Real-World Data Mining for Signal Detection of Antipsychotics-Associated Adverse Events Using the Korea Adverse Event Reporting System (KAERS) Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Study Medications
2.3. Data Acquisition and Definition of Adverse Drug Events
2.4. Statistical Analysis
3. Results
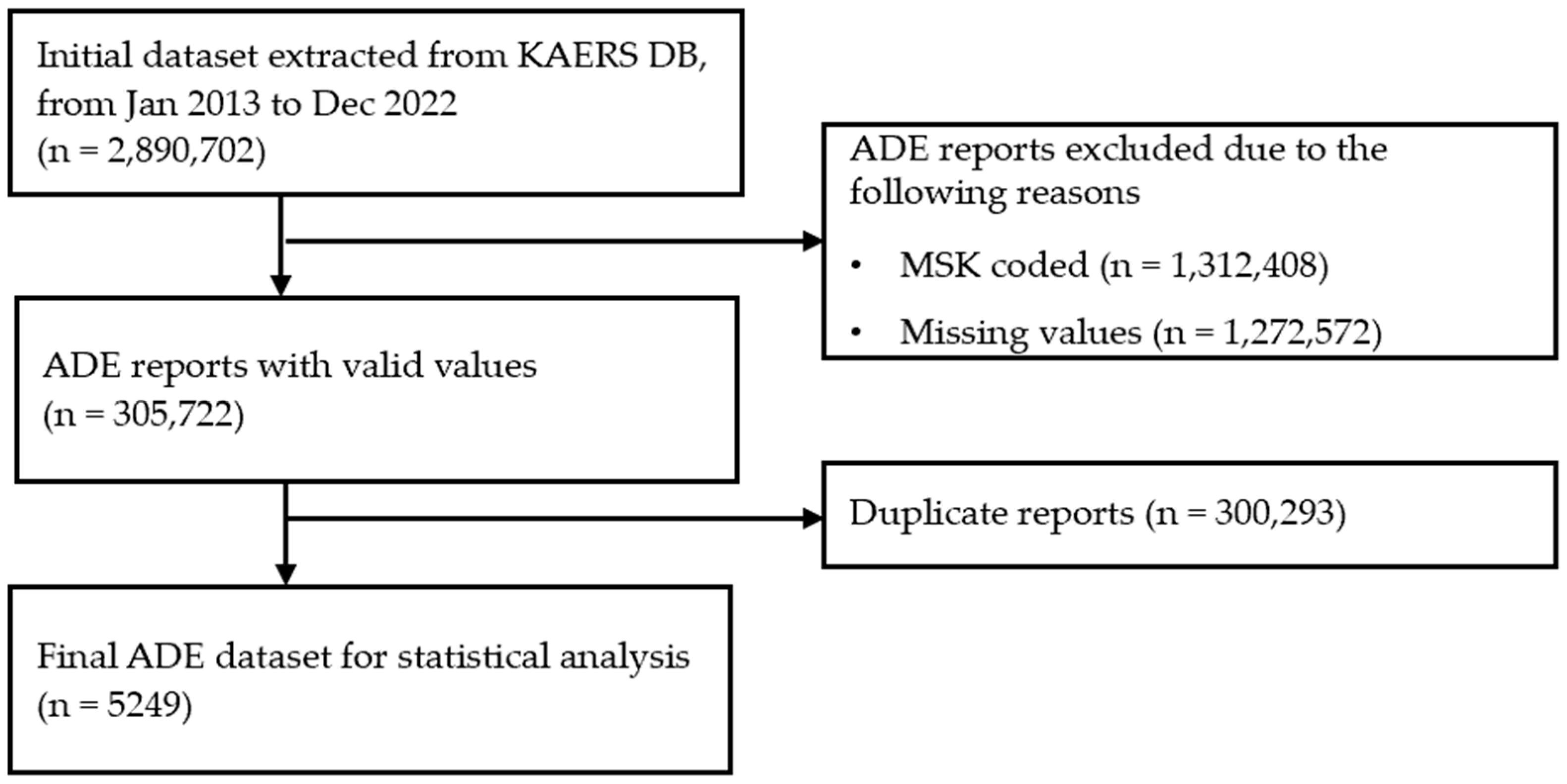
3.1. Data Filtering Process
3.2. Baseline Demographic Information of ADE Reports
3.3. Signal Detection through Data Mining Methods
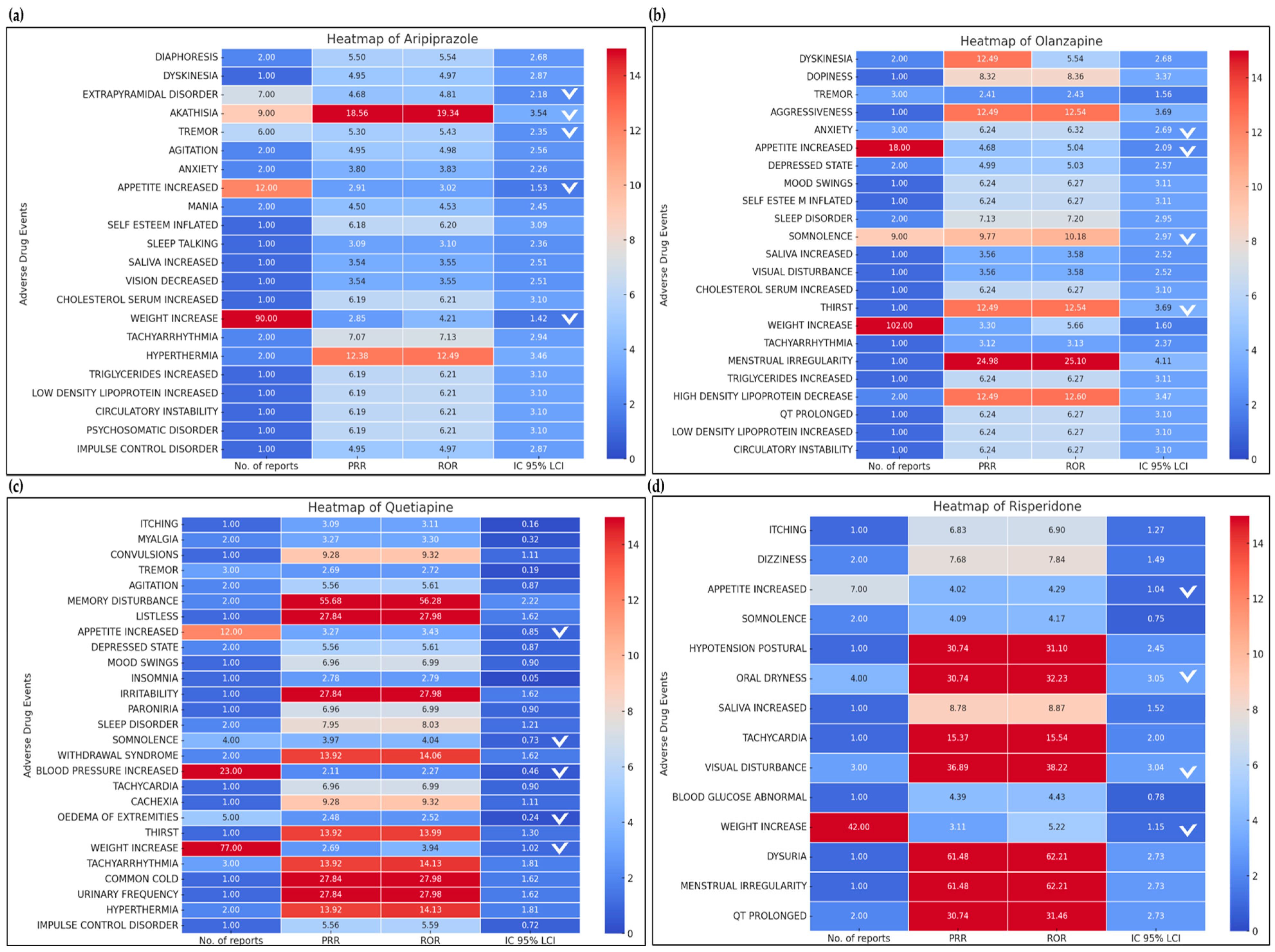
3.3.1. Second-Generation Antipsychotic: Aripiprazole
3.3.2. Second-Generation Antipsychotic: Olanzapine
3.3.3. Second-Generation Antipsychotic: Quetiapine
3.3.4. Second-Generation Antipsychotic: Risperidone
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Divac, N.; Prostran, M.; Jakovcevski, I.; Cerovac, N. Second-Generation Antipsychotics and Extrapyramidal Adverse Effects. BioMed Res. Int. 2014, 2014, 656370. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R. Antipsychotics in the Treatment of Schizophrenia: An Overview. J. Clin. Psychiatry 2011, 72, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Weiden, P.J. EPS Profiles: The Atypical Antipsychotics Are Not All the Same. J. Psychiatr. Pract. 2007, 13, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tarsy, D.; Baldessarini, R.J.; Tarazi, F.I. Effects of Newer Antipsychotics on Extrapyramidal Function. CNS Drugs 2002, 16, 23–45. [Google Scholar] [CrossRef]
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the Treatment-Resistant Schizophrenic. A Double-Blind Comparison with Chlorpromazine. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef]
- Kapur, S.; Seeman, P. Does Fast Dissociation from the Dopamine D2 Receptor Explain the Action of Atypical Antipsychotics?: A New Hypothesis. Am. J. Psychiatry 2001, 158, 360–369. [Google Scholar] [CrossRef]
- Kuroki, T.; Nagao, N.; Nakahara, T. Neuropharmacology of Second-Generation Antipsychotic Drugs: A Validity of the Serotonin-Dopamine Hypothesis. Prog. Brain Res. 2008, 172, 199–212. [Google Scholar] [CrossRef]
