Utilizing a Novel AI Tool to Detect the Posterior Superior Alveolar Artery’s Location’s Impact on Maxillary Sinus Mucosal Thickening in the Presence of Periapical Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Radiographic Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, S.C.; Pharoah, M.J. Oral Radiology-E-Book: Principles and Interpretation; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zadsirjan, S.; Sheikhi, M.; Dakhilalian, A.; Feli, M. Association of Inflammatory Periapical Lesions with Maxillary Sinus Abnormalities: A Retrospective Cone-Beam Computed Tomography Study. J. Dent. 2021, 22, 273–280. [Google Scholar] [CrossRef]
- Nair, A.K.; Jose, M.; Sreela, L.S.; Prasad, T.S.; Mathew, P. Prevalence and pattern of proximity of maxillary posterior teeth to maxillary sinus with mucosal thickening: A cone beam computed tomography based retrospective study. Ann. Afr. Med. 2023, 22, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Solar, P.; Geyerhofer, U.; Traxler, H.; Windisch, A.; Ulm, C.; Watzek, G. Blood supply to the maxillary sinus relevant to sinus floor elevation procedures. Clin. Oral Implants Res. 1999, 10, 34–44. [Google Scholar] [CrossRef]
- Kasahara, N.; Morita, W.; Tanaka, R.; Hayashi, T.; Kenmotsu, S.; Ohshima, H. The Relationships of the Maxillary Sinus with the Superior Alveolar Nerves and Vessels as Demonstrated by Cone-Beam CT Combined with mu-CT and Histological Analyses. Anat. Rec. 2016, 299, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Guncu, G.N.; Yildirim, Y.D.; Wang, H.L.; Tozum, T.F. Location of posterior superior alveolar artery and evaluation of maxillary sinus anatomy with computerized tomography: A clinical study. Clin. Oral Implants Res. 2011, 22, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Tehranchi, M.; Taleghani, F.; Shahab, S.; Nouri, A. Prevalence and location of the posterior superior alveolar artery using cone-beam computed tomography. Imaging Sci. Dent. 2017, 47, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Shiota, M.; Gao, S.; Imakita, C.; Tachikawa, N.; Kasugai, S. Verification of posterior superior alveolar artery distribution in lateral wall of maxillary sinus by location and defect pattern. Quintessence Int. 2014, 45, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Ella, B.; Sedarat, C.; Noble Rda, C.; Normand, E.; Lauverjat, Y.; Siberchicot, F.; Caix, P.; Zwetyenga, N. Vascular connections of the lateral wall of the sinus: Surgical effect in sinus augmentation. Int. J. Oral Maxillofac. Implants 2008, 23, 1047–1052. [Google Scholar]
- Flanagan, D. Arterial supply of maxillary sinus and potential for bleeding complication during lateral approach sinus elevation. Implant Dent. 2005, 14, 336–338. [Google Scholar] [CrossRef]
- Tong, D.C.; Rioux, K.; Drangsholt, M.; Beirne, O.R. A review of survival rates for implants placed in grafted maxillary sinuses using meta-analysis. Int. J. Oral Maxillofac. Implants 1998, 13, 175–182. [Google Scholar]
- Ilguy, D.; Ilguy, M.; Dolekoglu, S.; Fisekcioglu, E. Evaluation of the posterior superior alveolar artery and the maxillary sinus with CBCT. Braz. Oral Res. 2013, 27, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, J.B.; Ivanovic, M. Comparative dosimetry of dental CBCT devices and 64-slice CT for oral and maxillofacial radiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 106–114. [Google Scholar] [CrossRef]
