1. Introduction
In the human body, the anterior brachial region comprises many structures, including those associated with the muscular, nervous, and vascular systems. The muscles in this region include the coracobrachialis (CBM), biceps brachii (BBM), and brachialis muscles, which are responsible for elbow flexion. The neurovascular system of the anterior brachial region contains terminal branches of the brachial plexus, the brachial artery, and its branches. The terminal branches of the brachial plexus are the musculocutaneous, median, ulnar, radial, and axillary nerves. Among these, the nerves related to the anterior part of the brachial region are the median and the musculocutaneous nerves. The brachial artery is a continuation of the axillary artery, which supplies the anterior part of the brachial region.
In the anterior brachial region, the most powerful flexor muscle is the BBM, which is also a powerful supinator of the forearm [
1]. Anatomically, the BBM has two heads, namely, short and long heads. The origin of the short head of the BBM is the coracoid process, while that of the long head is the supraglenoid tubercle of the scapula. Additionally, this muscle is inserted into the radial tuberosity and bicipital aponeurosis. The BBM is innervated by the MCN and supplied by the brachial artery. Traditionally, the BBM is described as a two-headed muscle; however, variations of the BBM, such as supernumerary heads, have been reported [
2,
3,
4,
5]. Previous studies have suggested that such BBM variations may be related to the variation of the MCN [
6,
7], as the penetration site of the MCN differs according to the presence of the supernumerary head of the BBM. However, studies on the correlations between the BBM and other structures of the anterior brachial region in the upper extremities remain rare.
In this study, the anterior brachial region in 103 upper extremities was dissected to recognize the topography of the BBM, CBM, and MCN, as well as to investigate the association between the supernumerary head of the BBM and other structures in the brachial region. The findings of this study have clinical implications and can be helpful for accurate diagnostic interpretation.
2. Materials and Methods