- Casey, D.E. Implications of the CATIE Trial on Treatment: Extrapyramidal Symptoms. CNS Spectr. 2006, 11, 25–31. [Google Scholar] [CrossRef]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1230. [Google Scholar] [CrossRef]
- Abou-Setta, A.M.; Mousavi, S.S.; Spooner, C.; Schouten, J.R.; Pasichnyk, D.; Armijo-Olivo, S.; Beaith, A.; Seida, J.C.; Dursun, S.; Newton, A.S.; et al. First-Generation versus Second-Generation Antipsychotics in Adults: Comparative Effectiveness. In Comparative Effectiveness Review No. 63; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. [Google Scholar] [PubMed]
- Fischer-Barnicol, D.; Lanquillon, S.; Haen, E.; Zofel, P.; Koch, H.J.; Dose, M.; Klein, H.E.; Working Group ‘Drugs in Psychiatry’. Typical and Atypical Antipsychotics—The Misleading Dichotomy: Results from the Working Group “Drugs in Psychiatry” (AGATE). Neuropsychobiology 2008, 57, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Ahn, K.M.; Kang, H.R.; Cho, S.H. Past, present, and future of pharmacovigilance in Korea. Asia Pac. Allergy 2017, 7, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Masand, P.S.; Narasimhan, M. Improving adherence to antipsychotic pharmacotherapy. Curr. Clin. Pharmacol. 2006, 1, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Jankovic, J. Metoclopramide-induced movement disorders: Clinical findings with a review of the literature. Arch. Intern. Med. 1989, 149, 2486–2492. [Google Scholar] [CrossRef]
- Junqueira, D.R.; Bennett, D.; Huh, S.Y.; Fahrbach, K.; Neupane, B.; Betts, M. Risk of adverse events associated with domperidone and metoclopramide in gastroparesis: Systematic review and meta-analysis. Drugs R&D 2023, 23, 1–20. [Google Scholar] [CrossRef]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian Neural Network Method for Adverse Drug Reaction Signal Generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef]
- Evans, S.J.W.; Waller, P.C.; Davis, S. Use of Proportional Reporting Ratios (PRRs) for Signal Generation from Spontaneous Adverse Drug Reaction Reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef]
