Simultaneous Double-Vessel Coronary Thrombosis with Sudden Cardiac Arrest as the First Manifestation of COVID-19
Abstract
:1. Introduction
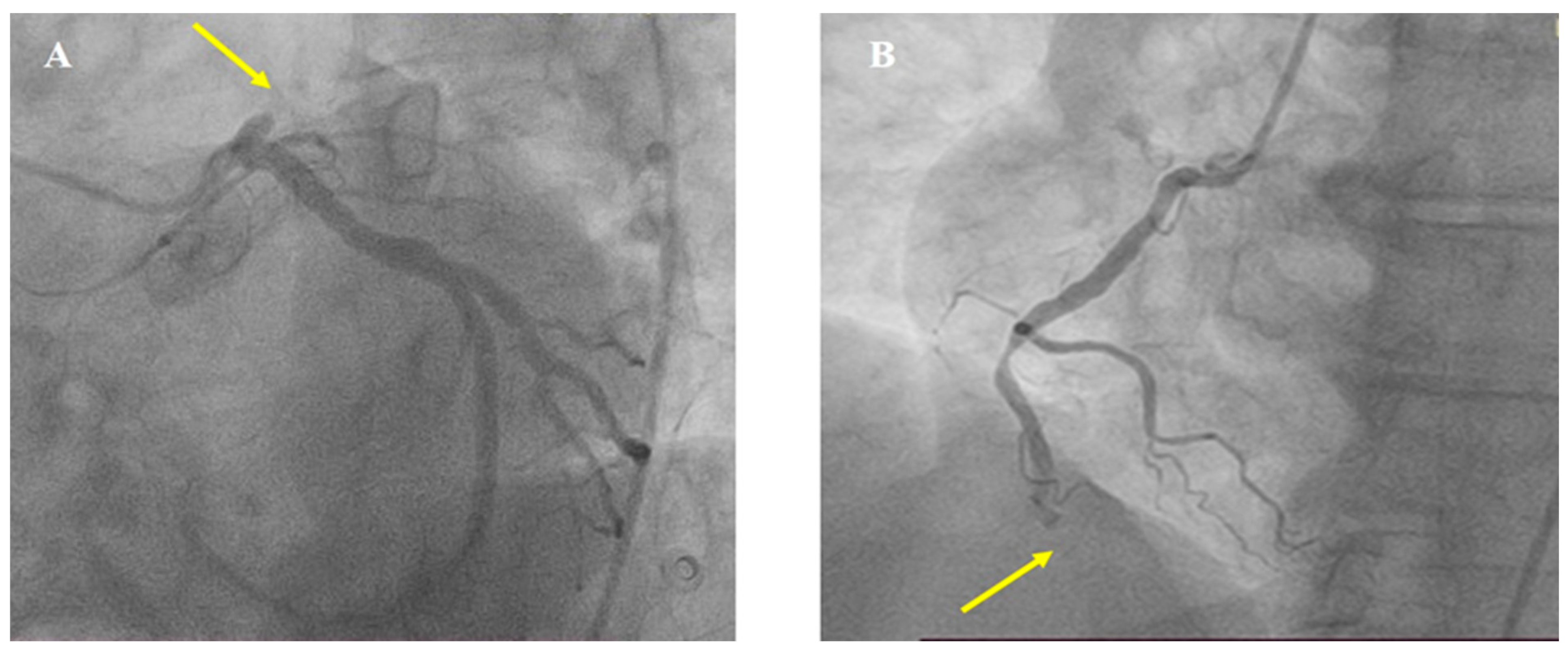
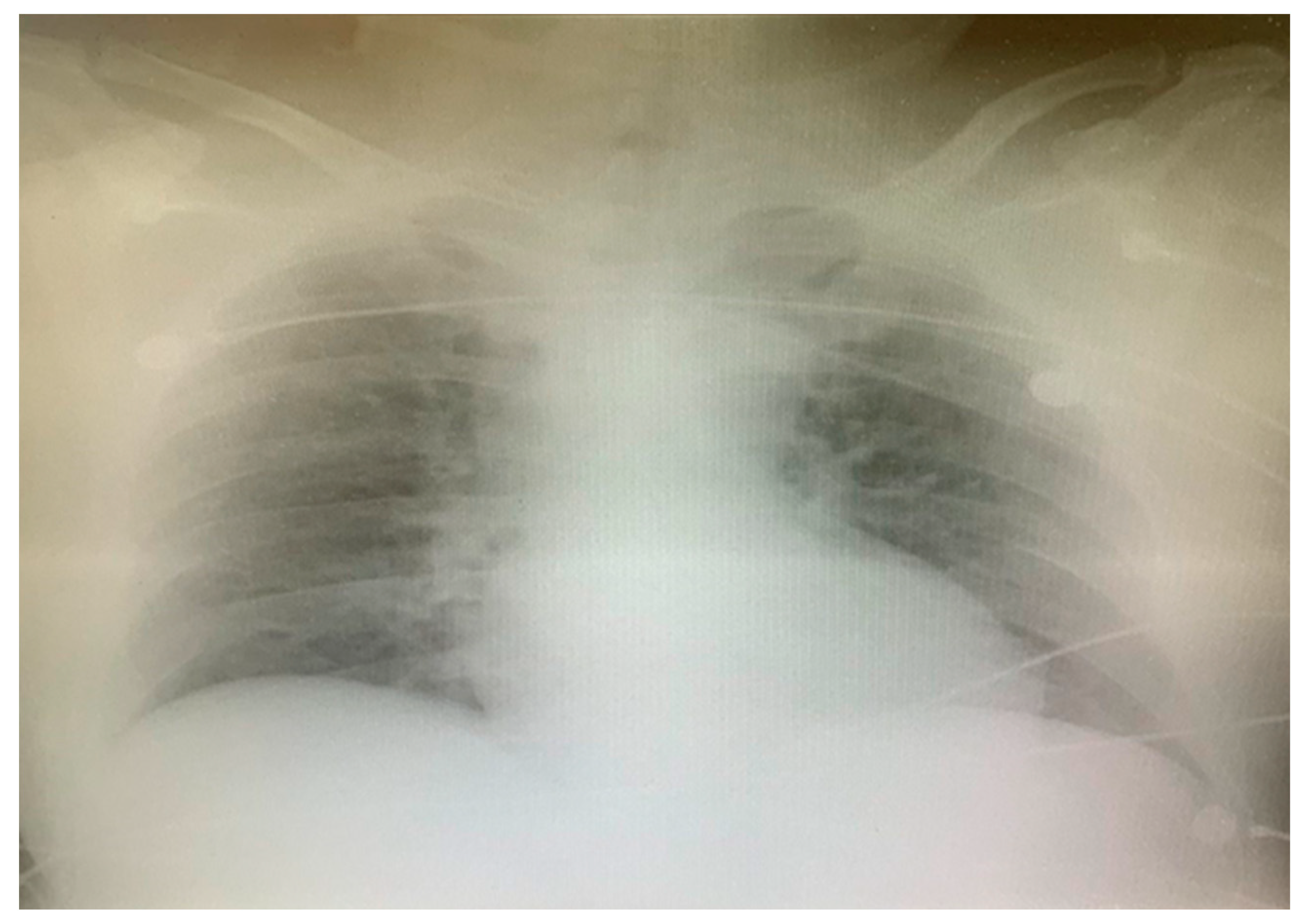
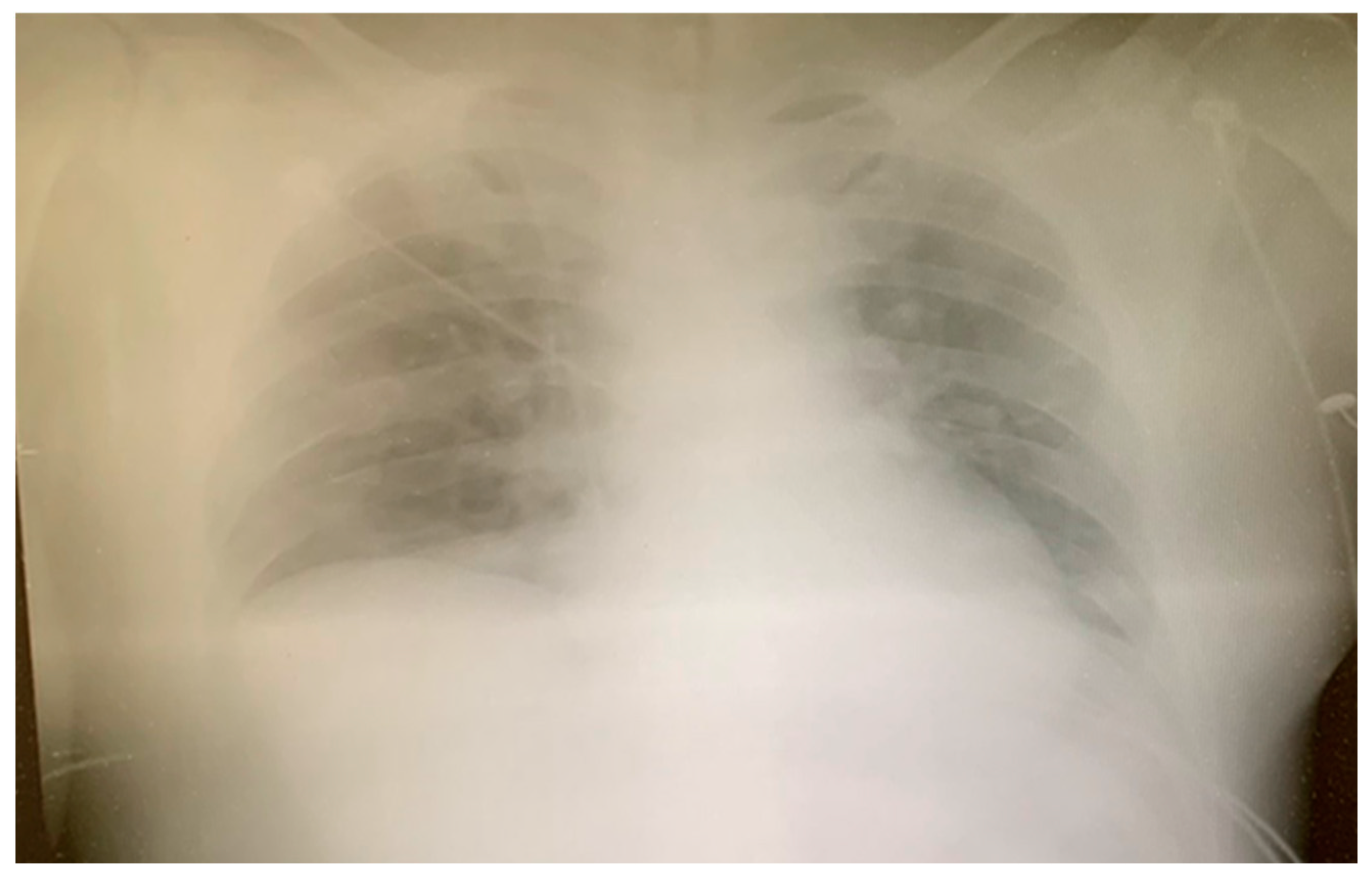
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chavez, S.; Huebinger, R.; Chan, H.K.; Gill, J.; White, L.; Mendez, D.; Jarvis, J.L.; Vithalani, V.D.; Tannenbaum, L.; Al-Araji, R.; et al. The impact of COVID-19 on incidence and outcomes from out-of-hospital cardiac arrest (OHCA) in Texas. Am. J. Emerg. Med. 2022, 57, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Karam, N.; Jost, D.; Perrot, D.; Frattini, B.; Derkenne, C.; Sharifzadehgan, A.; Waldmann, V.; Beganton, F.; Narayanan, K.; et al. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: A population-based, observational study. Lancet Public Health 2020, 5, e437–e443. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Sechi, G.M.; Mare, C.; Canevari, F.; Brancaglione, A.; Primi, R.; Klersy, C.; Palo, A.; Contri, E.; Ronchi, V.; et al. Out-of-Hospital Cardiac Arrest during the COVID-19 Outbreak in Italy. N. Engl. J. Med. 2020, 383, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guan, B.; Su, T.; Liu, W.; Chen, M.; Bin Waleed, K.; Guan, X.; Gary, T.; Zhu, Z. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: A systematic review and meta-analysis. Heart 2020, 106, 1142–1147. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Chiarito, M.; Ferrante, G.; Cannata, F.; Azzolini, E.; Viggiani, G.; De Marco, A.; Briani, M.; Bocciolone, M.; Bragato, R.; et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart 2020, 106, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Montorfano, M.; Trabattoni, D.; Andreini, D.; Ferrante, G.; Ancona, M.; Metra, M.; Curello, S.; Maffeo, D.; Pero, G.; et al. ST-Elevation Myocardial Infarction in Patients with COVID-19: Clinical and Angiographic Outcomes. Circulation 2020, 141, 2113–2116. [Google Scholar] [CrossRef]
- Bangalore, S.; Sharma, A.; Slotwiner, A.; Yatskar, L.; Harari, R.; Shah, B.; Ibrahim, H.; Friedman, G.H.; Thompson, C.; Alviar, C.L.; et al. ST-Segment Elevation in Patients with COVID-19—A Case Series. N. Engl. J. Med. 2020, 382, 2478–2480. [Google Scholar] [CrossRef]
- Kite, T.A.; Ludman, P.F.; Gale, C.P.; Wu, J.; Caixeta, A.; Mansourati, J.; Sabate, M.; Jimenez-Quevedo, P.; Candilio, L.; Sadeghipour, P.; et al. International Prospective Registry of Acute Coronary Syndromes in Patients with COVID-19. J. Am. Coll. Cardiol. 2021, 77, 2466–2476. [Google Scholar] [CrossRef]
- Ortega-Paz, L.; Capodanno, D.; Montalescot, G.; Angiolillo, D.J. Coronavirus Disease 2019-Associated Thrombosis and Coagulopathy: Review of the Pathophysiological Characteristics and Implications for Antithrombotic Management. J. Am. Heart Assoc. 2021, 10, e019650. [Google Scholar] [CrossRef]
- Cenko, E.; Badimon, L.; Bugiardini, R.; Claeys, M.J.; De Luca, G.; de Wit, C.; Derumeaux, G.; Dorobantu, M.; Duncker, D.J.; Eringa, E.C.; et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc. Res. 2021, 117, 2705–2729. [Google Scholar]
- Kurdi, H.; Obaid, D.R.; UlHaq, Z.; Ionescu, A.; Sekar, B. Multiple spontaneous coronary thrombosis causing ST-elevation myocardial infarction in a patient with COVID-19. Br. J. Hosp. Med. 2020, 81, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, D.; Rizzi, A.; Biscaglia, S.; Tumscitz, C. Acute myocardial infarction and large coronary thrombosis in a patient with COVID-19. Catheter. Cardiovasc. Interv. 