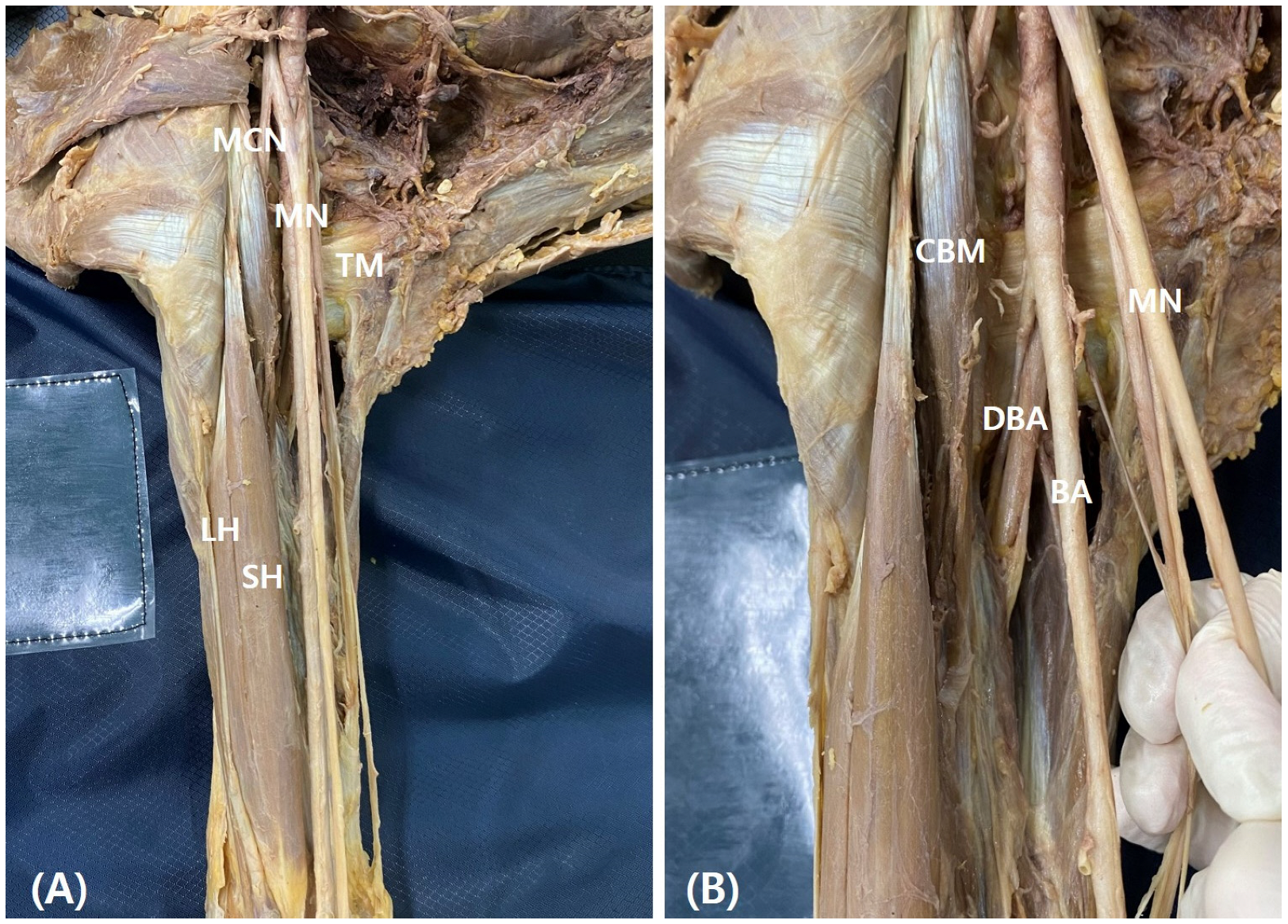
In this study, anterior brachial regions from 52 donated cadavers (103 cadaveric upper extremities) were dissected. Each cadaver was positioned in a supine position with the upper extremities extended. The skin and superficial fascia were removed by long deltopectoral incision and the deltopectoral fascia, pectoralis major, and deltoid muscles were identified. Then, the axillary sheath and adipose tissue were dissected to confirm the axillary and brachial arteries and the brachial plexus. The branches of the neurovascular structures were also identified (
Figure 1). The median nerve is formed by the connection of the lateral and medial cords, and this point was named the root of the median nerve. The brachial region was dissected and the BBM, CBM, and MCN were identified. The CBM perforates the MCN and inserts into the middle of the medial aspect of the humerus. The distance between the coracoid process and proximal and distal insertions of the CBM, BBM insertion (short head length), the root of the median nerve, the point of the CBM pierced by the MCN, and the origin of the deep brachial artery (DBA) were measured. These variables were compared according to the presence of the supernumerary head of the BBM. Furthermore, the correlation between the variation and other muscles and structures was analyzed. The length of the upper extremities from the coracoid process to the end of third finger was taken as the reference line, and the length of each variable was standardized with respect to the reference line and represented as a percentile. The length of each structure was measured using digital calipers (NA500-300S, Blue bird, Yongin-si, Korea). Each measurement was performed twice with an accuracy of up to 0.1 mm by two anatomists.
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) (Ver. 24.0, IBM Armonk, New York, NY, USA). To compare the variables, the Mann–Whitney U-test was used. Statistical significance was defined as a two-tailed p-value < 0.05.
3. Results
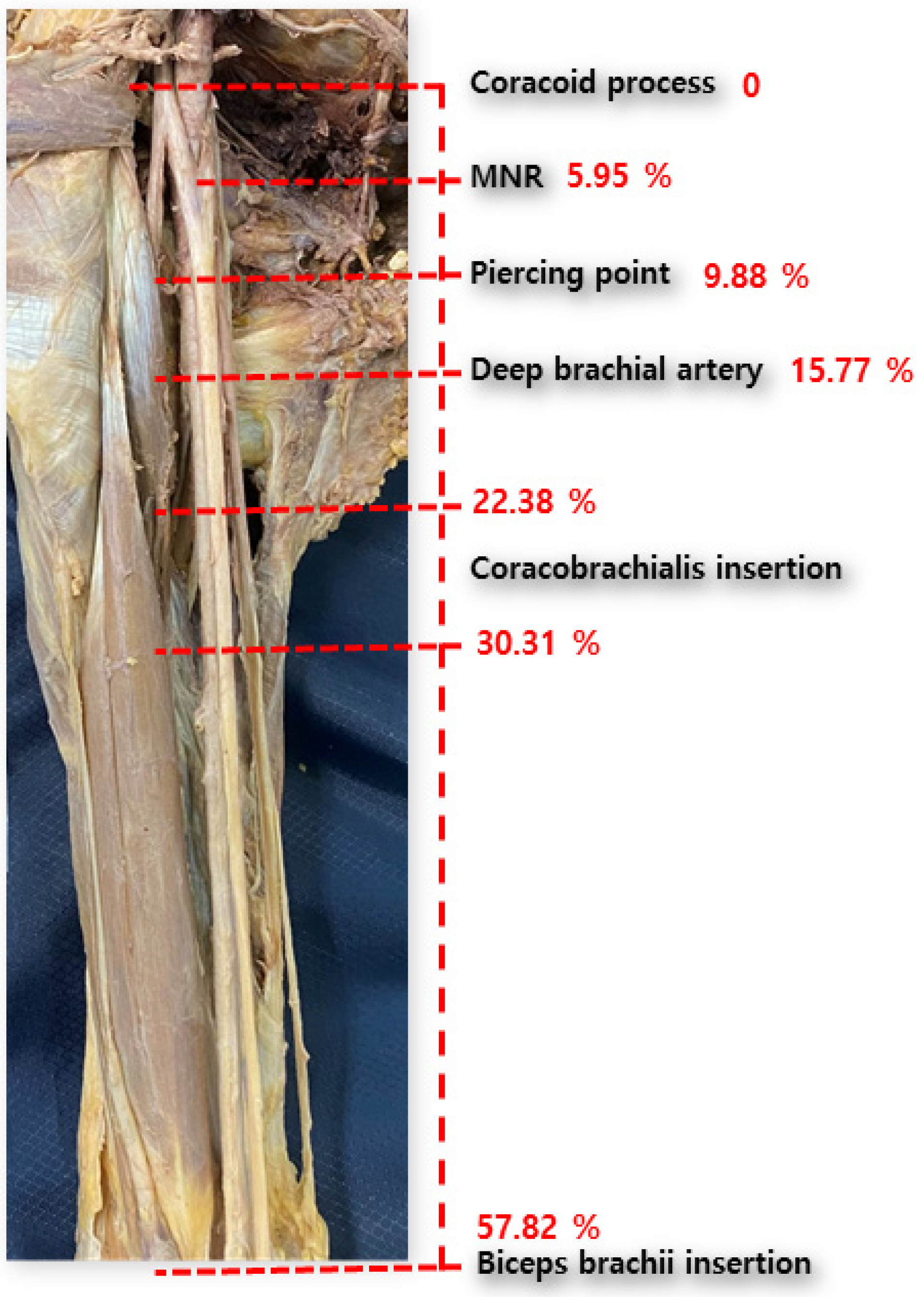