- Coloma, P.M.; Avillach, P.; Salvo, F.; Schuemie, M.J.; Ferrajolo, C.; Pariente, A.; Fourrier-Réglat, A.; Molokhia, M.; Patadia, V.; van der Lei, J.; et al. A Reference Standard for Evaluation of Methods for Drug Safety Signal Detection Using Electronic Healthcare Record Databases. Drug Saf. 2013, 36, 13–23. [Google Scholar] [CrossRef]
- Keks, N.A. Minimizing the non-extrapyramidal side-effects of antipsychotics. Acta Psychiatr. Scand. Suppl. 1996, 389, 18–24. [Google Scholar] [CrossRef]
- Caroff, S.N.; Mann, S.C.; Campbell, E.C.; Sullivan, K.A. Movement disorders associated with atypical antipsychotic drugs. J. Clin. Psychiatry 2002, 63 (Suppl. S4), 12–19. [Google Scholar] [PubMed]
- Raveendran, N.S.; Tharyan, P.; Alexander, J.; Adams, C.E. Rapid tranquillisation in psychiatric emergency settings in India: Pragmatic randomised controlled trial of intramuscular olanzapine versus intramuscular haloperidol plus promethazine. BMJ 2007, 335, 865. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.P.; McGovern, K.M.; Perreard, I.M.; Huang, V.; Moss, L.B.; Blike, G.T. Inpatient respiratory arrest associated with sedative and analgesic medications: Impact of continuous monitoring on patient mortality and severe morbidity. J. Patient Saf. 2021, 17, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Tschoner, A.; Engl, J.; Laimer, M.; Kaser, S.; Rettenbacher, M.; Fleischhacker, W.W.; Patsch, J.R.; Ebenbichler, C.F. Metabolic side effects of antipsychotic medication. Int. J. Clin. Pract. 2007, 61, 1356–1370. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Moon, S.; Wang, J.; Choi, Y.J. Impact of institutional quality improvement initiatives on metabolic monitoring in mental disorder in patients treated with antipsychotics: A meta-analysis of intervention studies. J. Glob. Health 2024, 14, 04074. [Google Scholar] [CrossRef]
- Glazer, W.M. Extrapyramidal side effects, tardive dyskinesia, and the concept of atypicality. J. Clin. Psychiatry 2000, 61 (Suppl. S3), 16–21. [Google Scholar] [PubMed]
