Napabucasin Inhibits Proliferation and Migration of Glioblastoma Cells (U87) by Regulating JAK2/STAT3 Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assay
2.3. Antagonistic–Synergistic Mechanism Detection
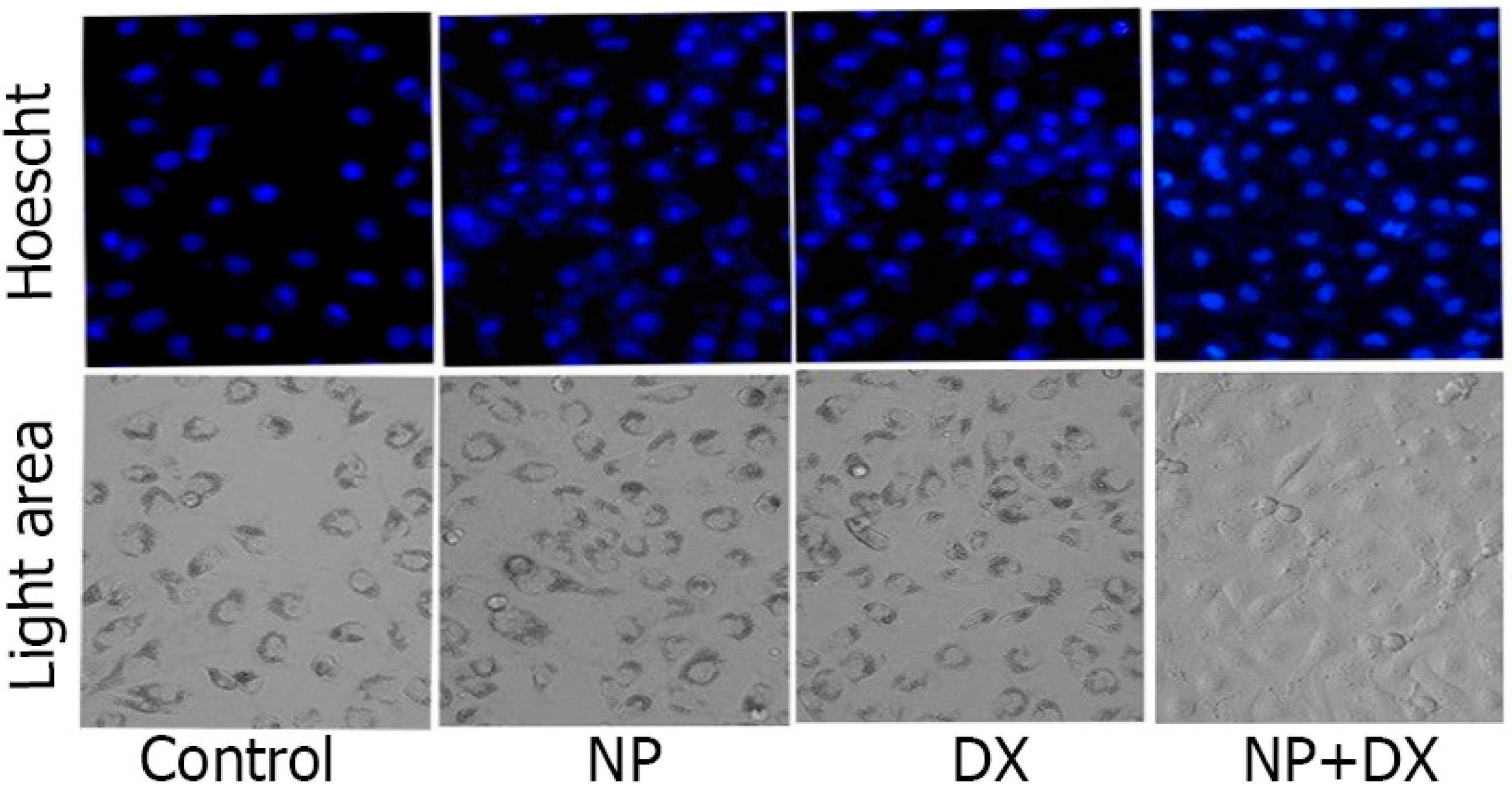
2.4. Apoptosis
2.5. Total RNA Isolation and cDNA Synthesis
2.6. Determination of Gene Expressions
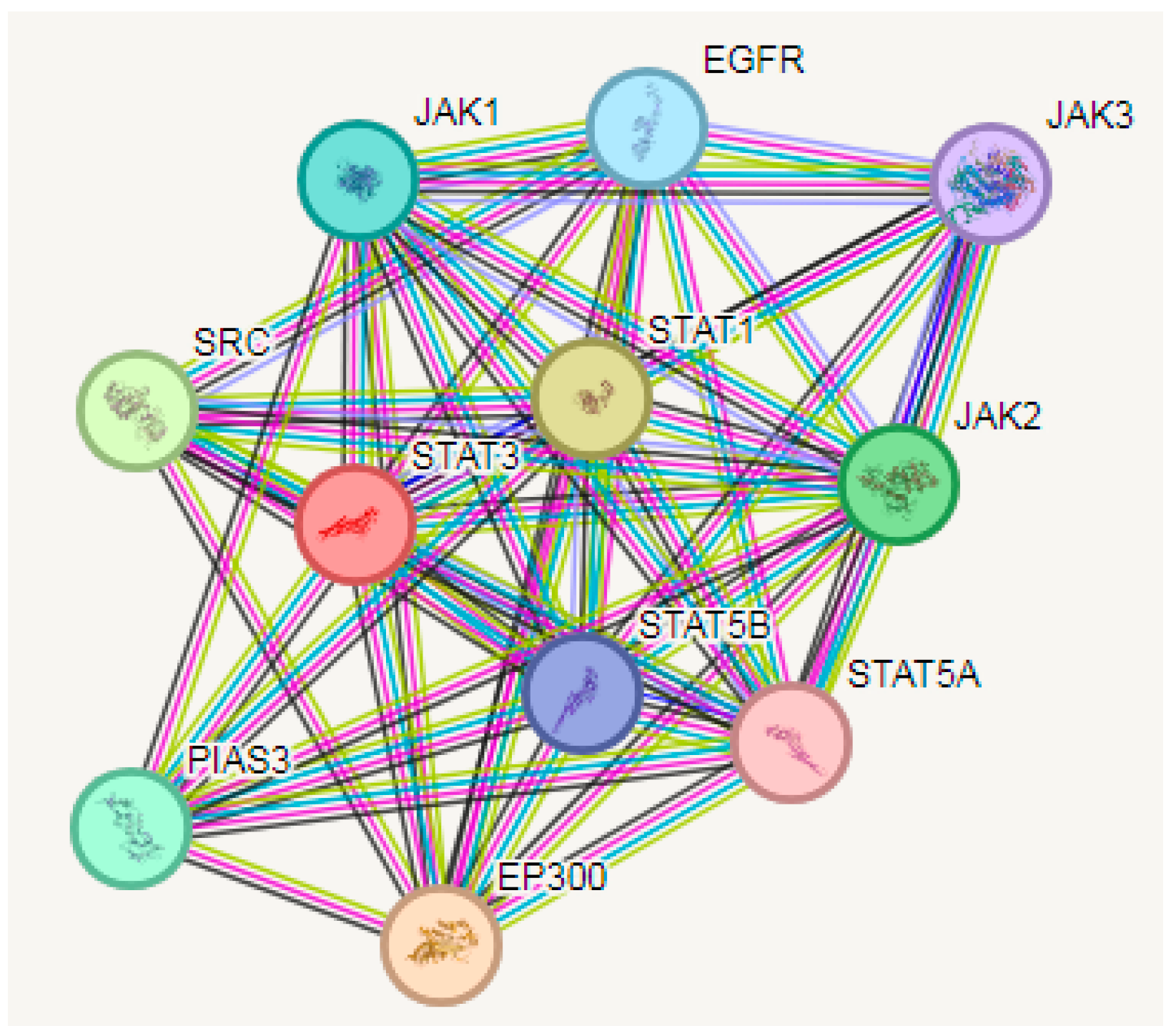
2.7. Protein–Protein Interaction (PPI) Analysis
2.8. Statistical Analysis
3. Results
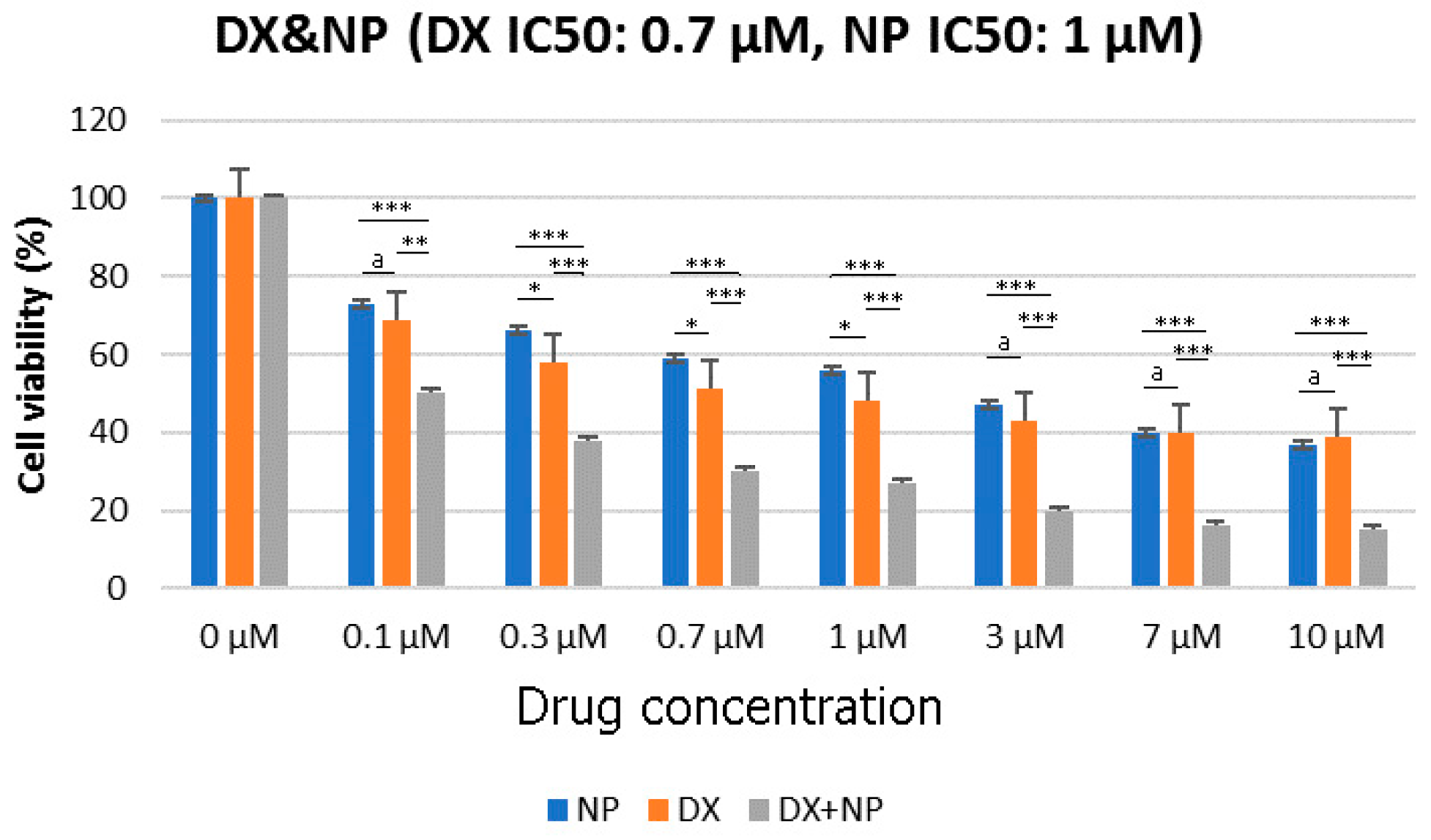
3.1. Cell Viability (%)
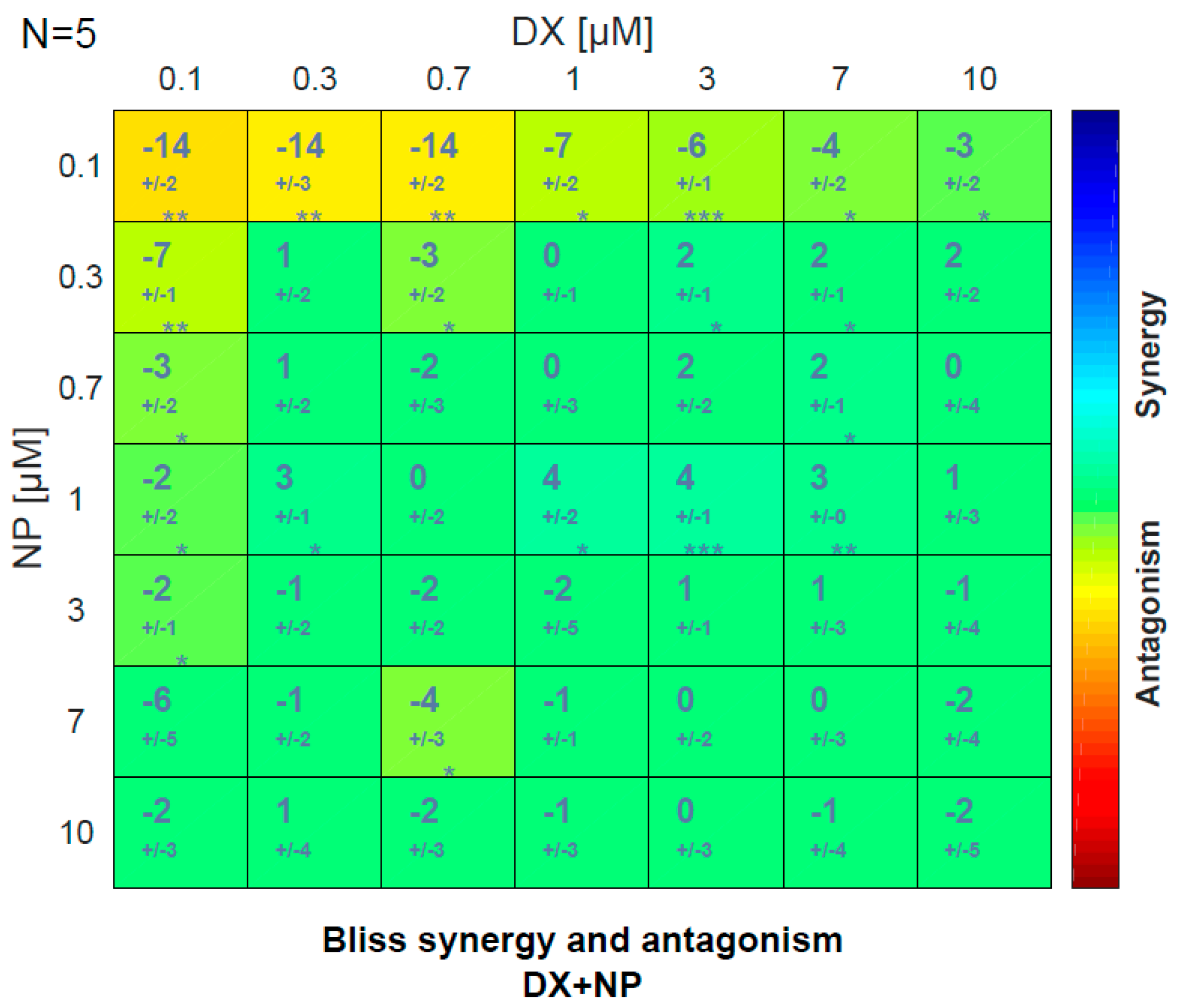
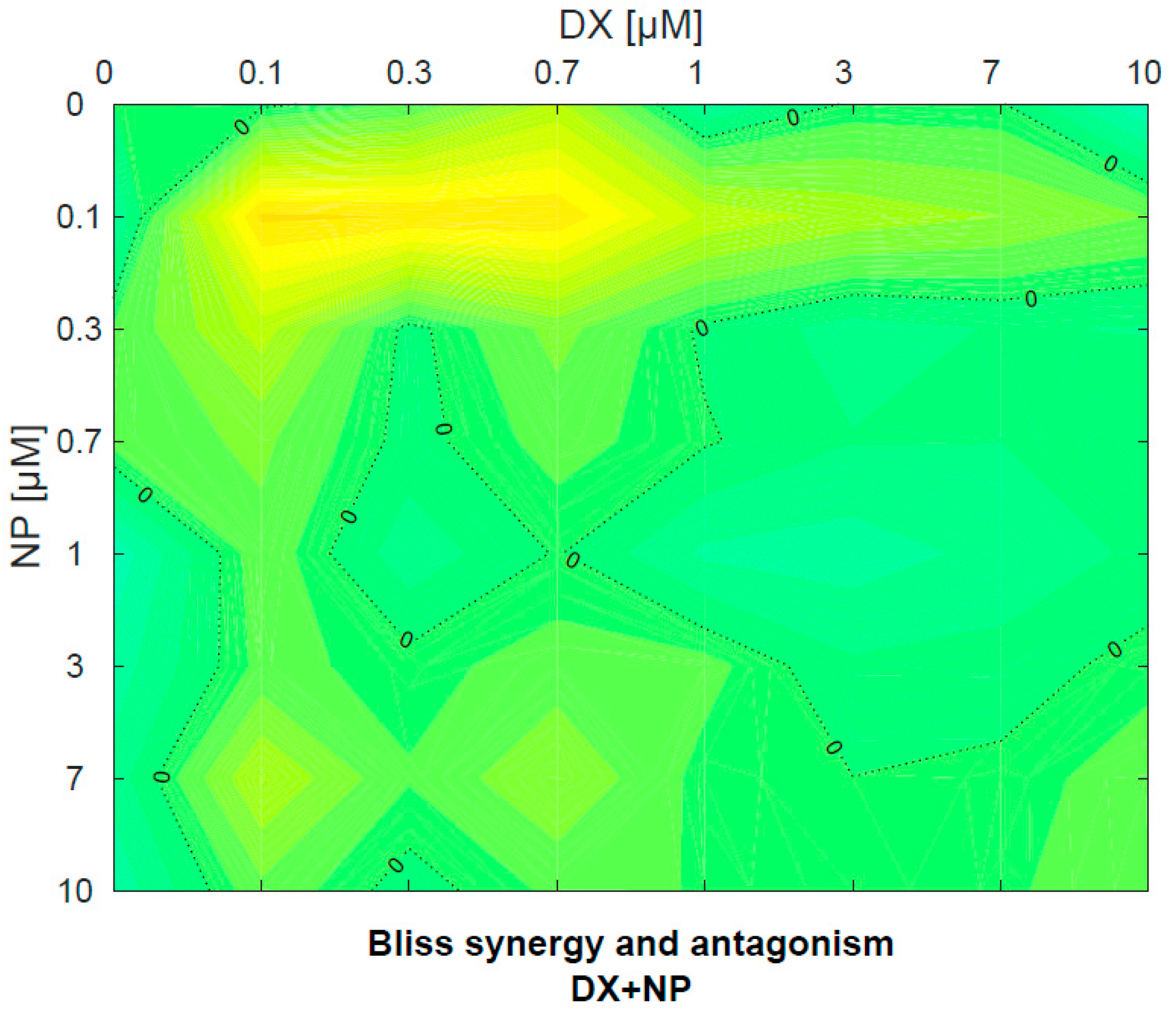
3.2. Antagonistic–Synergistic Effect
3.3. Apoptosis
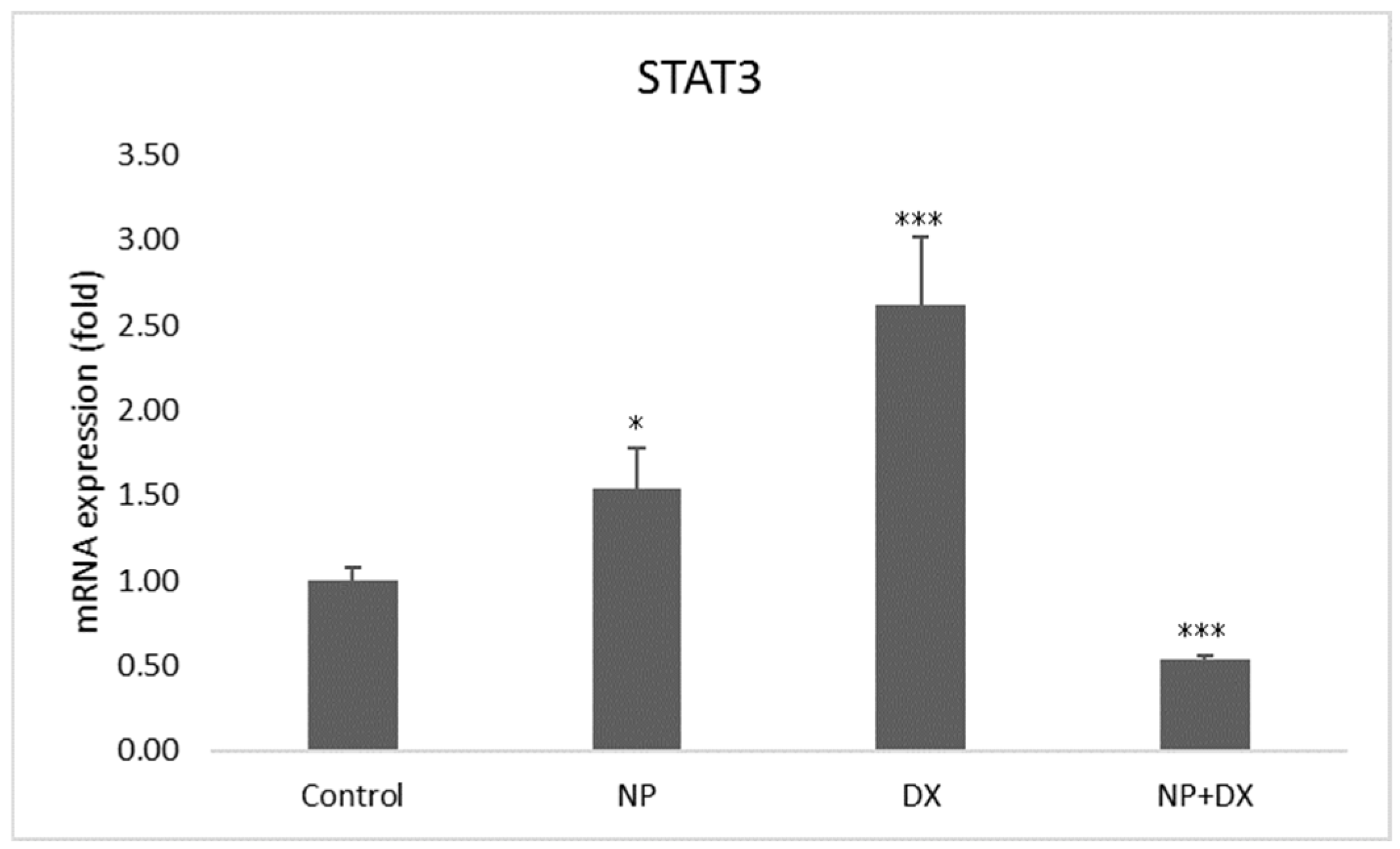
3.4. Gene Expression
3.5. PPI Analysis
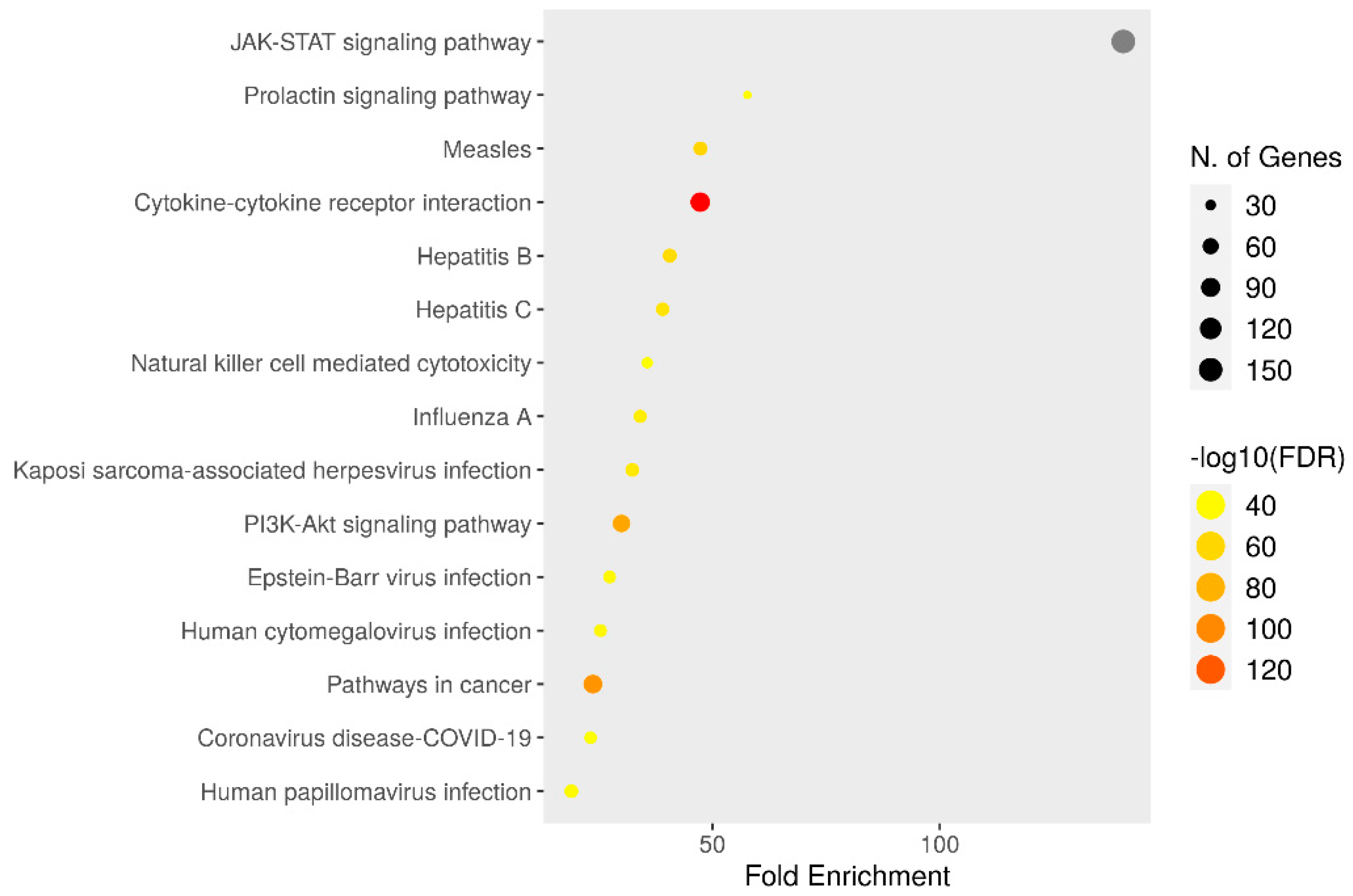
3.6. KEGG Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, R.K.; Calderon, C.; Vivas-Mejia, P.E. Targeting Non-coding RNA for Glioblastoma Therapy: The Challenge of Overcomes the Blood-Brain Barrier. Front. Med. Technol. 2021, 3, 678593. [Google Scholar] [CrossRef] [PubMed]
