The Prognostic Value of One-Year Changes in Biventricular Mechanics for Three-Year Survival in Patients with Precapillary Pulmonary Hypertension: A Cardiovascular Magnetic Resonance Feature Tracking Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Testing
2.2. Volumetric and Functional Measurements
2.3. Feature Tracking Mechanical Analysis
2.4. Data Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. Baseline cMRI Parameter Evaluation
3.3. One-Year Clinical and cMRI Parameter Changes within and between Groups
3.4. Survival Analysis
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Manes, A. The Right Ventricle in Pulmonary Arterial Hypertension. Eur. Respir. Rev. 2014, 23, 476. [Google Scholar] [CrossRef] [PubMed]
- Alabed, S.; Shahin, Y.; Garg, P.; Alandejani, F.; Johns, C.S.; Lewis, R.A.; Condliffe, R.; Wild, J.M.; Kiely, D.G.; Swift, A.J. Cardiac-MRI Predicts Clinical Worsening and Mortality in Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2021, 14, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Swift, A.J.; Rajaram, S.; Campbell, M.J.; Hurdman, J.; Thomas, S.; Capener, D.; Elliot, C.; Condliffe, R.; Wild, J.M.; Kiely, D.G. Prognostic Value of Cardiovascular Magnetic Resonance Imaging Measurements Corrected for Age and Sex in Idiopathic Pulmonary Arterial Hypertension. Circ. Cardiovasc. Imaging 2014, 7, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, A.; Mouratoglou, S.A.; Giannakoulas, G.; Finitsis, S.; Karvounis, H.; Sianos, G. Myocardial Deformation Assessment in Patients with Precapillary Pulmonary Hypertension: A Cardiac Magnetic Resonance Study. Diagn. Interv. Imaging 2021, 102, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Baggen, V.J.M.; Leiner, T.; Post, M.C.; van Dijk, A.P.; Roos-Hesselink, J.W.; Boersma, E.; Habets, J.; Sieswerda, G.T. Cardiac Magnetic Resonance Findings Predicting Mortality in Patients with Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. Eur. Radiol. 2016, 26, 3771. [Google Scholar] [CrossRef]
- van der Bruggen, C.E.; Handoko, M.L.; Bogaard, H.J.; Marcus, J.T.; Oosterveer, F.P.T.; Meijboom, L.J.; Westerhof, B.E.; Vonk Noordegraaf, A.; de Man, F.S. The Value of Hemodynamic Measurements or Cardiac MRI in the Follow-up of Patients with Idiopathic Pulmonary Arterial Hypertension. Chest 2021, 159, 1575–1585. [Google Scholar] [CrossRef]