- Tofangchiha, M.; Hematzadeh, S.; Vali, M.E.; Ghonche, M.R.A.; Mirzadeh, M.; Reda, R.; Testarelli, L. Anatomical localization of posterior superior alveolar artery: A retrospective study by cone-beam computed tomography. Dent. Med. Probl. 2022, 59, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, S.A.; Movahed, A.; ElChaar, E.S.; Chong Chan, K.; Amintavakoli, N. Radiographic Evaluation of Maxillary Sinus Lateral Wall and Posterior Superior Alveolar Artery Anatomy: A Cone-Beam Computed Tomographic Study. Clin. Implant Dent. Relat. Res. 2017, 19, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.S.; Kim, J.E.; Hwang, J.J.; Han, S.S.; Kim, J.S.; Yi, W.J.; Park, I.W. Artificial intelligence in oral and maxillofacial radiology: What is currently possible? Dentomaxillofac. Radiol. 2021, 50, 20200375. [Google Scholar] [CrossRef] [PubMed]
- Mall, P.K.; Singh, P.K.; Srivastav, S.; Narayan, V.; Paprzycki, M.; Jaworska, T.; Ganzha, M. A comprehensive review of deep neural networks for medical image processing: Recent developments and future opportunities. Healthc. Anal. 2023, 4, 100216. [Google Scholar] [CrossRef]
- Yalcin, E.D.; Akyol, S. Relationship Between the Posterior Superior Alveolar Artery and Maxillary Sinus Pathology: A Cone-Beam Computed Tomography Study. J. Oral Maxil Surg. 2019, 77, 2494–2502. [Google Scholar] [CrossRef]
- Ang, K.Y.; Ang, K.L.; Ngeow, W.C. The prevalence and location of the posterior superior alveolar artery in the maxillary sinus wall: A preliminary computed-cone beam study. Saudi Dent. J. 2022, 34, 629–635. [Google Scholar] [CrossRef]
- Ren, S.; Zhao, H.; Liu, J.; Wang, Q.; Pan, Y. Significance of maxillary sinus mucosal thickening in patients with periodontal disease. Int. Dent. J. 2015, 65, 303–310. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Wasmer, J.; Sendi, P.; Janner, S.F.; Buser, D.; von Arx, T. Characteristics and dimensions of the Schneiderian membrane and apical bone in maxillary molars referred for apical surgery: A comparative radiographic analysis using limited cone beam computed tomography. J. Endod. 2012, 38, 51–57. [Google Scholar] [CrossRef]