The mean total length of the upper extremity (taken as the reference line) was found to be 486.83 ± 31.31 mm. The distances between the coracoid process and proximal and distal insertions of the CBM were 109.03 ± 16.78 mm and 147.36 ± 16.04 mm, respectively. The length of the short head of the BBM was 281.33 ± 23.97 mm. The origin of the DBA and the root of the MN were 76.5 ± 24.45 mm and 29.06 ± 24.81 mm, respectively. The point at which the MCN pierces the CBM was located at 48.43 ± 20.11 mm (
Figure 2). Detailed information is presented in
Supplementary Table S1. In addition, the detailed topographies of the upper structures, according to the presence or absence of the accessory head, are presented in
Table 1. Its difference according to gender is summarized in
Supplementary Table S2.
The supernumerary head of the BBM was observed in 11.65% (12/103) of upper extremities; moreover, four heads of BBM were found (
Supplementary Figure S1). The differences between upper structures, according to this variation, are summarized in
Table 1. The accessory head was associated with a longer length of the upper limb (506.25 ± 32.55 mm vs. 484.27 ± 30.41 mm,
p = 0.022). The point of the CBM pierced by the MCN also differed between these groups; however, this difference was not statistically significant (47.23 ± 20.1 mm vs. 57.5 ± 18.58 mm,
p = 0.097). Other variables did not present any significant differences according to the presence of the supernumerary head.
The muscular and neurovascular topographies, according to the presence or absence of the supernumerary head, are presented as percentile data in
Table 2. Considering the mean of the percentile with respect to the reference line, the distances between the coracoid process and proximal and distal insertions of the CBM were 22.38 ± 3.07 and 30.31 ± 3.03, respectively. The length of short head of the BBM was 57.82 ± 3.66. The origin of the DBA and the root of the MN were 15.77 ± 5.16 and 5.95 ± 5.30, respectively. The point of the CBM pierced by the MCN was at 9.88 ± 3.88.
The distal insertion of the CBM was located more proximally in arms with a supernumerary head (28.18 ± 3.54%) than in those without it (30.59 ± 2.94%, p = 0.011). The length of the short head of the BBM was shorter in arms with a supernumerary head (55.11 ± 2.17%) than in those without it (58.18 ± 3.72%, p = 0.006). The other structures did not vary according to the presence of the accessory head.