- Miyamoto, S.; Duncan, G.E.; Marx, C.E.; Lieberman, J.A. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 2005, 10, 79–104. [Google Scholar] [CrossRef]
- Misdrahi, D.; Tessier, A.; Daubigney, A.; Meissner, W.G.; Schurhoff, F.; Boyer, L.; Godin, O.; Bulzacka, E.; Aouizerate, B.; Andrianarisoa, M.; et al. Prevalence of and risk factors for extrapyramidal side effects of antipsychotics: Results from the national FACE-SZ cohort. J. Clin. Psychiatry 2019, 80, 7055. [Google Scholar] [CrossRef]
- Kannarkat, G.T.; Caroff, S.N.; Morley, J.F. Risk of drug-induced movement disorders with newer antipsychotic agents. Tremor Other Hyperkinet. Mov. 2022, 12, 19. [Google Scholar] [CrossRef]
| Signal Index | Definition | Criteria of Signal |
|---|---|---|
| PRR | PRR ≥ 2, χ2 ≥ 4, A ≥ 3 | |
| ROR | ROR ≥ 2, χ2 ≥ 4, A ≥ 3 | |
| IC | Lower limit of 95% CI ≥ 0 | |
| Specific adverse event | All other adverse events | |
| Specific drug | A | B |
| All other drugs | C | D |
| Sex (n = 4997) | |
| Men | 2871 (57.5%) |
| Women | 2126 (42.5%) |
| Age in years (n = 4192) | |
| <20 | 83 (2.0%) |
| 20–39 | 1050 (25.0%) |
| 40–59 | 1940 (46.3%) |
| 60–79 | 1089 (26.0%) |
| 80–99 | 30 (0.7%) |
| Causality (n = 2830) | |
| Certain | 74 (1.4%) |
| Probable/likely | 100 (1.9%) |
| Possible | 2656 (50.6%) |
| Reporter type (n = 5249) | |
| Pharmaceutical company | 4341 (82.7%) |
| Regional pharmacovigilance center | 893 (17.0%) |
| Medical professional | 13 (0.2%) |
| Others (e.g., distributors or other organizations) | 2 (0.0%) |
| Report type (n = 5249) | |
| Report from study/research | 3834 (73.0%) |
| Voluntary report | 1071 (20.4%) |
| Others | 344 (6.6%) |
| Final disposition of patients (n = 5249) | |
| Recovered | 3435 (65.4%) |
| Not recovered | 793 (15.1%) |
| Recovered with sequelae | 75 (1.4%) |
| Unknown | 946 (18.0%) |
| Serious ADE | |
| Hospitalization | 129/5249 (2.5%) |
| Significant medical situation | 114/5249 (2.2%) |
| Adverse Event | No. of Reports | PRR | ROR | IC 95% LCI | MFDS | FDA |
|---|---|---|---|---|---|---|