2021, 97, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Madjid, M.; Vela, D.; Khalili-Tabrizi, H.; Casscells, S.W.; Litovsky, S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: Clues to the triggering effect of acute infections on acute coronary syndromes. Tex. Heart Inst. J. 2007, 34, 11–18. [Google Scholar] [PubMed]
- Choudry, F.A.; Hamshere, S.M.; Rathod, K.S.; Akhtar, M.M.; Archbold, R.A.; Guttmann, O.P.; Woldman, S.; Jain, A.K.; Knight, C.J.; Baumbach, A.; et al. High Thrombus Burden in Patients with COVID-19 Presenting with ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Tanabe, Y.; Chinushi, M.; Hayashi, Y.; Yoshida, T.; Ito, E.; Izumi, D.; Iijima, K.; Yagihara, N.; Watanabe, H.; et al. Analysis of J waves during myocardial ischaemia. Europace 2012, 14, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Jastrzebski, M.; Ozawa, T.; Kawecka-Jaszcz, K.; Kukla, P.; Mitsuma, W.; Chinushi, M.; Ida, T.; Aizawa, Y.; Ojima, K.; et al. Characteristics of electrocardiographic repolarization in acute myocardial infarction complicated by ventricular fibrillation. J. Electrocardiol. 2012, 45, 252–259. [Google Scholar] [CrossRef]
- Cipriani, A.; D’Amico, G.; Brunello, G.; Perazzolo Marra, M.; Migliore, F.; Cacciavillani, L.; Tarantini, G.; Bauce, B.; Iliceto, S.; Corrado, D.; et al. The electrocardiographic “triangular QRS-ST-T waveform” pattern in patients with ST-segment elevation myocardial infarction: Incidence, pathophysiology and clinical implications. J. Electrocardiol. 2018, 51, 8–14. [Google Scholar] [CrossRef]
