Combined Area of Left and Right Atria May Outperform Atrial Volumes as a Predictor of Recurrences after Ablation in Patients with Persistent Atrial Fibrillation—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Image Acquisition
2.3. Image Analysis
2.4. Ablation Procedure
2.5. Follow-Up
2.6. Reproducibility of MRI Measurements
2.7. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiology 2014, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 473–498. [Google Scholar]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [PubMed]
- Calvo, N.; Ramos, P.; Montserrat, S.; Guasch, E.; Coll-Vinent, B.; Domenech, M.; Bisbal, F.; Hevia, S.; Vidorreta, S.; Borras, R.; et al. Emerging risk factors and the dose-response relationship between physical activity and lone atrial fibrillation: A prospective case-control study. Europace 2016, 18, 57–63. [Google Scholar] [CrossRef]
- Neilan, T.G.; Farhad, H.; Dodson, J.A.; Shah, R.V.; Abbasi, S.A.; Bakker, J.P.; Michaud, G.F.; van der Geest, R.; Blankstein, R.; Steigner, M.; et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J. Am. Heart Assoc. 2013, 2, e000421. [Google Scholar] [CrossRef]
- Pathak, R.K.; Middeldorp, M.E.; Meredith, M.; Mehta, A.B.; Mahajan, R.; Wong, C.X.; Twomey, D.; Elliott, A.D.; Kalman, J.M.; Abhayaratna, W.P.; et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J. Am. Coll. Cardiol. 2015, 65, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, M.Y.; Oh, W.J.; Hong, S.J.; Pak, H.N.; Song, W.H.; Lim, D.S.; Kim, Y.H.; Shim, W.J. Left atrial volume is a predictor of atrial fibrillation recurrence after catheter ablation. J. Am. Soc. Echocardiogr. 2008, 21, 697–702. [Google Scholar] [CrossRef]
- Bisbal, F.; Guiu, E.; Calvo, N.; Marin, D.; Berruezo, A.; Arbelo, E.; Ortiz-Pérez, J.; de Caralt, T.M.; Tolosana, J.M.; Borràs, R.; et al. Left Atrial Sphericity: A New Method to Assess Atrial Remodeling. Impact on the Outcome of Atrial Fibrillation Ablation. J. Cardiovasc. Electrophysiol. 2013, 24, 752–759. [Google Scholar] [CrossRef]
- McGann, C.; Akoum, N.; Patel, A.; Kholmovski, E.; Revelo, P.; Damal, K.; Wilson, B.; Cates, J.; Harrison, A.; Ranjan, R.; et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ. Arrhythmia Electrophysiol. 2014, 7, 23–30. [Google Scholar] [CrossRef]
