ||
| Antithrombotics | 0.13 | 0.22 | ||
| Salvage RT | 0.05 | 4.4 (1.00–20.48) | 0.04 | 8.3 (1.03–47.4) |
| RT dose | 0.8 | 0.99 | ||
| Time to first TUBF | 0.76 | 0.77 |
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age | 66 | 72 | 82 |
| Comorbid conditions | Ischemic heart disease Type 2 diabetes mellitus Hypertension | Hypertension Hyperlipidemia | Atrial fibrillation Interstitial lung disease Hypertension Chronic kidney disease |
| Initial diagnosis | Gleason 3 + 4, T3aN0M0 | Gleason 4 + 3, T2bN0M0 | Gleason 3 + 4 T3aN0M0 |
| Treatment | Robot-assisted radical prostatectomy Salvage RT (1 year post prostatectomy) IMRT 66 grey | Primary RT IGRT 74 grey | Primary RT IGRT 66 grey |
| Time to first TUBF | 7 years and 10 months | 3 years and 9 months | 4 years 6 months |
| Time from first TUBF to Cystectomy | 2 years and 2 months | 3 years and 5 months | 1 year 5 months |
| Number of TUBF prior to cystectomy | 6 | 4 | 3 |
| Number of hospital admissions prior to cystectomy | 4 | 11 | 8 |
| Mode of urinary diversion | Ileal Conduit | Ileal Conduit | Ileal conduit |
| Complications | Clavien–Dindo IIIB
| Clavien–Dindo I
| Clavien–Dindo V
|
| Mortality | No | No | Yes |
| Cause of death | NA | NA | Interstitial lung disease flare |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatsinga, R.; Lim, B.J.H.; Kumar, N.; Tan, J.G.G.; Li, Y.; Wang, M.L.C.; Tan, T.W.K.; Tuan, J.K.L.; Tan, Y.G.; Chen, K.; et al. Radiation-Induced Hemorrhagic Cystitis in Prostate Cancer Survivors: The Hidden Toll. Medicina 2024, 60, 1746. https://doi.org/10.3390/medicina60111746
Gatsinga R, Lim BJH, Kumar N, Tan JGG, Li Y, Wang MLC, Tan TWK, Tuan JKL, Tan YG, Chen K, et al. Radiation-Induced Hemorrhagic Cystitis in Prostate Cancer Survivors: The Hidden Toll. Medicina. 2024; 60(11):1746. https://doi.org/10.3390/medicina60111746
Chicago/Turabian StyleGatsinga, René, Benjamin J. H. Lim, Navin Kumar, Jacinda G. G. Tan, Youquan Li, Michael L. C. Wang, Terence W. K. Tan, Jeffrey K. L. Tuan, Yu Guang Tan, Kenneth Chen, and et al. 2024. "Radiation-Induced Hemorrhagic Cystitis in Prostate Cancer Survivors: The Hidden Toll" Medicina 60, no. 11: 1746. https://doi.org/10.3390/medicina60111746
APA StyleGatsinga, R., Lim, B. J. H., Kumar, N., Tan, J. G. G., Li, Y., Wang, M. L. C., Tan, T. W. K., Tuan, J. K. L., Tan, Y. G., Chen, K., & Yuen, J. S. P. (2024). Radiation-Induced Hemorrhagic Cystitis in Prostate Cancer Survivors: The Hidden Toll. Medicina, 60(11), 1746. https://doi.org/10.3390/medicina60111746