- Pollak, P.M.; Parikh, S.V.; Kizilgul, M.; Keeley, E.C. Multiple culprit arteries in patients with ST segment elevation myocardial infarction referred for primary percutaneous coronary intervention. Am. J. Cardiol. 2009, 104, 619–623. [Google Scholar] [CrossRef]
- Davies, M.J.; Thomas, A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N. Engl. J. Med. 1984, 310, 1137–1140. [Google Scholar] [CrossRef]
- Asano, M.; Kurabayashi, M.; Yamauchi, Y.; Sasano, T. Relationship between D-dimer level upon emergency room arrival and the duration of cardiac arrest in patients with witnessed out-of-hospital cardiac arrest. Heart Vessel. 2021, 36, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, W.; Liu, K.; Fang, Y.Y.; Shang, J.; Zhou, L.; Wang, K.; Leng, F.; Wei, S.; Chen, L.; et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: A retrospective study. Chin. Med. J. 2020, 133, 1261–1267. [Google Scholar] [CrossRef]
- Milovančev, A.; Petrović, M.; Popadić, V.; Miljković, T.; Klašnja, S.; Djuran, P.; Ilić, A.; Kovačević, M.; Stojšić Milosavljević, A.; Brajković, M.; et al. Characteristics and Outcomes of Patients with Acute Coronary Syndrome and COVID-19. J. Clin. Med. 2022, 11, 1791. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Dehghani, P.; Grines, C.; Davidson, L.; Nayak, K.R.; Saw, J.; Waksman, R.; Blair, J.; Akshay, B.; Garberich, R.; et al. Society for Cardiac Angiography and Interventions, the Canadian Association of Interventional Cardiology, and the American College of Cardiology Interventional Council. Initial Findings from the North American COVID-19 Myocardial Infarction Registry. J. Am. Coll. Cardiol. 2021, 77, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, H.; Gheshlaghi, M.; Shah Hosseini, S.; Javedani Masroor, M.; Daliri, S. Myocardial Infarction in Patients with and without COVID-19: Comparisons of Characteristics, Clinical Courses, and Outcomes. J. Tehran Univ. Heart Cent. 2023, 18, 16–23. [Google Scholar] [CrossRef]