- Ayanlaja, A.A.; Ji, G.; Wang, J.; Gao, Y.; Cheng, B.; Kanwore, K.; Zhang, L.; Xiong, Y.; Kambey, P.A.; Gao, D. Doublecortin undergo nucleocytoplasmic transport via the RanGTPase signaling to promote glioma progression. Cell Commun. Signal. 2020, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, X.; Zhou, H.; Yao, D.; Wei, R.; Muhammad, S. Pediatric angiocentric glioma with acute intracerebral hemorrhage: A case report with 36 months follow-up. Surg. Neurol. Int. 2021, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Mao, L.; Mo, W.; Wang, R.; Zhang, Y. Resveratrol Enhances the Radiosensitivity by Inducing DNA Damage and Antitumor Immunity in a Glioblastoma Rat Model under 3 T MRI Monitoring. J. Oncol. 2022, 2022, 9672773. [Google Scholar] [CrossRef] [PubMed]
- Gojo, J.; Pavelka, Z.; Zapletalova, D.; Schmook, M.T.; Mayr, L.; Madlener, S.; Kyr, M.; Vejmelkova, K.; Smrcka, M.; Czech, T.; et al. Personalized Treatment of H3K27M-Mutant Pediatric Diffuse Gliomas Provides Improved Therapeutic Opportunities. Front. Oncol. 2020, 9, 1436. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Grothey, A. Napabucasin: An update on the first-in-class cancer stemness inhibitor. Drugs 2017, 77, 1091–1103. [Google Scholar] [CrossRef]
