Serum Glial Fibrillary Acidic Protein Can Predict Cross-Sectional Vasculitis Activity by Reflecting Renal Involvement in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Data
2.3. Measurement of Serum GFAP
2.4. Statistical Analyses
3. Results
3.1. Characteristics
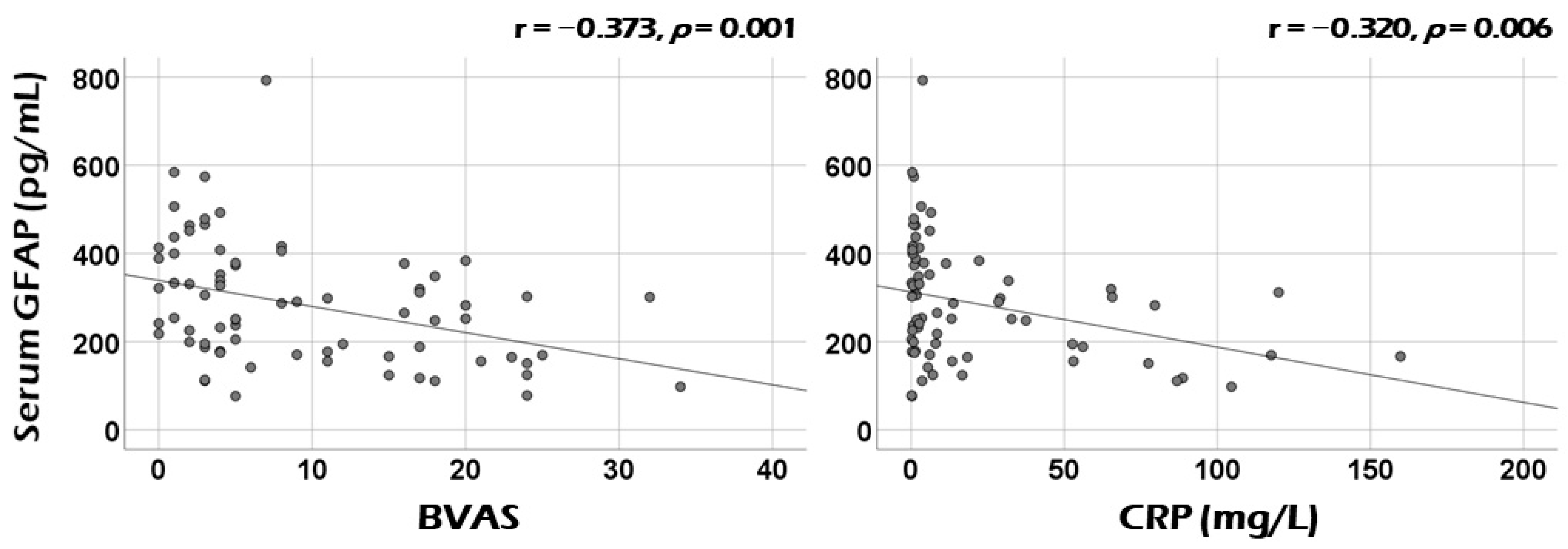
3.2. Correlation Analysis of Serum GFAP with Continuous Variables at Diagnosis
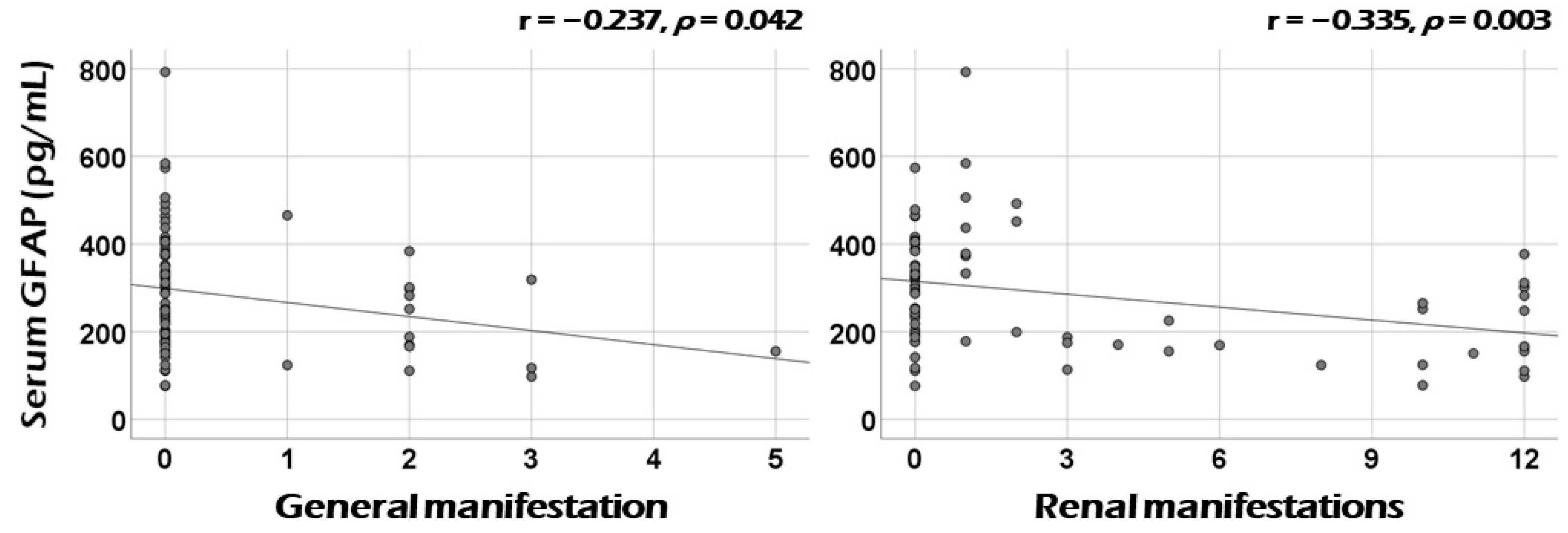
3.3. Correlation Analysis of Serum GFAP with the Sum of Scores of Each Systemic Item of BVAS at Diagnosis
3.4. Association between Serum GFAP and Renal Manifestation at Diagnosis
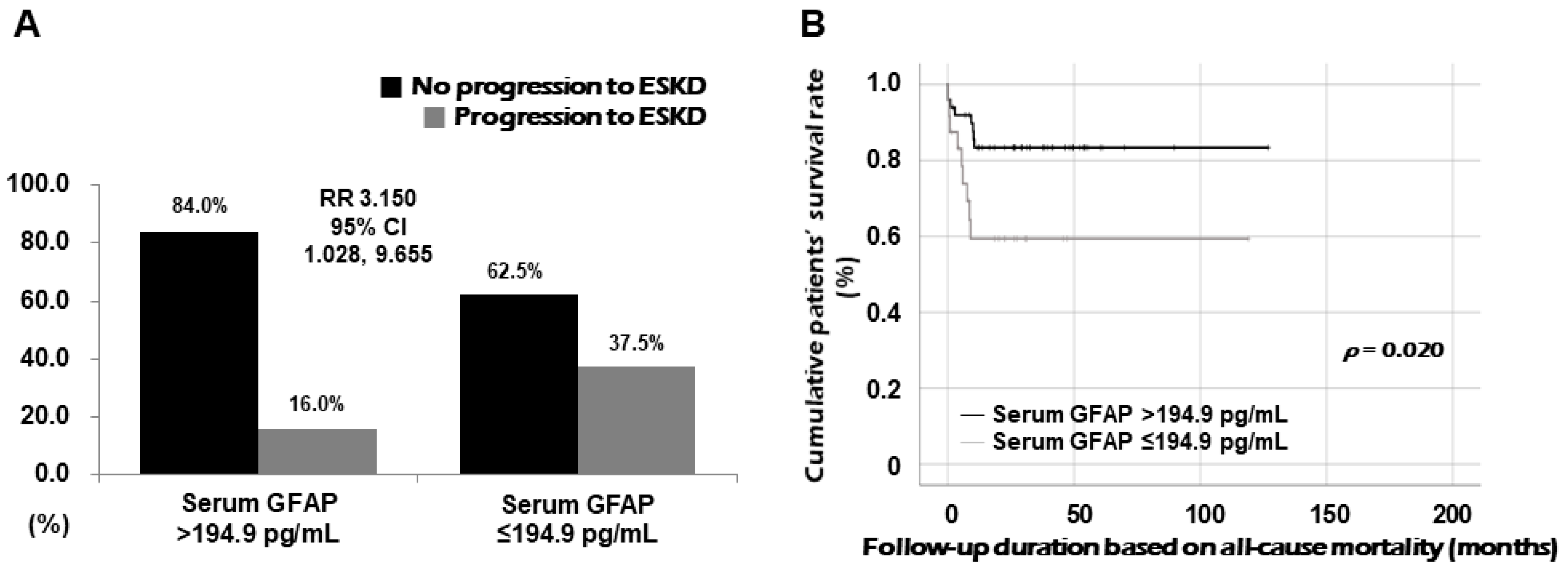
3.5. Relative Risk of Serum GFAP for Progression to ESKD
3.6. Comparison of Cumulative Survival Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, R.; Robson, J.C.; Grayson, P.C.; Ponte, C.; Craven, A.; Khalid, S.; Judge, A.; Hutchings, A.; Merkel, P.A.; Luqmani, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann. Rheum. Dis. 2022, 81, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Robson, J.C.; Grayson, P.C.; Ponte, C.; Suppiah, R.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Watts, R.A.; Merkel, P.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann. Rheum. Dis. 2022, 81, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef]
- Park, P.G.; Song, J.J.; Park, Y.B.; Lee, S.W. Clinical application of low erythrocyte sedimentation rate/high C-reactive protein to antineutrophil cytoplasmic antibody-associated vasculitis. J. Clin. Lab. Anal. 2022, 36, e24237. [Google Scholar] [CrossRef]
- Kim, J.S. Protein biomarkers in multiple sclerosis. Encephalitis 2023, 3, 54–63. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Amalia, L. Glial Fibrillary Acidic Protein (GFAP): Neuroinflammation Biomarker in Acute Ischemic Stroke. J. Inflamm. Res. 2021, 14, 7501–7506. [Google Scholar] [CrossRef]