4. Discussion
The anterior compartment of the upper extremity contains the BBM, CBM, and brachialis muscles, and variations in the BBM and CBM are frequently observed. Muscles in the anterior brachial region of the upper extremity act to promote flexion of the elbow joint, and these muscles are innervated by the musculocutaneous nerve (MCN). The axillary and brachial arteries are responsible for blood supply to this region [
8]. Variations in these neurovascular structures are also observed, and are often accompanied by variations in the abovementioned muscles. Therefore, the topography of neurovascular structures in the axilla and arm should be assessed according to major muscle variations.
First, we demonstrated the overall topography of the axilla and arm. From the coracoid process, the median nerve is formed from the lateral and medial cords at the 5 percentile level. Then, the MCN pierces the CBM at the 10 percentile level and the deep brachial artery originates from the brachial artery at the 15 percentile level. The CBM is widely inserted at the 20–30 percentile level of the upper limb. These data are similar to those reported in previous studies [
6,
9], and these major structures appear at regular intervals (about 5 percentile intervals). The landmarks of major anatomical structures at such regular intervals are clinically important and helpful. However, our results represent a relative value to the total length of upper limb and, thus, may differ from previous results [
10,
11], due to differences in the race or age of donated cadavers. A larger-scale study should be conducted using a clinically meaningful reference line.
Many authors have studied muscular and neurovascular variations in the upper extremities [
2,
3,
12,
13,
14,
15,
16]. Of these variations, the number and pattern of the BBM heads are the most variable. The reported frequency of the accessory head of BBM ranges from 8% to 37.5% [
17]. This variant muscle may cause idiopathic pain by neurovascular compression [
18,
19]. And it may be mistaken as a soft tissue tumor in ultrasound scans. Considering the high frequency and clinical importance of this variation, the topography of neurovascular structures was compared according to the presence of the accessory head. In this study, we found the supernumerary head of the BBM in 11.65% of cadavers. Interestingly, the upper limb was significantly longer in limbs with a supernumerary head than in those without it. The anterior compartment with a longer length requires more muscles; therefore, supernumerary muscles may have developed in response to the length. Embryologically, the mesoderm invades the upper limb bud and divides into ventral and dorsal muscular masses in the fifth week of development. The BBM is derived from the ventral mass, and its inappropriate cleavage may produce the supernumerary head. However, its embryological mechanism should be further investigated. Furthermore, the percentile level between the coracoid process and the coracobrachialis differed according to the variation of the BBM. In addition, the supernumerary head was associated with a shorter percentile of BBM length. This suggests that the total amount of muscle in the anterior part of the brachial region is fixed, such that the length of the original muscles can be shortened when additional muscles are present.
As a limitation in this study, no mention was made of differences by sex or age, as we sought to understand the overall correlation between the structures. Furthermore, when measuring the BBM, the short and long heads were not measured separately. Additionally, the length of the humerus is normally used as a reference line in research on the upper limb; however, in our study, the length of the entire upper limb was used as the reference line, given that the result was not statistically significant. As the upper limb length is not a clinically meaningful reference line, additional studies using other reference lines are needed. Considering that variations in this region are diverse, it is necessary to compare the topographies of these structures according to the pattern of the variations.
In this study, we demonstrated the topography of structures in the upper extremities and examined their correlations. It was demonstrated that variations in the upper extremities may affect the neuromuscular structures in this region. Differences in anatomical positions of neuromuscular structures due to the presence of a supernumerary head may cause confusion to clinicians.
5. Conclusions
Variations in the biceps brachii may affect the topography of other structures, such as the length of the upper extremities, insertion of the coracobrachialis, and length of the long head of the biceps brachii. Our results allowed for the determination of an embryological basis for the observed relationship between the muscular and neurovascular structures. Further studies should be carried out to explain and validate the possible clinical implications.
Supplementary Materials
The following supporting information can be downloaded at
https://www.mdpi.com/article/10.3390/medicina60111726/s1. Supplementary Table S1: Anatomical location of the muscle and neurovascular structures; Supplementary Table S2: Gender difference of anatomical location of the muscle and neurovascular structures; Supplementary Figure S1: Representative image of supernumerary head of the biceps brachii muscle (BBM). Between two heads, superior head (*) was found. Infero-lateral head of BBM was shown in middle level of upper arm, and this cadaver had four heads of BBM. CBM, coracobrachialis muscle; MNR, median nerve root.