- Linhart, M.; Lewalter, T.; Mittmann-Braun, E.L.; Karbach, N.C.; Andrié, R.P.; Hammerstingl, C.; Fimmers, R.; Kreuz, J.; Nickenig, G.; Schrickel, J.W.; et al. Left atrial pressure as predictor for recurrence of atrial fibrillation after pulmonary vein isolation. J. Interv. Card. Electrophysiol. 2013, 38, 107–114. [Google Scholar] [CrossRef]
- Akutsu, Y.; Kaneko, K.; Kodama, Y.; Suyama, J.; Li, H.L.; Hamazaki, Y.; Tanno, K.; Gokan, T.; Kobayashi, Y. Association between left and right atrial remodeling with atrial fibrillation recurrence after pulmonary vein catheter ablation in patients with paroxysmal atrial fibrillation: A pilot study. Circ. Cardiovasc. Imaging 2011, 4, 524–531. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, W.; Zhou, L.; Gu, J.; Wang, Y.; Liu, Y.; Zhang, X.; Wu, S.; Liu, X. Why atrial fibrillation recurs in patients who obtained current ablation endpoints with longstanding persistent atrial fibrillation. J. Interv. Card. Electrophysiol. 2013, 37, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, H.J.; Shim, J.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Kim, Y.J.; Joung, B. Distinct prognostic impacts of both atrial volumes on outcomes after radiofrequency ablation of nonvalvular atrial fibrillation: Three-dimensional imaging study using multidetector computed tomography. Int. J. Cardiol. 2013, 168, 5430–5436. [Google Scholar] [CrossRef] [PubMed]
- Luong, C.; Thompson, D.J.; Bennett, M.; Gin, K.; Jue, J.; Barnes, M.E.; Colley, P.; Tsang, T.S. Right atrial volume is superior to left atrial volume for prediction of atrial fibrillation recurrence after direct current cardioversion. Can. J. Cardiol. 2015, 31, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, H.J.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Kim, Y.J.; Joung, B. Prognostic Implications of Right and Left Atrial Enlargement after Radiofrequency Catheter Ablation in Patients with Nonvalvular Atrial Fibrillation. Korean Circ. J. 2015, 45, 301–309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasaki, T.; Nakamura, K.; Naito, S.; Minami, K.; Koyama, K.; Yamashita, E.; Kumagai, K.; Oshima, S. The Right to Left Atrial Volume Ratio Predicts Outcomes after Circumferential Pulmonary Vein Isolation of Longstanding Persistent Atrial Fibrillation. Pacing Clin. Electrophysiol. 2016, 39, 1181–1190. [Google Scholar] [CrossRef]