- Petsri, K.; Thongsom, S.; Racha, S.; Chamni, S.; Jindapol, S.; Kaekratoke, N.; Zou, H.; Chanvorachote, P. Novel mechanism of napabucasin, a naturally derived furanonaphthoquinone: Apoptosis and autophagy induction in lung cancer cells through direct targeting on Akt/mTOR proteins. BMC Complement. Med. Ther. 2022, 22, 250. [Google Scholar] [CrossRef]
- Shih, P.C. The role of the STAT3 signaling transduction pathways in radioresistance. Pharmacol. Ther. 2022, 234, 108118. [Google Scholar] [CrossRef]
- Froeling, F.E.M.; Swamynathan, M.M.; Deschênes, A.; Chio, I.I.C.; Brosnan, E.; Yao, M.A.; Alagesan, P.; Lucito, M.; Li, J.; Chang, A.Y.; et al. Bioactivation of Napabucasin Triggers Reactive Oxygen Species-Mediated Cancer Cell Death. Clin. Cancer Res. 2019, 25, 7162–7174. [Google Scholar] [CrossRef]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar]
- Zhang, Y.; Jin, Z.; Zhou, H.; Ou, X.; Xu, Y.; Li, H.; Liu, C.; Li, B. Suppression of prostate cancer progression by cancer cell stemness inhibitor napabucasin. Cancer Med. 2016, 5, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Y.; Wei, X. Napabucasin, a novel inhibitor of STAT3, inhibits growth and synergises with doxorubicin in diffuse large B-cell lymphoma. Cancer Lett. 2020, 491, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Farran, B.; Farren, M.; Chalikonda, G.; Wu, C.; Lesinski, G.B.; El-Rayes, B.F. Napabucasin (BBI 608), a potent chemoradiosensitizer in rectal cancer. Cancer 2020, 126, 3360–3371. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, A.; Kuboki, Y.; Shinozaki, E.; Hara, H.; Nishina, T.; Komatsu, Y.; Yuki, S.; Wakabayashi, M.; Nomura, S.; Sato, A.; et al. Multicenter phase I/II trial of napabucasin and pembrolizumab in patients with metastatic colorectal cancer (EPOC1503/SCOOP trial). Clin. Cancer Res. 2020, 26, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer. 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Man, J.; Yu, X.; Huang, H.; Zhou, W.; Xiang, C.; Huang, H.; Miele, L.; Liu, Z.; Bebek, G.; Bao, S.; et al. Hypoxic Induction of Vasorin Regulates Notch1 Turnover to Maintain Glioma Stem-like Cells. Cell Stem Cell 2018, 22, 104–118.e6. [Google Scholar] [CrossRef]