Author Contributions
Conceptualization, J.-H.L. and Y.-R.H.; methodology, Y.-R.H.; software, Y.-R.H. and H.L.; validation, S.-W.L., B.-S.K. and H.-T.K.; formal analysis, Y.-R.H., S.-W.L. and B.-S.K.; investigation, J.-H.L. and Y.-R.H.; writing—original draft preparation, J.-H.L.; writing—review and editing, all authors; supervision, J.-H.L. and Y.-R.H.; funding acquisition, J.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Research Foundation of Korea (NRF-2021R1I1A3048089).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the ethical review and approval were waived for this study as this study started in 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Landin, D.; Thompson, M.; Jackson, M.R. Actions of the Biceps Brachii at the Shoulder: A Review. J. Clin. Med. Res. 2017, 9, 667–670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asvat, R.; Candler, P.; Sarmiento, E.E. High incidence of the third head of biceps brachii in south african populations. J. Anat. 1993, 182 Pt 1, 101–104. [Google Scholar]
- Abu-Hijleh, M.F. Three-headed biceps brachii muscle associated with duplicated musculocutaneous nerve. Clin. Anat. 2005, 18, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Kervancioglu, P.; Orhan, M. An anatomical study on the three-headed biceps brachii in human foetuses, and clinical relevance. Folia Morphol. 2011, 70, 116–120. [Google Scholar]
- Saluja, S.; Das, S.S.; Kumar, D.; Goswami, P. Bilateral three-headed biceps brachii muscle and its clinical implications. Int. J. Appl. Basic Med. Res. 2017, 7, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Cho, B.P.; Whang, T.S.; Yang, Y.C. The relation of the musculocutaneous nerve to the coracobrachialis muscle in Korean adults. Korean J. Phys. Anthropol. 1992, 5, 139–148. [Google Scholar] [CrossRef]
- Darvishi, M.; Moayeri, A. Anatomical variations of the musculocutaneous and median nerves: A case report. Folia. Med. 2019, 61, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.D.; Arora, Y.; Mahajan, K. Anatomy, Blood Vessels; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ozturk, A.; Bayraktar, B.; Taskara, N.; Kale, A.C.; Kutlu, C.; Cecen, A. Morphometric study of the nerves entering into the coracobrachialis muscle. Surg. Radiol. Anat. 2005, 27, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Satyapal, K.S.; Naidoo, N.; Lazarus, L. Long head of biceps brachii tendon and transverse humeral ligament morphometry and their associated pathology. Folia Morphol. 2020, 79, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B.; Sanudo, J.R.; Podgórski, M.; Zielinska, N.; Pires, M.B.; Aragonés, P.; Olewnik, Ł. A Proposal for a New Classification of the Supernumerary Heads of the Biceps Brachii Muscle. Biomed Res Int. 2022, 2022, 1510363. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Annaswamy, T.M. Trifid median nerve: A rare variant in a patient with carpal tunnel syndrome. Am. J. Phys. Med. Rehabil. 2019, 98, e67–e68. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Wysiadecki, G.; Haładaj, R.; Skrzat, J. Fusion between the median and musculocutaneous nerve: A case study. Folia. Med. Cracov. 2019, 59, 45–52. [Google Scholar] [PubMed]
- Guerri-Guttenberg, R.A.; Ingolotti, M. Classifying musculocutaneous nerve variations. Clin. Anat. 2009, 22, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Kawai, K.; Koizumi, M.; Kodama, K. The superficial brachial artery passing superficially to the pectoral ansa, the highest superficial brachial artery (arteria brachialis superficialis suprema). Anat. Sci. Int. 2011, 86, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kojyo, U.; Yanagisawa, N.; Mitomo, K.; Takayama, T.; Sakiyama, K.; Abe, S. Morphology and relationships of the biceps brachii and brachialis with the musculocutaneous nerve. Surg. Radiol. Anat. 2018, 40, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Manjatika, A.T.; Davimes, J.G.; Mazengenya, P. The third head of the biceps brachii muscle exhibiting variable shape presentation: Prevalence, variability and clinical considerations. Transl. Res. Anat. 2024, 34, 100282. [Google Scholar] [CrossRef]
- Snow, E.L.; Lanik, W.E. Structural and functional analysis of bilateral five-headed biceps brachii muscles with clinical insights. Transl. Res. Anat. 2024, 35, 100289. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).