- Vos, J.L.; Leiner, T.; van Dijk, A.P.J.; van der Zwaan, H.B.; Sieswerda, G.T.; Snijder, R.J.; Post, M.C.; Vonk, M.C.; van Leuven, S.; Vart, P.; et al. Right Atrial and Ventricular Strain Detects Subclinical Changes in Right Ventricular Function in Precapillary Pulmonary Hypertension. Int. J. Cardiovasc. Imaging 2022, 38, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, X.; Yin, G.; Li, S.; Zhao, S.; Liu, Z.; Lu, M. Risk Stratification and Outcomes in Patients with Pulmonary Hypertension: Insights into Right Ventricular Strain by MRI Feature Tracking. J. Magn. Reson. Imaging 2023, 57, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.C.W.; Seale, H.; Hamilton-Craig, C.; Morris, N.R.; Strugnell, W. Quantification of Biventricular Strain and Assessment of Ventriculo-Ventricular Interaction in Pulmonary Arterial Hypertension Using Exercise Cardiac Magnetic Resonance Imaging and Myocardial Feature Tracking. J. Magn. Reson. Imaging 2019, 49, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Kallianos, K.; Brooks, G.C.; Mukai, K.; Seguro de Carvalho, F.; Liu, J.; Naeger, D.M.; De Marco, T.; Ordovas, K.G. Cardiac Magnetic Resonance Evaluation of Left Ventricular Myocardial Strain in Pulmonary Hypertension. Acad. Radiol. 2018, 25, 129–135. [Google Scholar] [CrossRef]
- De Siqueira, M.E.M.; Pozo, E.; Fernandes, V.R.; Sengupta, P.P.; Modesto, K.; Gupta, S.S.; Barbeito-Caamaño, C.; Narula, J.; Fuster, V.; Caixeta, A.; et al. Characterization and Clinical Significance of Right Ventricular Mechanics in Pulmonary Hypertension Evaluated with Cardiovascular Magnetic Resonance Feature Tracking. J. Cardiovasc. Magn. Reson. 2016, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Miller, D.P.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An Evaluation of Long-Term Survival from Time of Diagnosis in Pulmonary Arterial Hypertension from the Reveal Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef]
- Farber, H.W.; Miller, D.P.; Poms, A.D.; Badesch, D.B.; Frost, A.E.; Muros-Le Rouzic, E.; Romero, A.J.; Benton, W.W.; Elliott, C.G.; McGoon, M.D.; et al. Five-Year Outcomes of Patients Enrolled in the REVEAL Registry. Chest 2015, 148, 1043–1054. [Google Scholar] [CrossRef]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; MacIntyre, N.R.; McKay, R.T.; Johnson, D.; Wanger, J.S.; Zeballos, R.J.; Bittner, V.; et al. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; Von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized Image Interpretation and Post-Processing in Cardiovascular Magnetic Resonance—2020 Update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Padervinskienė, L.; Krivickienė, A.; Hoppenot, D.; Miliauskas, S.; Basevičius, A.; Nedzelskienė, I.; Jankauskas, A.; Šimkus, P.; Ereminienė, E. Prognostic Value of Left Ventricular Function and Mechanics in Pulmonary Hypertension: A Pilot Cardiovascular Magnetic Resonance Feature Tracking Study. Medicina 2019, 55, 73. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Sitbon, O.; Yaïci, A.; Montani, D.; O’Callaghan, D.S.; Jaïs, X.; Parent, F.; Savale, L.; Natali, D.; Günther, S.; et al. Survival in Incident and Prevalent Cohorts of Patients with Pulmonary Arterial Hypertension. Eur. Respir. J. 2010, 36, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Padervinskiene, L.; Hoppenot, D.; Krivickiene, A.; Gumauskiene, B.; Nedzelskiene, I.; Simkus, P.; Miliauskas, S.; Jankauskas, A.; Basevicius, A.; Ereminiene, E. Identification of Cardiac Mri and Bio-Marker Thresholds for One-Year Survival in Pre-Capillary Pulmonary Hypertension: Prospective Study. Medicina 2020, 56, 167. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczyk, R.; Malek, L.A.; Szumowski, P.; Nekolla, S.G.; Blaszczak, P.; Jurgilewicz, D.; Hladunski, M.; Sobkowicz, B.; Mysliwiec, J.; Grzywna, R.; et al. Multimodal Assessment of Right Ventricle Overload-Metabolic and Clinical Consequences in Pulmonary Arterial Hypertension. J. Cardiovasc. Magn. Reson. 2021, 23, 49. [Google Scholar] [CrossRef] [PubMed]

| Parameter | All Patients (n = 36) | Survival Group (n = 30) | Non-Survival Group (n = 6) | p-Value |
|---|---|---|---|---|
| Age (years) | 56.0 [45.3–68.8] | 52.0 [41.8–63.3] | 70.50 [56.5–72.3] | 0.015 ** |
| Women/men (N (%)) | 27/9 (75.0/25.0) | 23/7 (76.7/23.3) | 4/2 (66.7/33.3) | 0.606 * |
| NYHA class 2/3/4 (N (%)) | 2/31/3 (5.6/86.1/8.3) | 2/26/2 (6.7/86.7/6.7) | 0/5/1 (83.3/16.7) | 0.605 * |
| 6MWT (m) | 339.0 [210.0–414.0] | 342.5 [261.8–420.0] | 180.0 [116.0–240.0] | 0.012 ** |
| NT-proBNP (ng/L) | 1472.0 [398.0–3484.0] | 1433.0 [308.5–2915.5] | 3131.5 [1308.0–6963.5] | 0.096 ** |
| mPAP (mmHg) | 55.0 [46.3–70.5] | 54.5 [45.8–67.5] | 59.5 [49.3–80.3) | 0.442 ** |
| Parameter at Baseline | Survival Group (n = 30) | Non-Survival Group (n = 6) | p-Value between the Groups |
|---|---|---|---|
| LV GLS (%) | −17.9 [−24.2–(−15.4)] | −17.1 [−20.4–(−11.9)] | 0.497 |
| LV GCS (%) | −31.6 [−37.3–(−26.4)] | −31.1 [−38.3–(−23.8)] | 0.799 |
| LV EDVI (mL/m2) | 64.0 [52.5–81.3] | 58.0 [54.0–67.3] | 0.384 |
| LV ESVI (mL/m2) | 28.0 [18.8–40.0] | 26.5 [19.0–34.0] | 0.702 |
| LV SVI (mL/m2) | 34.5 [31.0–45.0] | 34.0 [29.5–38.3] | 0.782 |
| LV EF (%) | 59.0 [48.5–65.5] | 56.5 [41.5–66.5] | 0.865 |
| RV free wall LS (%) | −19.6 [−24.6–(−13.4)] | −15.0 [−19.5–(−12.7)] | 0.243 |
| RV septum LS (%) | −11.3 [−13.9–(−6.7)] | −12.4 [−16.3–(−7.5)] | 0.552 |