- Gunturiz-Beltrán, C.; Nuñez-Garcia, M.; Althoff, T.F.; Borràs, R.; Figueras I Ventura, R.M.; Garre, P.; Caixal, G.; Prat-González, S.; Perea, R.J.; Benito, E.M.; et al. Progressive and Simultaneous Right and Left Atrial Remodeling Uncovered by a Comprehensive Magnetic Resonance Assessment in Atrial Fibrillation. J. Am. Heart Assoc. 2022, 11, e026028. [Google Scholar] [CrossRef]
- Döring, C.; Richter, U.; Ulbrich, S.; Wunderlich, C.; Ebert, M.; Richter, S.; Linke, A.; Sveric, K.M. The Impact of Right Atrial Size to Predict Success of Direct Current Cardioversion in Patients With Persistent Atrial Fibrillation. Korean Circ. J. 2023, 53, 331–343. [Google Scholar] [CrossRef]
- Mărgulescu, A.D.; Nuñez-Garcia, M.; Alarcón, F.; Benito, E.M.; Enomoto, N.; Cozzari, J.; Chipa, F.; Fernandez, H.; Borras, R.; Guasch, E.; et al. Reproducibility and accuracy of late gadolinium enhancement cardiac magnetic resonance measurements for the detection of left atrial fibrosis in patients undergoing atrial fibrillation ablation procedures. Europace 2019, 21, 724–731. [Google Scholar] [CrossRef]
- Xiong, Q.; Proietti, M.; Senoo, K.; Lip, G.Y. Asymptomatic versus symptomatic atrial fibrillation: A systematic review of age/gender differences and cardiovascular outcomes. Int. J. Cardiol. 2015, 191, 172–177. [Google Scholar] [CrossRef]
- Narayan, S.M.; Baykaner, T.; Clopton, P.; Schricker, A.; Lalani, G.G.; Krummen, D.E.; Shivkumar, K.; Miller, J.M. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: Extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J. Am. Coll. Cardiol. 2014, 63, 1761–1768. [Google Scholar] [PubMed]

| Age, Years (Mean, SD) | 56.9 ± 9.1 |
|---|---|
| Male sex (n, %) | 70 (82.4%) |
| Height, cm (mean, SD) | 174.0 ± 9.6 |
| Weight, kg (mean, SD) | 84.2 ± 14.8 |
| Persistent AF (n, %) | 40 (47.1%) |
| Hypertension (n, %) | 40 (47.1%) |
| OAS (n, %) | 12 (14.1%) |
| Endurance training (n, %) | 12 (14.3%) |
| LVEF, % (mean, SD) | 59.3 ± 8.1 |
| LV end-diastolic diameter, mm (mean, SD) | 52.2 ± 5.0 |
| Follow-up, months (median, IQR) | 11.0 (12.0) |
| Recurrence (n, %) | 35 (41.1%) |
| General | Paroxysmal AF | Persistent AF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Recurrence | Recurrence | p Value | No Recurrence | Recurrence | p Value | No Recurrence | Recurrence | p Value | |
| N = 50 | N = 35 | N = 28 | N = 17 | N = 22 | N = 18 | ||||
| Clinical characteristics | |||||||||
| Age, years (mean, SD) | 57.6 ± 9.6 | 55.9 ± 8.3 | 0.42 | 57.1 ± 11.0 | 57.5 ± 9.1 | 0.89 | 58.3 ± 7.8 | 54.2 ± 7.4 | 0.11 |