- MacDonagh, L.; Gray, S.G.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018, 428, 117–126. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Takemura, G.; Fujiwara, H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007, 49, 330–35252. [Google Scholar] [CrossRef]
- Alves, A.C.; Magarkar, A.; Horta, M.; Lima, J.L.F.C.; Bunker, A.; Nunes, C.; Reis, S. Influence of doxorubicin on model cell membrane properties: Insights from in vitro and in silico studies. Sci. Rep. 2017, 7, 6343. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Amelio, D.; Amichetti, M.; Salvati, M.; Muni, R.; Bozzao, A.; Lanzetta, G.; Scarpino, S.; Arcella, A.; Enrici, R.M. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother. Oncol. 2010, 97, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Fraass, B.A.; Marsh, L.H.; Herbort, K.; Gebarski, S.S.; Martel, M.K.; Radany, E.H.; Lichter, A.S.; Sandler, H.M. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int. J. Rad. Onc. Bio. Phy. 1999, 43, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Mesas, C.; Chico, M.A.; Doello, K.; Lara, P.; Moreno, J.; Melguizo, C.; Perazzoli, G.; Prados, J. Experimental Tumor Induction and Evaluation of Its Treatment in the Chicken Embryo Chorioallantoic Membrane Model: A Systematic Review. Int. Mol. Sci. 2024, 25, 837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cui, X.; Zhu, W.; Chen, X.; Shen, C.; Liu, Z.; Yang, G.; Liu, Y.; Zhao, S. Synergistic regulatory effects of microRNAs on brain glioma cells. Mol. Med. Rep. 2017, 16, 1409–1416. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, H.; Ren, H.; Sun, Y.; Chen, X. The Anticancer Effect of Napabucasin (BBI608), a Natural Naphthoquinone. Molecules 2023, 28, 5678. [Google Scholar] [CrossRef]
- Li, H.; Qian, Y.; Wang, X.; Pi, R.; Zhao, X.; Wei, X. Targeted activation of Stat3 in combination with paclitaxel results in increased apoptosis in epithelial ovarian cancer cells and a reduced tumour burden. Cell Prolif. 2020, 53, e12719. [Google Scholar] [CrossRef]
- Li, J.M.; Hsu, P.C.; Kuan, F.C.; Shi, C.S.; Yang, C.T. The cancer stemness inhibitor napabucasin suppresses small cell lung cancer growth through SOX2 expression. Am. J. Cancer Res. 2022, 12, 4637–4651. [Google Scholar]