| RV GLS (%) | −14.0 [−16.6–(−10.8)] | −14.0 [−17.0–(−9.3)] | 0.734 |
| RV EDVI (mL/m2) | 86.5 [64.8–108.8] | 86.0 [69.5–111.5] | 0.782 |
| RV ESVI (mL/m2) | 51.0 [33.3–69.8] | 62.0 [45.0–88.5] | 0.234 |
| RV SVI (mL/m2) | 33.0 [26.8–41.3] | 25.0 [20.8–33.8] | 0.078 |
| RV Mass (g/m2) | 46.5 [35.5–57.3] | 50.0 [40.0–62.8] | 0.432 |
| RV EF (%) | 41.0 [33.75–47.25] | 28.0 [23.5–36.3] | 0.044 |
| Parameter | Survival Group (n = 30/n = 24 *) | Non-Survival Group (n = 6) | p-Value between the Groups | ||
|---|---|---|---|---|---|
| Δ | p-Value within the Group | Δ | p-Value within the Group | ||
| 6MWT (m) | 15.0 [−25.0–52.5] | 0.271 | −60.0 [−100.0–] ** | 0.109 | 0.081 |
| NT-proBNP (ng/L) | 18.7 [−582.3–362.0] | 0.495 | 481.0 [−183.5–4576.0] | 0.138 | 0.098 |
| LV GLS (%) *** | −1.35 [−8.9–2.1] | 0.067 | −1.8 [−21.1–5.3] | 0.463 | 0.832 |
| LV GCS (%) *** | −2.7 [−8.4–(−0.5)] | 0.002 | −2.8 [−3.7–4.4] | 0.463 | 0.396 |
| LV EDVI (mL/m2) | 1.5 [−1.0–6.5] | 0.101 | 0.0 [−11.3–19.0] | 0.674 | 0.882 |
| LV ESVI (mL/m2) | 0.0 [−4.0–4.0] | 0.810 | 2.5 [−6.0–18.0] | 0.600 | 0.457 |
| LV SVI (mL/m2) | 4.0 [−1.5–7.3] | 0.058 | −1.5 [−20.3–7.3] | 0.600 | 0.327 |
| LV EF (%) | 2.0 [−6.8–8.3] | 0.532 | −1.0 [−22.8–8.5] | 0.600 | 0.339 |
| RV free wall LS (%) *** | −3.5 [−9.3–3.8] | 0.043 | −0.45 [−6.6–5.9] | 0.917 | 0.445 |
| RV septum LS (%) *** | −1.2 [−6.4–1.6]/ −1.8 [−6.1–0.8] | 0.165/ 0.054 | 4.9 [1.5–6.7]/ 4.9 [1.5–6.7] | 0.075/ 0.075 | 0.038 0.020 |
| RV GLS (%) *** | −3.1 [−9.1–2.6]/ −3.6 [−8.7–1.6] | 0.049/ 0.027 | 4.5 [−2.1–8.5]/ 4.5 [−2.1–8.5] | 0.249/ 0.249 | 0.048 0.028 |
| RV EDVI (mL/m2) | 0.5 [−7.0–10.8] | 0.737 | 8.0 [−7.3–17.8] | 0.400 | 0.470 |
| RV ESVI (mL/m2) | 3.0 [−5.0–11.0] | 0.179 | 11.5 [−3.8–19.3] | 0.173 | 0.298 |
| RV SVI (mL/m2) | −2.0 [−7.0–5.8] | 0.436 | −0.50 [−8.0–10.5] | 0.752 | 0.766 |
| RV Mass (g/m2) | 3.5 [−2.3–9.3] | 0.015 | 8.0 [−1.5–15.0] | 0.136 | 0.419 |
| RV EF (%) | −1.5 [−8.0–5.0] | 0.249 | −2.5 [−9.8–8.0] | 0.753 | 0.949 |
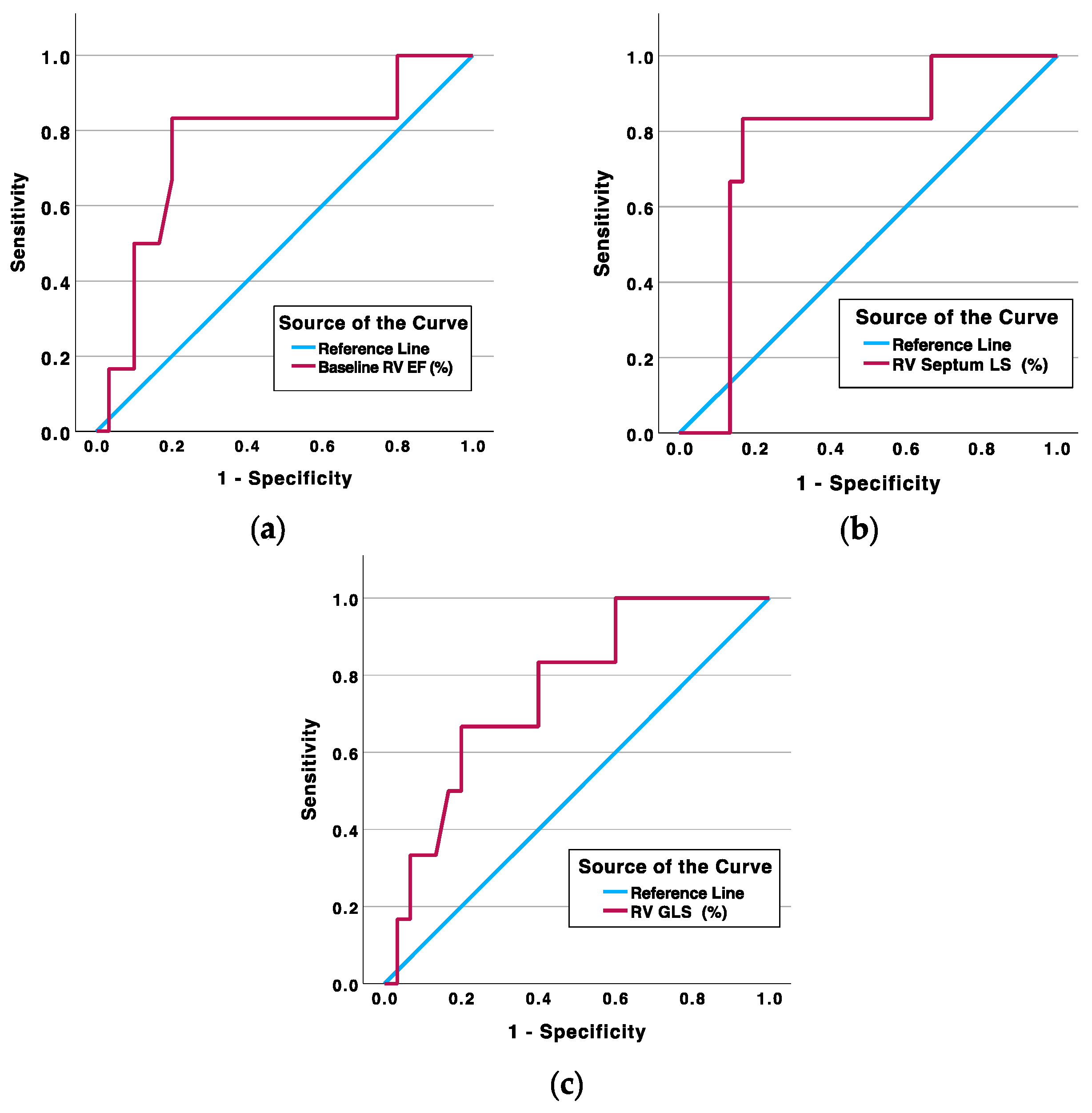
| Parameter (Threshold Value) | Area Under the Curve (%) | Sensitivity/Specificity (%) | Survival/Non-Survival (n (%)) | p-Value |
|---|---|---|---|---|
| Baseline RV EF ≤ 32.5% | 76.4 | 83.3/80.0 | 6/5 | 0.006 |
| RV septum LS Δ ≥ 2.95% | 77.2 | 83.3/83.3 | 5/5 | 0.003 |
| RV GLS Δ ≥ 3.60% | 76.1 | 66.7/80.0 | 6/4 | 0.039 |
| Parameter (Threshold Value) | Odds Ratio [95% CI] | p-Value | Odds Ratio Adjusted by Age [95% CI] | p-Value |
|---|---|---|---|---|
| Baseline RV EF ≤ 32.5% | 20.0 [1.954–204.728] | 0.012 | 22.067 [1.739–279.963] | 0.017 |