- Sultanian, P.; Lundgren, P.; Strömsöe, A.; Aune, S.; Bergström, G.; Hagberg, E.; Hollenberg, J.; Lindqvist, J.; Djärv, T.; Castelheim, A.; et al. Cardiac arrest in COVID-19: Characteristics and outcomes of in- and out-of-hospital cardiac arrest. A report from the Swedish Registry for Cardiopulmonary Resuscitation. Eur. Heart J. 2021, 42, 1094–1106. [Google Scholar] [CrossRef]
- Lai, P.H.; Lancet, E.A.; Weiden, M.D.; Webber, M.P.; Zeig-Owens, R.; Hall, C.B.; Prezant, D.J. Characteristics Associated with Out-of-Hospital Cardiac Arrests and Resuscitations during the Novel Coronavirus Disease 2019 Pandemic in New York City. JAMA Cardiol. 2020, 5, 1154–1163. [Google Scholar] [CrossRef]
- Chan, P.S.; Girotra, S.; Tang, Y.; Al-Araji, R.; Nallamothu, B.K.; McNally, B. Outcomes for Out-of-Hospital Cardiac Arrest in the United States during the Coronavirus Disease 2019 Pandemic. JAMA Cardiol. 2021, 6, 296–303. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef] [PubMed]





| Reference Range | Day 1 | Day 2 | Day 3 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|
| RBC | 4–10 × 1012 | 4.49 | 5.01 | 4.6 | 4.0 | 4.0 | 3.72 |
| Hb | 140–180 g/L | 139 | 157 | 143 | 123 | 123 | 115 |
| WBC | 4.00–10.00 × 109 | 11.9 | 19.17 | 13.9 | 8.7 | 8.76 | 7.40 |
| NEUT | 2.00–7.00 × 109 | 3.60 | 16.11 | 12.1 | 7.6 | 7.02 | 5.47 |
| LYMPH | 0.80–4.00 × 109 | 7.75 | 1.22 | 0.9 | 0.6 | 0.75 | 0.79 |
| PLT | 150–450 × 109 | 165 | 270 | 199 | 160 | 215 | 237 |
| CRP | <8.0 mg/L | 3.5 | 8.2 | 163 | 241 | 249.5 | 238.6 |
| Glucose | 3.3–6.1 mmol/L | 12.5 | 8.7 | 7.7 | 6.8 | 7.0 | |