- Goller-Bulut, D.; Sekerci, A.E.; Kose, E.; Sisman, Y. Cone beam computed tomographic analysis of maxillary premolars and molars to detect the relationship between periapical and marginal bone loss and mucosal thickness of maxillary sinus. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e572–e579. [Google Scholar] [CrossRef] [PubMed]
- Kasikcioglu, A.; Gulsahi, A. Relationship between maxillary sinus pathologies and maxillary posterior tooth periapical pathologies. Oral Radiol. 2016, 32, 180–186. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Z.; Zhang, L.; Zhou, X.; Zheng, Q.; Duan, X.; Zheng, G.; Wang, H.; Huang, D. Associations between maxillary sinus mucosal thickening and apical periodontitis using cone-beam computed tomography scanning: A retrospective study. J. Endod. 2012, 38, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Guedes, O.A.; Alencar, A.H.; Peters, O.A.; Estrela, C.R.; Estrela, C. Evaluation of Periapical Lesions and Their Association with Maxillary Sinus Abnormalities on Cone-beam Computed Tomographic Images. J. Endod. 2016, 42, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ciftci, M.E.; Kamak, G.; Aktan, A.M. Evaluation of the relationship between maxillary sinus floor position and maxillary sinusitis using cone beam computed tomography. Oral Radiol. 2017, 33, 16–22. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Xu, Q.; Shu, J.; Xu, B.; Liu, L.; Chen, H.; Hu, Y.; Li, Y.; Song, L. Increased risks of maxillary sinus mucosal thickening in Chinese patients with periapical lesions. Heliyon 2023, 9, e18050. [Google Scholar] [CrossRef] [PubMed]