| BSA, m2 (mean, SD) | 2.00 ± 0.21 | 2.03 ± 0.23 | 0.93 | 1.96 ± 0.22 | 2.00 ± 0.23 | 0.60 | 2.05 ± 0.19 | 2.06 ± 0.22 | 0.94 |
| Men, n (%) | 41/50 (82.0%) | 29/35 (82.9%) | 1.00 | 22/28 (78.6%) | 12/17 (70.6%) | 0.72 | 19/22 (86.4%) | 17/18 (94.4%) | 0.61 |
| Hypertension, n (%) | 23/50 (46.0%) | 17/35 (48.6%) | 0.83 | 11/28 (39.3%) | 7/17 (41.2%) | 1.00 | 12/22 (54.6%) | 10/18 (55.6%) | 0.97 |
| Diabetes, n (%) | 3/41 (7.3%) | 3/28 (10.7%) | 0.68 | 1/24 (4.2%) | 1/15 (6.7%) | 1.00 | 2/17 (11.8%) | 2/13 (15.4%) | 1.00 |
| OSA, n (%) | 7/50 (14.0%) | 5/35 (14.3%) | 1.00 | 3/28 (10.7%) | 3/17 (17.7%) | 0.66 | 4/22 (18.2%) | 2/18 (11.1%) | 0.56 |
| Endurance training, n (%) | 8/50 (16.0%) | 4/34 (11.8%) | 0.75 | 5/28 (17.9%) | 1/16 (6.3%) | 0.39 | 3/22 (13.6%) | 3/18 (16.7%) | 1.00 |
| Cardiomyopathy, n (%) | 8/48 (16.7%) | 6/32 (18.8%) | 1.00 | 4/28 (14.3%) | 1/16 (6.3%) | 0.64 | 4/20 (20.0%) | 5/16 (31.3%) | 0.47 |
| Echocardiographic data | |||||||||
| LVEF, % (median, IQR) | 60.0 (5.0) | 60.0 (0.0) | 0.57 | 63.0 (5.0) | 60.0 (5.0) | 0.81 | 60.0 (10.5) | 60.0 (4.0) | 0.58 |
| LVEDD, mm (mean, SD) | 49.5 ± 5.7 | 51.5 ± 6.7 | 0.12 | 49.4 ± 5.3 | 51.7 ± 5.3 | 0.22 | 49.8 ± 6.4 | 51.3 ± 7.8 | 0.56 |
| LA diameter, mm (mean, SD) | 41.5 ± 5.4 | 43.3 ± 4.8 | 0.19 | 41.2 ± 6.2 | 42.5 ± 5.3 | 0.51 | 41.2 ± 4.4 | 44.1 ± 4.3 | 0.13 |
| Procedure-related parameters | |||||||||
| Duration of procedure, hours (median, IQR) | 180.0 (67.5) | 200 (62.5) | 0.93 | 180.0 (100.0) | 200.0 (80.0) | 0.46 | 195.0 (60.0) | 187.5 (52.5) | 0.95 |
| Fluoroscopy time, min (median, IQR) | 23.0 (17.8) | 24.0 (11.5) | 0.75 | 23.0 (16.5) | 46.0 ± 75.2 | 0.25 | 23.4 ± 11.1 | 22.9 ± 9.7 | 0.88 |
| CMR parameters | |||||||||
| AILA, cm2/m2 (mean, SD) | 13.0 (2.9) | 14.5 (3.2) | 0.021 | 12.6 (2.5) | 13.8 (2.9) | 0.16 | 13.4 (3.2) | 15.3 (3.4) | 0.094 |
| AIRA, cm2/m2 (mean, SD) | 11.9 (2.2) | 13.0 (3.1) | 0.07 | 12.0 (2.3) | 12.0 (2.1) | 0.98 | 11.8 (2.1) | 14.0 (3.6) | 0.026 |
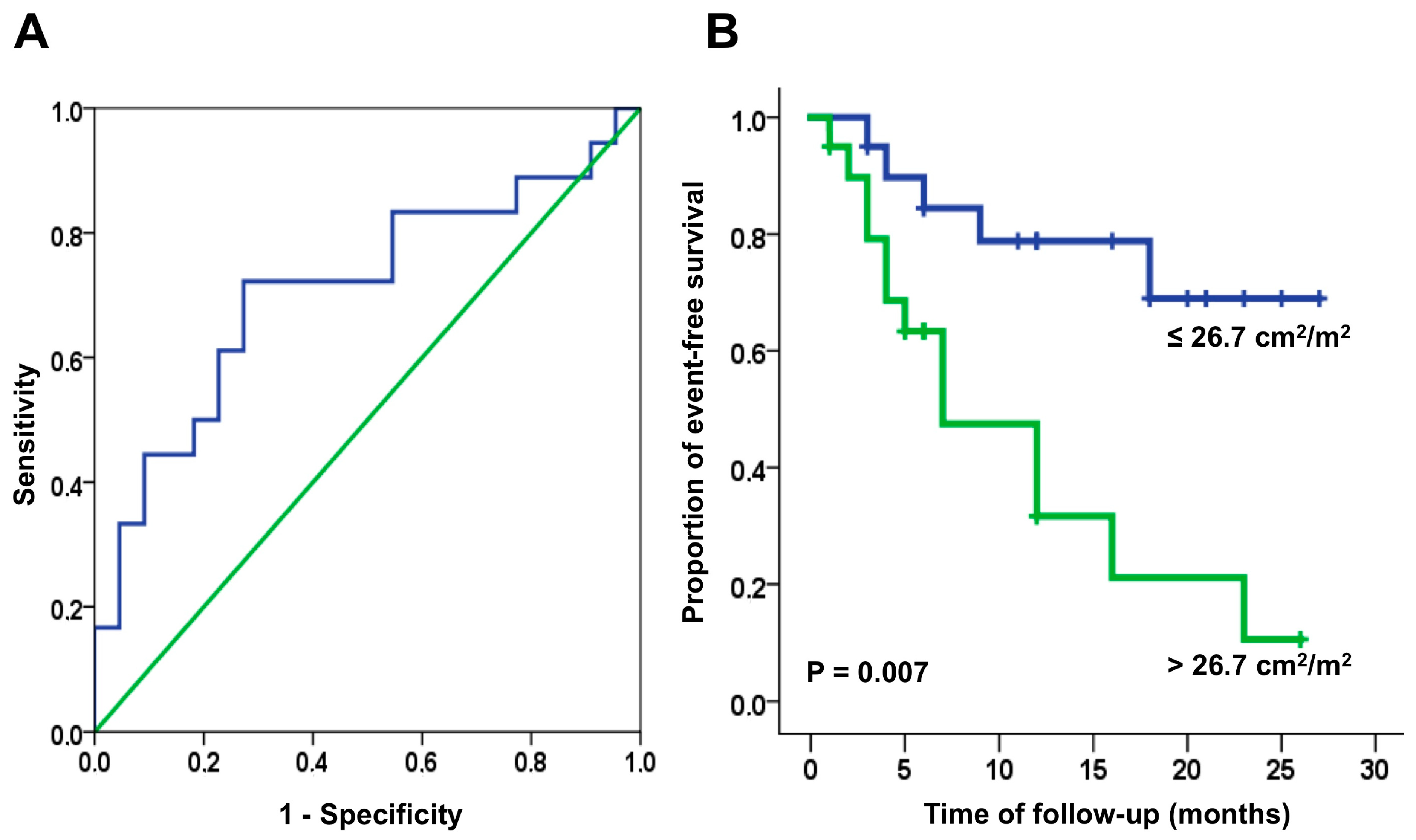
| AILA+RA, cm2/m2 (mean, SD) | 24.9 (4.2) | 27.5 (5.6) | 0.015 | 24.6 (4.1) | 25.8 (4.1) | 0.36 | 25.3 (4.4) | 29.2 (6.3) | 0.026 |
| LA indexed volume (mL/m2) (mean, SD) | 44.3 (12.1) | 49.5 (14.8) | 0.08 | 40.6 (8.4) | 44.4 (10.5) | 0.19 | 49.0 (14.4) | 54.2 (16.9) | 0.30 |
| RA indexed volume (mL/m2) (mean, SD) | 49.0 (13.0) | 55.7 (20.0) | 0.07 | 45.4 (10.0) | 46.7 (11.9) | 0.70 | 53.8 (15.0) | 64.2 (22.5) | 0.09 |