- Neubauer, K.; Knittel, T.; Aurisch, S.; Fellmer, P.; Ramadori, G. Glial fibrillary acidic protein--a cell type specific marker for Ito cells in vivo and in vitro. J. Hepatol. 1996, 24, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Buniatian, G.H.; Hartmann, H.J.; Traub, P.; Wiesinger, H.; Albinus, M.; Nagel, W.; Shoeman, R.; Mecke, D.; Weser, U. Glial fibrillary acidic protein-positive cells of the kidney are capable of raising a protective biochemical barrier similar to astrocytes: Expression of metallothionein in podocytes. Anat. Rec. 2002, 267, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Huang, W.; Wang, L.; ZhangBao, J.; Zhou, L.; Lu, C.; Wang, M.; Yu, J.; Li, H.; Li, Y.; et al. Serum Neurofilament Light and GFAP Are Associated With Disease Severity in Inflammatory Disorders With Aquaporin-4 or Myelin Oligodendrocyte Glycoprotein Antibodies. Front. Immunol. 2021, 12, 647618. [Google Scholar] [CrossRef]
- Valletta, M.; Vetrano, D.L.; Rizzuto, D.; Winblad, B.; Canevelli, M.; Andersson, S.; Dale, M.; Fredolini, C.; Fratiglioni, L.; Grande, G. Blood biomarkers of Alzheimer’s disease in the community: Variation by chronic diseases and inflammatory status. Alzheimers Dement. 2024, 20, 4115–4125. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, J.; Hou, Y.; Shi, X.; Liu, K. Prediction of clinical progression in nervous system diseases: Plasma glial fibrillary acidic protein (GFAP). Eur. J. Med. Res. 2024, 29, 51. [Google Scholar] [CrossRef] [PubMed]
- Batu, E.D.; Ozen, S. Measuring Vasculitis with Numbers: Outcome Scores. Curr. Rheumatol. Rev. 2020, 16, 21–28. [Google Scholar] [CrossRef]
- Watts, R.A.; Scott, D.G. Vasculitis. Baillieres Clin. Rheumatol. 1995, 9, 529–554. [Google Scholar] [CrossRef]
- Bhamra, K.; Luqmani, R. Damage assessment in ANCA-associated vasculitis. Curr. Rheumatol. Rep. 2012, 14, 494–500. [Google Scholar] [CrossRef]
- Gairing, S.J.; Danneberg, S.; Kaps, L.; Nagel, M.; Schleicher, E.M.; Quack, C.; Engel, S.; Bittner, S.; Galle, P.R.; Schattenberg, J.M.; et al. Elevated serum levels of glial fibrillary acidic protein are associated with covert hepatic encephalopathy in patients with cirrhosis. JHEP Rep. 2023, 5, 100671. [Google Scholar] [CrossRef]