- Eggmann, F.; Connert, T.; Bühler, J.; Dagassan-Berndt, D.; Weiger, R.; Walter, C. Do periapical and periodontal pathologies affect Schneiderian membrane appearance? Systematic review of studies using cone-beam computed tomography. Clin. Oral Investig. 2017, 21, 1611–1630. [Google Scholar] [CrossRef] [PubMed]
- Janner, S.F.; Caversaccio, M.D.; Dubach, P.; Sendi, P.; Buser, D.; Bornstein, M.M. Characteristics and dimensions of the Schneiderian membrane: A radiographic analysis using cone beam computed tomography in patients referred for dental implant surgery in the posterior maxilla. Clin. Oral Implants Res. 2011, 22, 1446–1453. [Google Scholar] [CrossRef]
- Rege, I.C.; Sousa, T.O.; Leles, C.R.; Mendonca, E.F. Occurrence of maxillary sinus abnormalities detected by cone beam CT in asymptomatic patients. BMC Oral Health 2012, 12, 30. [Google Scholar] [CrossRef]
- Schneider, A.C.; Bragger, U.; Sendi, P.; Caversaccio, M.D.; Buser, D.; Bornstein, M.M. Characteristics and dimensions of the sinus membrane in patients referred for single-implant treatment in the posterior maxilla: A cone beam computed tomographic analysis. Int. J. Oral Maxillofac. Implants 2013, 28, 587–596. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Block, M.S.; Dastoury, K. Prevalence of sinus membrane thickening and association with unhealthy teeth: A retrospective review of 831 consecutive patients with 1,662 cone-beam scans. J. Oral Maxillofac. Surg. 2014, 72, 2454–2460. [Google Scholar] [CrossRef] [PubMed]
- Eggers, G. Cone beam computer tomography for paranasal sinus imaging. Int. J. Comput. Assisted Radiol. Surg. 2011, 6, 205–206. [Google Scholar]