| Diaphoresis | 2 | 5.50 | 5.54 | 2.68 | Y | Y |
| Dyskinesia | 1 | 4.95 | 4.97 | 2.87 | Y | Y |
| Extrapyramidal disorder * | 7 | 4.68 * | 4.81 * | 2.18 * | Y | Y |
| Akathisia * | 9 | 18.56 * | 19.34 * | 3.54 * | Y | Y |
| Tremor * | 6 | 5.30 * | 5.43 * | 2.35 * | Y | Y |
| Agitation | 2 | 4.95 | 4.98 | 2.56 | Y | Y |
| Anxiety | 2 | 3.80 | 3.83 | 2.26 | Y | Y |
| Appetite increased * | 12 | 2.91 * | 3.02 * | 1.53 * | Y | Y |
| Mania | 2 | 4.50 | 4.53 | 2.45 | Y | Y |
| Self-esteem inflated | 1 | 6.18 | 6.2 | 3.09 | Y | Y |
| Sleep talking | 1 | 3.09 | 3.10 | 2.35 | Y | Y |
| Saliva increased | 1 | 3.53 | 3.54 | 2.51 | Y | Y |
| Vision decreased | 1 | 3.53 | 3.54 | 2.51 | Y | Y |
| Cholesterol serum increased | 1 | 6.18 | 6.2 | 3.09 | Y | Y |
| Weight increased * | 90 | 2.84 * | 4.21 * | 1.41 * | Y | Y |
| Tachyarrhythmia | 2 | 7.07 | 7.13 | 2.94 | Y | Y |
| Hyperthermia | 2 | 12.37 | 12.48 | 3.45 | Y | Y |
| Triglyceride increased | 1 | 6.18 | 6.21 | 3.09 | Y | Y |
| Low-density lipoprotein increased | 1 | 6.18 | 6.21 | 3.09 | Y | Y |
| Circulatory instability | 1 | 6.18 | 6.21 | 3.09 | Y | Y |
| Psychosomatic disorder | 1 | 6.18 | 6.21 | 3.09 | Y | Y |
| Impulse control disorder | 1 | 4.95 | 4.97 | 2.87 | Y | Y |
| Adverse Event | No. of Reports | PRR | ROR | IC 95% LCI | MFDS | FDA |
|---|---|---|---|---|---|---|
| Dyskinesia | 2 | 12.49 | 5.54 | 2.68 | Y | Y |
| Dopiness | 1 | 8.32 | 8.36 | 3.37 | Y | Y |
| Tremor | 3 | 2.41 | 2.43 | 1.56 | Y | Y |
| Aggressiveness | 1 | 12.49 | 12.54 | 3.69 | Y | N |
| Anxiety * | 3 | 6.24 * | 6.32 * | 2.69 * | Y | Y |
| Appetite increased * | 18 | 4.68 * | 5.04 * | 2.09 * | Y | Y |
| Depressed state | 2 | 4.99 | 5.03 | 2.57 | Y | Y |
| Mood swings | 1 | 6.24 | 6.27 | 3.11 | Y | Y |
| Self-esteem inflated | 1 | 6.24 | 6.27 | 3.11 | N | N |
| Sleep disorder | 2 | 7.13 | 7.2 | 2.95 | Y | Y |
| Somnolence * | 9 | 9.77 * | 10.18 * | 2.97 * | Y | Y |
| Saliva increased | 1 | 3.56 | 3.58 | 2.52 | Y | Y |
| Visual disturbance | 1 | 3.56 | 3.58 | 2.52 | Y | Y |
| Cholesterol serum increased | 1 | 6.24 | 6.27 | 3.10 | Y | Y |