- Kammeyer, R.; Chapman, K.; Furniss, A.; Hsieh, E.; Fuhlbrigge, R.; Ogbu, E.A.; Boackle, S.; Zell, J.; Nair, K.V.; Borko, T.L.; et al. Blood-based biomarkers of neuronal and glial injury in active major neuropsychiatric systemic lupus erythematosus. Lupus 2024, 33, 1116–1129. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Omar, H.R.; Sweiss, N. Hypoalbuminemia is related to inflammation rather than malnutrition in sarcoidosis. Eur. J. Intern. Med. 2018, 53, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, R.; Wang, Y.; Li, H.; Han, Q.; Wu, Y.; Wang, T.; Liu, F. The Level of Serum Albumin Is Associated with Renal Prognosis in Patients with Diabetic Nephropathy. J. Diabetes Res. 2019, 2019, 7825804. [Google Scholar] [CrossRef] [PubMed]

| Variables | Values |
|---|---|
| At diagnosis | |
| Demographic data | |
| Age (years) | 63.5 (51.8−73.3) |
| Male sex (N, (%)) | 32 (43.2) |
| Female sex (N, (%)) | 42 (56.8) |
| AAV subtype (N, (%)) | |
| MPA | 35 (47.3) |
| GPA | 23 (31.1) |
| EGPA | 16 (21.6) |
| ANCA positivity (N, (%)) | |
| MPO-ANCA titre | 0 (0−24.5) |
| PR3-ANCA titre | 0 (0−0) |
| MPO-ANCA (or P-ANCA) positive | 40 (54.1) |
| PR3-ANCA (or C-ANCA) positive | 12 (16.2) |
| Both ANCA positive | 3 (4.1) |
| AAV-specific indices | |
| BVAS | 5.0 (3.0−17.0) |
| FFS | 0 (0−1.0) |
| SF-36 PCS | 52.5 (35.9−67.9) |
| SF-36 MCS | 57.0 (39.9−72.1) |
| VDI | 3.0 (2.0−4.0) |
| Comorbidities (N, (%)) | |
| Type 2 diabetes mellitus | 16 (21.6) |
| Hypertension | 22 (29.7) |
| Dyslipidaemia | 14 (18.9) |
| Acute-phase reactants | |
| ESR (mm/h) | 23.0 (7.0−85.0) |
| CRP (mg/L) | 3.8 (0.9−28.6) |
| Laboratory results | |
| White blood cell count (/mm3) | 7710.0 (5975.0−10,515.0) |
| Neutrophil count (/mm3) | 4930.0 (3595.0−8345.0) |
| Lymphocyte count (/mm3) | 1630.0 (1225.0−2145.0) |
| Monocyte count (/mm3) | 480.0 (390.0−600.0) |
| Eosinophil count (/mm3) | 120.0 (70.0−330.0) |
| Haemoglobin (g/dL) | 12.5 (10.4−13.6) |
| Platelet count (×1000/mm3) | 248.0 (193.0−370.5) |