| RV septum LS Δ ≥ 2.95% | 25.0 [2.380–262.653] | 0.007 | 27.034 [1.982–368.747] | 0.013 |
| RV GLS Δ ≥ 3.60% | 8.0 [1.174–54.497] | 0.034 | 13.989 [1.243–157.400] | 0.033 |
| Parameter (Threshold Value) | Hazard Ratio [95% CI] | p-Value | Hazard Ratio Adjusted by Age [95% CI] | p-Value |
|---|---|---|---|---|
| Baseline RV EF ≤ 32.5% | 12.721 [1.485–108.932] | 0.020 | 9.883 [1.109–88.072] | 0.040 |
| RV septum LS Δ ≥ 2.95% | 16.513 [1.924–141.767] | 0.011 | 14.744 [1.686–128.925] | 0.015 |
| RV GLS Δ ≥ 3.60% | 5.996 [1.097–32.775] | 0.039 | 7.210 [1.303–39.895] | 0.024 |
| Regressors | OR [95% CI], p-Value |
|---|---|
| Model No. 1 (correct prediction 88.9%, Nagelkerke determination coefficient 0.527) | |
| Baseline RV EF ≤ 32.5% | 28.163 [1.836–431.973], 0.017 |
| RV GLS Δ ≥ 3.60% * | 12.285 [0.96–156.905], 0.054 |
| Model constant | −4.419, p = 0.0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padervinskienė, L.; Ažukaitė, J.; Hoppenot, D.; Krivickienė, A.; Šimkus, P.; Nedzelskienė, I.; Miliauskas, S.; Ereminienė, E. The Prognostic Value of One-Year Changes in Biventricular Mechanics for Three-Year Survival in Patients with Precapillary Pulmonary Hypertension: A Cardiovascular Magnetic Resonance Feature Tracking Study. Medicina 2024, 60, 141. https://doi.org/10.3390/medicina60010141
Padervinskienė L, Ažukaitė J, Hoppenot D, Krivickienė A, Šimkus P, Nedzelskienė I, Miliauskas S, Ereminienė E. The Prognostic Value of One-Year Changes in Biventricular Mechanics for Three-Year Survival in Patients with Precapillary Pulmonary Hypertension: A Cardiovascular Magnetic Resonance Feature Tracking Study. Medicina. 2024; 60(1):141. https://doi.org/10.3390/medicina60010141
Chicago/Turabian StylePadervinskienė, Lina, Joana Ažukaitė, Deimantė Hoppenot, Aušra Krivickienė, Paulius Šimkus, Irena Nedzelskienė, Skaidrius Miliauskas, and Eglė Ereminienė. 2024. "The Prognostic Value of One-Year Changes in Biventricular Mechanics for Three-Year Survival in Patients with Precapillary Pulmonary Hypertension: A Cardiovascular Magnetic Resonance Feature Tracking Study" Medicina 60, no. 1: 141. https://doi.org/10.3390/medicina60010141
APA StylePadervinskienė, L., Ažukaitė, J., Hoppenot, D., Krivickienė, A., Šimkus, P., Nedzelskienė, I., Miliauskas, S., & Ereminienė, E. (2024). The Prognostic Value of One-Year Changes in Biventricular Mechanics for Three-Year Survival in Patients with Precapillary Pulmonary Hypertension: A Cardiovascular Magnetic Resonance Feature Tracking Study. Medicina, 60(1), 141. https://doi.org/10.3390/medicina60010141