| Thirst | 1 | 12.49 | 12.54 | 3.69 | Y | Y |
| Weight increased * | 102 | 3.30 * | 5.66 * | 1.60 * | Y | Y |
| Tachyarrhythmia | 1 | 3.12 | 3.13 | 2.37 | Y | Y |
| Menstrual irregularity | 1 | 24.98 | 25.10 | 4.11 | Y | Y |
| Triglyceride increased | 1 | 6.24 | 6.27 | 3.11 | Y | Y |
| High-density lipoprotein decreased | 1 | 6.24 | 6.27 | 3.11 | Y | Y |
| QT prolonged | 2 | 12.49 | 12.60 | 3.47 | Y | Y |
| Low-density lipoprotein increased | 1 | 6.24 | 6.27 | 3.10 | Y | Y |
| Circulatory instability | 1 | 6.24 | 6.27 | 3.10 | N | N |
| Adverse Event | No. of Reports | PRR | ROR | IC 95% LCI | MFDS | FDA |
|---|---|---|---|---|---|---|
| Itching | 1 | 3.09 | 3.11 | 0.16 | N | N |
| Myalgia | 2 | 3.27 | 3.30 | 0.32 | Y | Y |
| Convulsions | 1 | 9.28 | 9.32 | 1.11 | Y | N |
| Tremor | 3 | 2.69 | 2.72 | 0.19 | Y | Y |
| Agitation | 2 | 5.56 | 5.61 | 0.87 | Y | Y |
| Memory disturbance | 2 | 55.68 | 56.28 | 2.22 | N | Y |
| Listless | 1 | 27.84 | 27.98 | 1.62 | Y | Y |
| Appetite increased * | 12 | 3.27 * | 3.43 * | 0.85 * | Y | Y |
| Depressed state | 2 | 5.56 | 5.61 | 0.87 | Y | Y |
| Mood swings | 1 | 6.96 | 6.99 | 0.90 | Y | Y |
| Insomnia | 1 | 2.78 | 2.79 | 0.05 | Y | Y |
| Irritability | 1 | 27.84 | 27.98 | 1.62 | Y | Y |
| Paroniria | 1 | 6.96 | 6.99 | 0.90 | Y | Y |
| Sleep disorder | 2 | 7.95 | 8.03 | 1.21 | Y | Y |
| Somnolence * | 4 | 3.97 * | 4.04 * | 0.73 * | Y | Y |
| Withdrawal syndrome | 2 | 13.92 | 14.06 | 1.62 | Y | Y |
| Blood pressure increased * | 23 | 2.11 * | 2.27 * | 0.46 * | Y | Y |
| Tachycardia | 1 | 6.96 | 6.99 | 0.90 | Y | Y |
| Cachexia | 1 | 9.28 | 9.32 | 1.11 | N | N |
| Oedema of extremities * | 5 | 2.48 * | 2.52 * | 0.24 * | Y | Y |
| Thirst | 1 | 13.92 | 13.99 | 1.3 | Y | Y |
| Weight increased * | 77 | 2.69 * | 3.94 * | 1.02 * | Y | Y |
| Tachyarrhythmia * | 3 | 13.92 * | 14.13 * | 1.81 * | Y | Y |
| Common cold | 1 | 27.84 | 27.98 | 1.62 | N | N |
| Urinary frequency | 1 | 27.84 | 27.98 | 1.62 | N | N |
| Hyperthermia | 2 | 13.92 | 14.13 | 1.81 | N | N |