| Blood urea nitrogen (mg/dL) | 19.2 (13.7−28.7) |
| Serum creatinine (mg/dL) | 0.8 (0.6−1.6) |
| Total serum protein (g/dL) | 6.8 (6.5−7.3) |
| Serum albumin (g/dL) | 4.2 (3.7−4.4) |
| Serum GFAP (pg/mL) | 259.4 (176.4−377.4) |
| During follow-up | |
| Poor outcomes | |
| All-cause mortality (N, (%)) | 6 (8.1) |
| Follow-up duration based on all-cause mortality (months) | 27.1 (12.0−46.4) |
| ESKD (N, (%)) | 17 (23.0) |
| Follow-up duration based on ESKD (months) | 26.6 (8.9−46.4) |
| Medications (N, (%)) | |
| Glucocorticoids | 73 (98.6) |
| Cyclophosphamide | 47 (63.5) |
| Rituximab | 14 (18.9) |
| Mycophenolate mofetil | 19 (25.7) |
| Azathioprine | 45 (60.8) |
| Tacrolimus | 7 (9.5) |
| Methotrexate | 3 (4.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.E.; Yoon, T.; Chung, J.; Ha, J.W.; Park, Y.-B.; Lee, S.-W. Serum Glial Fibrillary Acidic Protein Can Predict Cross-Sectional Vasculitis Activity by Reflecting Renal Involvement in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina 2024, 60, 1639. https://doi.org/10.3390/medicina60101639
Lee LE, Yoon T, Chung J, Ha JW, Park Y-B, Lee S-W. Serum Glial Fibrillary Acidic Protein Can Predict Cross-Sectional Vasculitis Activity by Reflecting Renal Involvement in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina. 2024; 60(10):1639. https://doi.org/10.3390/medicina60101639
Chicago/Turabian StyleLee, Lucy Eunju, Taejun Yoon, Jihye Chung, Jang Woo Ha, Yong-Beom Park, and Sang-Won Lee. 2024. "Serum Glial Fibrillary Acidic Protein Can Predict Cross-Sectional Vasculitis Activity by Reflecting Renal Involvement in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis" Medicina 60, no. 10: 1639. https://doi.org/10.3390/medicina60101639
APA StyleLee, L. E., Yoon, T., Chung, J., Ha, J. W., Park, Y.-B., & Lee, S.-W. (2024). Serum Glial Fibrillary Acidic Protein Can Predict Cross-Sectional Vasculitis Activity by Reflecting Renal Involvement in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Medicina, 60(10), 1639. https://doi.org/10.3390/medicina60101639