| Urea | 2.8–7.2 mmol/L | 5.8 | 8.5 | 10.7 | 7.4 | 6.7 | 10.4 |
| Creatine | 53–120 μmol/L | 133 | 140 | 143 | 102 | 65 | 78 |
| AST | 7–55 U/L | 137 | 1436 | 630 | 341 | 137 | 97 |
| ALT | <45 U/L | 214 | 440 | 334 | 257 | 142 | 104 |
| LDH | 241 U/L | 311 | 2458 | 2396 | 1582 | 1076 | 817 |
| Hs-cTnI | <34.2 ng/L | 832 | >50,000 | 18,091.2 | |||
| CK | 55–170 U/L | 217 | 4000 | 6321 | 2604 | 1219 | 538 |
| CK-MB | <4.87 ng/mL | 60 | 500 | 711 | 123 | 42 | 27 |
| D-dimer | <500 ng/ml | >10,000 | 4702 | 3266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jokšić-Mazinjanin, R.; Marić, N.; Đuričin, A.; Bjelobrk, M.; Bjelić, S.; Trajković, M.; Kovačević, M. Simultaneous Double-Vessel Coronary Thrombosis with Sudden Cardiac Arrest as the First Manifestation of COVID-19. Medicina 2024, 60, 39. https://doi.org/10.3390/medicina60010039
Jokšić-Mazinjanin R, Marić N, Đuričin A, Bjelobrk M, Bjelić S, Trajković M, Kovačević M. Simultaneous Double-Vessel Coronary Thrombosis with Sudden Cardiac Arrest as the First Manifestation of COVID-19. Medicina. 2024; 60(1):39. https://doi.org/10.3390/medicina60010039
Chicago/Turabian StyleJokšić-Mazinjanin, Radojka, Nikolina Marić, Aleksandar Đuričin, Marija Bjelobrk, Snežana Bjelić, Miloš Trajković, and Mila Kovačević. 2024. "Simultaneous Double-Vessel Coronary Thrombosis with Sudden Cardiac Arrest as the First Manifestation of COVID-19" Medicina 60, no. 1: 39. https://doi.org/10.3390/medicina60010039
APA StyleJokšić-Mazinjanin, R., Marić, N., Đuričin, A., Bjelobrk, M., Bjelić, S., Trajković, M., & Kovačević, M. (2024). Simultaneous Double-Vessel Coronary Thrombosis with Sudden Cardiac Arrest as the First Manifestation of COVID-19. Medicina, 60(1), 39. https://doi.org/10.3390/medicina60010039