| LA + RA indexed volume (mL/m2) (mean, SD) | 93.4 (22.0) | 105.2 (30.4) | 0.043 | 86.1 (15.0) | 91.1 (19.0) | 0.33 | 103.2 (26.1) | 118.5 (33.6) | 0.12 |
| General | Paroxysmal AF | Persistent AF | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| AILA (cm2/m2) | 1.14 (1.02–1.28) | 0.021 | 1.19 (0.98–1.44) | 0.09 | 1.13 (0.97–1.32) | 0.13 |
| AIRA (cm2/m2) | 1.12 (0.98–1.23) | 0.09 | 0.96 (0.75–1.24) | 0.78 | 1.16 (1.00–1.33) | 0.043 |
| AILA+RA (cm2/m2) | 1.08 (1.01–1.16) | 0.017 | 1.08 (0.94–1.25) | 0.27 | 1.08 (1.00–1.17) | 0.048 |
| LA indexed volume (mL/m2) | 1.02 (1.00–1.05) | 0.11 | 1.04 (0.98–1.10) | 0.19 | 1.02 (0.99–1.05) | 0.30 |
| RA indexed volume (mL/m2) | 1.02 (1.00–1.03) | 0.12 | 1.01 (0.96–1.06) | 0.77 | 1.02 (0.99–1.04) | 0.18 |
| LA + RA indexed volume (mL/m2) | 1.01 (1.00–1.02) | 0.07 | 1.02 (0.99–1.05) | 0.33 | 1.01 (1.00–1.03) | 0.18 |
| Study | N | Follow-Up | Type of AF | Intervention | Method | Parameter | Results |
|---|---|---|---|---|---|---|---|
| Shin 2008 [7] | 68 | 6 months | 59% paroxysmal | Ablation | 2D echo | RAVi, LAVi | Univariate analysis: RAVi and LAVi predicted recurrences. Multivariate analysis: only LAVi predicted recurrences. Cut-off value 34 mL/m2: Ss 70%, Sp 91%. |
| Akutsu 2011 [11] | 65 | 6 months | Paroxysmal | Ablation | CT angiogram | RAV, LAV | RAV correlated with LAV (r = 0.4, p < 0.01). RA volume [OR, 1.04; 95% confidence interval (CI), 1.02 to 1.07, p < 0.0001],
Cut-off values:
|
| Zhao 2013 [12] | 208 | 19.9 months (average) | Long-term persistent | Ablation | 2D echo | RA enlargement (categorical value) | Patients with AF recurrence had higher rates of:
|
| Moon 2013 [13] | 242 | 18 months (average) | 66% paroxysmal | Ablation | CT angiogram | RAVi, LAVi | RAVi correlated with LAVi in both paroxysmal and persistent AF (r = 0.6; p < 0.01 for both) Overall:
|
| Luong 2015 [14] | 95 | 6 months | Persistent | Cardioversion | 2D echo | RAVi, LAVi | RAVi was superior to LAVi in predicting recurrences:
|
| Moon 2015 [15] | 111 | 12 months | 61% paroxysmal | Ablation | CT angiogram | RAVi, LAVi |
|
| Sazaki 2016 [16] | 70 | 15 months | Long-term persistent | Ablation | CT angiogram | RAVi, LAVi RA/LA volume ratio | RA (but not LA) dilatation are risk factors for AF recurrence after ablation (AUC 0.64; 95% CI, 0.49–0.79). RA/LA volume ratio is a better predictor of recurrences than RA volume alone: RA/LA volume ratio cut-off 100.1%; hazard ratio, 1.05; 95% CI, 1.00–1.10; p = 0.039; Ss 85.7%, Sp 71.4%, AUC 0.77, 95% CI, 0.66–0.88. |