- Han, D.; Yu, T.; Dong, N.; Wang, B.; Sun, F.; Jiang, D. Napabucasin, a novel STAT3 inhibitor suppresses proliferation, invasion and stemness of glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 289. [Google Scholar] [CrossRef]
- Bi, S.; Chen, K.; Feng, L.; Fu, G.; Yang, Q.; Deng, M.; Zhao, H.; Li, Z.; Yu, L.; Fang, Z.; et al. Napabucasin (BBI608) eliminate AML cells in vitro and in vivo via inhibition of Stat3 pathway and induction of DNA damage. Eur. J. Pharmacol. 2019, 855, 252–261. [Google Scholar] [CrossRef]
- Bitsch, R.; Kurzay, A.; Özbay Kurt, F.; De La Torre, C.; Lasser, S.; Lepper, A.; Siebenmorgen, A.; Müller, V.; Altevogt, P.; Utikal, J.; et al. STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J. Immunother. Cancer. 2022, 10, e004384. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, M.; Woo, D.H.; Shin, Y.; Shin, J.; Chang, N.; Oh, Y.T.; Kim, H.; Rheey, J.; Nakano, I.; et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell 2013, 23, 839–852. [Google Scholar] [CrossRef] [PubMed]


| JAK 2: F: CAGTGGTCAAGAGGGAAACA, R: TGTCTGAGCGAACAGTTTCC |