| Impulse control disorder | 1 | 5.56 | 5.59 | 0.72 | Y | Y |
| Adverse Event | No. of Reports | PRR | ROR | IC 95% LCI | MFDS | FDA |
|---|---|---|---|---|---|---|
| Itching | 1 | 6.83 | 6.90 | 1.27 | Y | N |
| Dizziness | 2 | 7.68 | 7.84 | 1.49 | Y | Y |
| Appetite increased * | 7 | 4.02 * | 4.29 * | 1.04 * | Y | Y |
| Somnolence | 2 | 4.09 | 4.17 | 0.75 | Y | Y |
| Hypotension postural | 1 | 30.74 | 31.10 | 2.45 | Y | Y |
| Oral Dryness * | 4 | 30.74 * | 32.23 * | 3.05 * | Y | Y |
| Saliva increased | 1 | 8.78 | 8.87 | 1.52 | Y | Y |
| Tachycardia | 1 | 15.37 | 15.54 | 2.00 | Y | Y |
| Visual disturbance * | 3 | 36.89 * | 38.22 * | 3.04 * | Y | Y |
| Blood glucose abnormal | 1 | 4.39 | 4.43 | 0.78 | Y | Y |
| Weight increased * | 42 | 3.11 * | 5.22 * | 1.15 * | Y | Y |
| Dysuria | 1 | 61.48 | 62.21 | 2.73 | Y | Y |
| Menstrual irregularity | 1 | 61.48 | 62.21 | 2.73 | Y | Y |
| QT Prolonged | 2 | 30.74 | 31.46 | 2.73 | Y | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Ko, M.; Choi, Y.-J.; Shin, S. Real-World Data Mining for Signal Detection of Antipsychotics-Associated Adverse Events Using the Korea Adverse Event Reporting System (KAERS) Database. Medicina 2024, 60, 1714. https://doi.org/10.3390/medicina60101714
Moon S, Ko M, Choi Y-J, Shin S. Real-World Data Mining for Signal Detection of Antipsychotics-Associated Adverse Events Using the Korea Adverse Event Reporting System (KAERS) Database. Medicina. 2024; 60(10):1714. https://doi.org/10.3390/medicina60101714
Chicago/Turabian StyleMoon, Suhyeon, Minjung Ko, Yeo-Jin Choi, and Sooyoung Shin. 2024. "Real-World Data Mining for Signal Detection of Antipsychotics-Associated Adverse Events Using the Korea Adverse Event Reporting System (KAERS) Database" Medicina 60, no. 10: 1714. https://doi.org/10.3390/medicina60101714
APA StyleMoon, S., Ko, M., Choi, Y.-J., & Shin, S. (2024). Real-World Data Mining for Signal Detection of Antipsychotics-Associated Adverse Events Using the Korea Adverse Event Reporting System (KAERS) Database. Medicina, 60(10), 1714. https://doi.org/10.3390/medicina60101714