| Gunturiz-Beltrán 2022 [17] | 100 | 24 months (median) | 55% paroxysmal | Ablation | CMR | RAVi, LAVi Sphericity index | Pooled results suggest only the sphericity index was predictive of AF recurrences after ablation, while RA and LA area and volumes were not. Results not split between paroxysmal and persistent AF. |
| Döring 2023 [18] | 589 | Immediate success | Persistent (86%) and long-term persistent (14%) | Cardioversion | 2D echo | RAi area, LAVi | iRA area (OR, 0.27; 95% CI, 0.12–0.69; AUC 0.71) but not LAVi (OR, 1.16; 95% CI, 1.05–1.56) is a strong echocardiographic indicator of cardioversion success. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mărgulescu, A.D.; Mas-Lladó, C.; Prat-Gonzàlez, S.; Perea, R.J.; Borras, R.; Benito, E.; Alarcón, F.; Guasch, E.; Tolosana, J.M.; Arbelo, E.; et al. Combined Area of Left and Right Atria May Outperform Atrial Volumes as a Predictor of Recurrences after Ablation in Patients with Persistent Atrial Fibrillation—A Pilot Study. Medicina 2024, 60, 151. https://doi.org/10.3390/medicina60010151
Mărgulescu AD, Mas-Lladó C, Prat-Gonzàlez S, Perea RJ, Borras R, Benito E, Alarcón F, Guasch E, Tolosana JM, Arbelo E, et al. Combined Area of Left and Right Atria May Outperform Atrial Volumes as a Predictor of Recurrences after Ablation in Patients with Persistent Atrial Fibrillation—A Pilot Study. Medicina. 2024; 60(1):151. https://doi.org/10.3390/medicina60010151
Chicago/Turabian StyleMărgulescu, Andrei D., Caterina Mas-Lladó, Susanna Prat-Gonzàlez, Rosario Jesus Perea, Roger Borras, Eva Benito, Francisco Alarcón, Eduard Guasch, Jose María Tolosana, Elena Arbelo, and et al. 2024. "Combined Area of Left and Right Atria May Outperform Atrial Volumes as a Predictor of Recurrences after Ablation in Patients with Persistent Atrial Fibrillation—A Pilot Study" Medicina 60, no. 1: 151. https://doi.org/10.3390/medicina60010151
APA StyleMărgulescu, A. D., Mas-Lladó, C., Prat-Gonzàlez, S., Perea, R. J., Borras, R., Benito, E., Alarcón, F., Guasch, E., Tolosana, J. M., Arbelo, E., Sitges, M., Brugada, J., & Mont, L. (2024). Combined Area of Left and Right Atria May Outperform Atrial Volumes as a Predictor of Recurrences after Ablation in Patients with Persistent Atrial Fibrillation—A Pilot Study. Medicina, 60(1), 151. https://doi.org/10.3390/medicina60010151