| STAT3: F: GGAGGAGTTGCAGCAAAAAG, R: TGTGTTTGTGCCCAGAATGT |
| β-actin: F: CCTCTGAACCCTAAGGCCAAC, R: TGCCACAGGATTCCATACCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ünlü, İ.; Özdemir, İ.; Tuncer, M.C. Napabucasin Inhibits Proliferation and Migration of Glioblastoma Cells (U87) by Regulating JAK2/STAT3 Signaling Pathway. Medicina 2024, 60, 1715. https://doi.org/10.3390/medicina60101715
Ünlü İ, Özdemir İ, Tuncer MC. Napabucasin Inhibits Proliferation and Migration of Glioblastoma Cells (U87) by Regulating JAK2/STAT3 Signaling Pathway. Medicina. 2024; 60(10):1715. https://doi.org/10.3390/medicina60101715
Chicago/Turabian StyleÜnlü, İlker, İlhan Özdemir, and Mehmet Cudi Tuncer. 2024. "Napabucasin Inhibits Proliferation and Migration of Glioblastoma Cells (U87) by Regulating JAK2/STAT3 Signaling Pathway" Medicina 60, no. 10: 1715. https://doi.org/10.3390/medicina60101715
APA StyleÜnlü, İ., Özdemir, İ., & Tuncer, M. C. (2024). Napabucasin Inhibits Proliferation and Migration of Glioblastoma Cells (U87) by Regulating JAK2/STAT3 Signaling Pathway. Medicina, 60(10), 1715. https://doi.org/10.3390/medicina60101715