- Nurbakhsh, B.; Friedman, S.; Kulkarni, G.V.; Basrani, B.; Lam, E. Resolution of maxillary sinus mucositis after endodontic treatment of maxillary teeth with apical periodontitis: A cone-beam computed tomography pilot study. J. Endod. 2011, 37, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Godil, A.Z.; Devadiga, T.J.; Supnekar, S.C.; Kazi, A.I.; Wadwan, S.A.; Dugal, R. Position of posterior superior alveolar artery in relation to the maxillary sinus using cone beam computed tomography in Indian sub-population. J. Oral Med. Oral Surg. 2021, 27, 34. [Google Scholar] [CrossRef]
- Khojastehpour, L.; Dehbozorgi, M.; Tabrizi, R.; Esfandnia, S. Evaluating the anatomical location of the posterior superior alveolar artery in cone beam computed tomography images. Int. J. Oral Maxillofac. Surg. 2016, 45, 354–358. [Google Scholar] [CrossRef]
- Rathod, R.; Singh, M.P.; Nahar, P.; Mathur, H.; Daga, D. Assessment of Pathway and Location of Posterior Superior Alveolar Artery: A Cone-Beam Computed Tomography Study. Cureus 2022, 14, e22028. [Google Scholar] [CrossRef]
- Bedeloğlu, E.; Yalçın, M. Evaluation of the Posterior Superior Alveolar Artery Prior to Sinus Floor Elevation via Lateral Window Technique: A Cone-Beam Computed Tomography Study. J. Adv. Oral Res. 2020, 11, 215–223. [Google Scholar] [CrossRef]
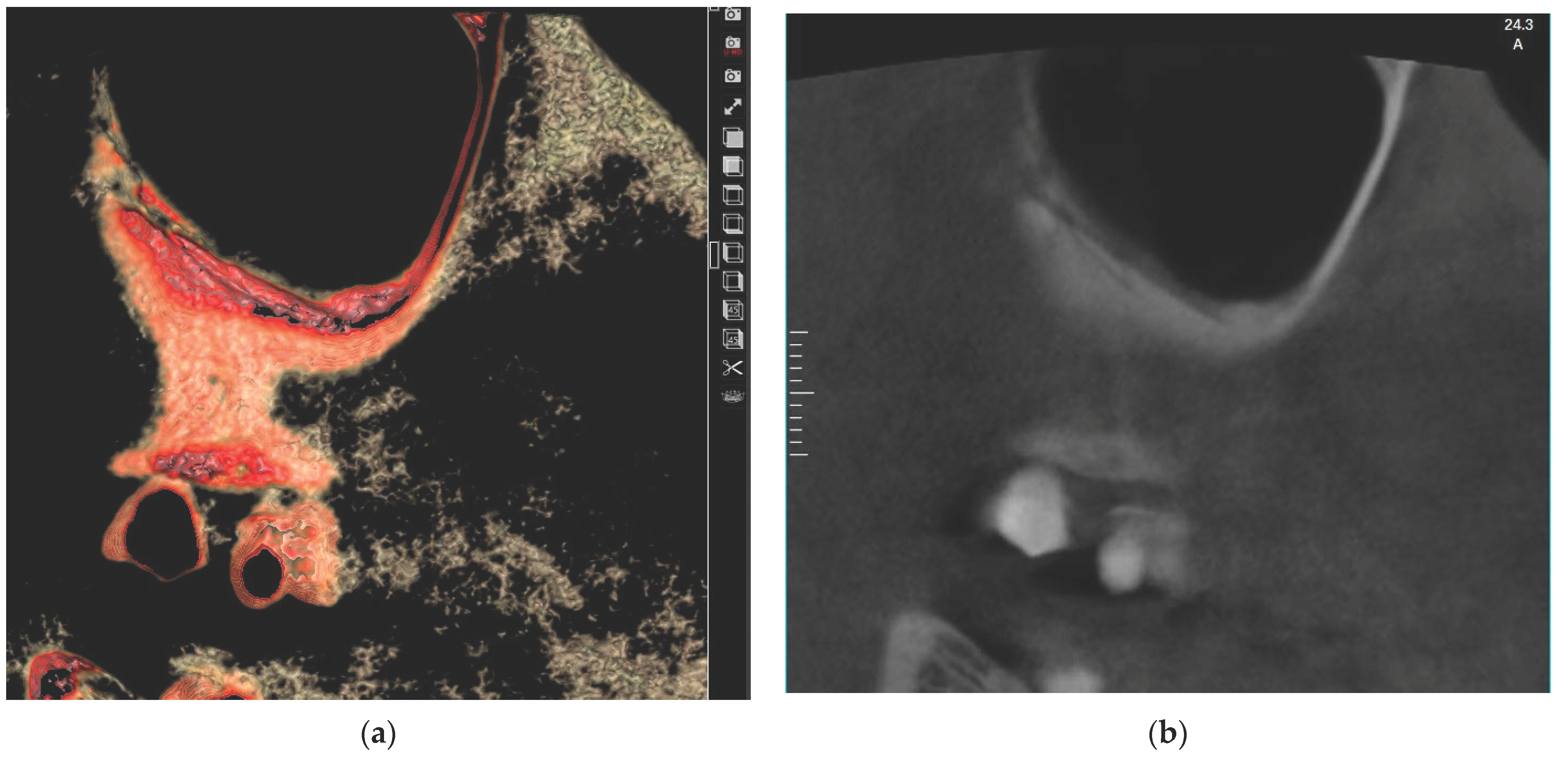

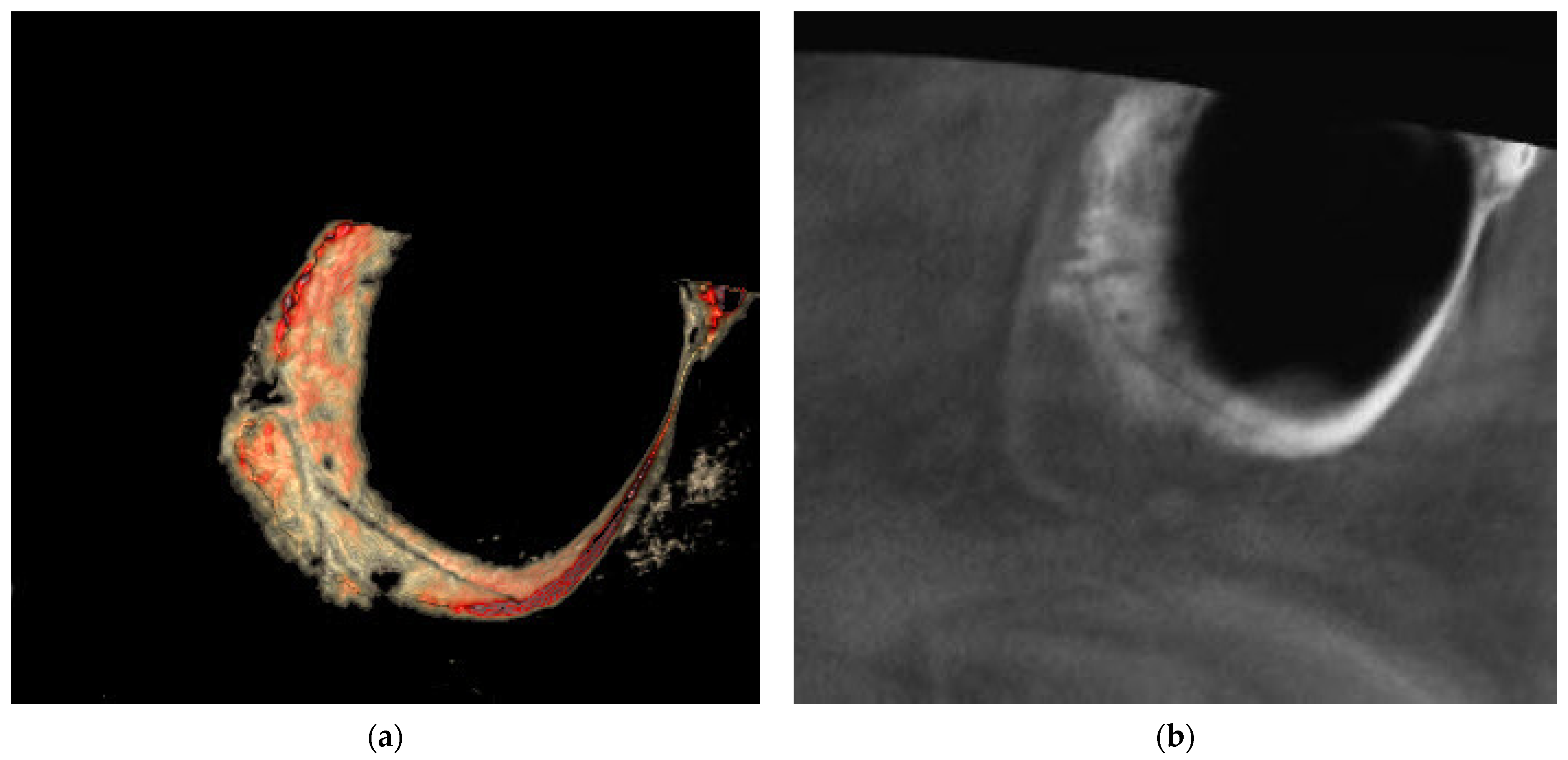
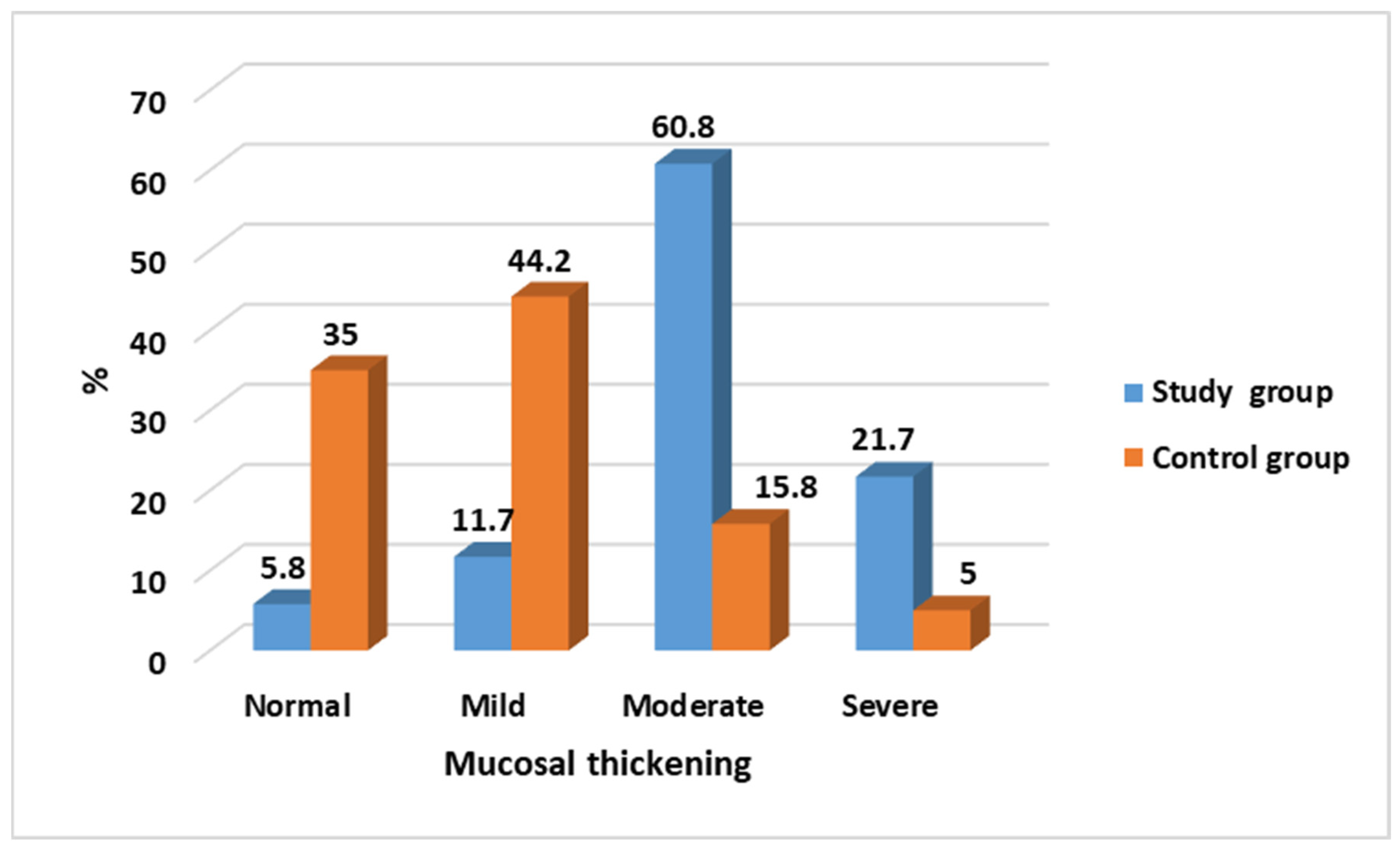

| Mucosal Thickening | Study Group N = 120 (%) | Control Group N = 120 (%) | Test of Significance | Significance for Every Category |
|---|---|---|---|---|
| Normal | 7 (5.8) | 42 (35.0) | χ2 = 91.89 p < 0.001 * | p < 0.001 * |
| Mild | 14 (11.7) | 53 (44.2) | p < 0.001 * | |
| Moderate | 73 (60.8) | 19 (15.8) | p < 0.001 * | |
| Severe | 26 (21.7) | 6 (5.0) | p = 0.001 * |
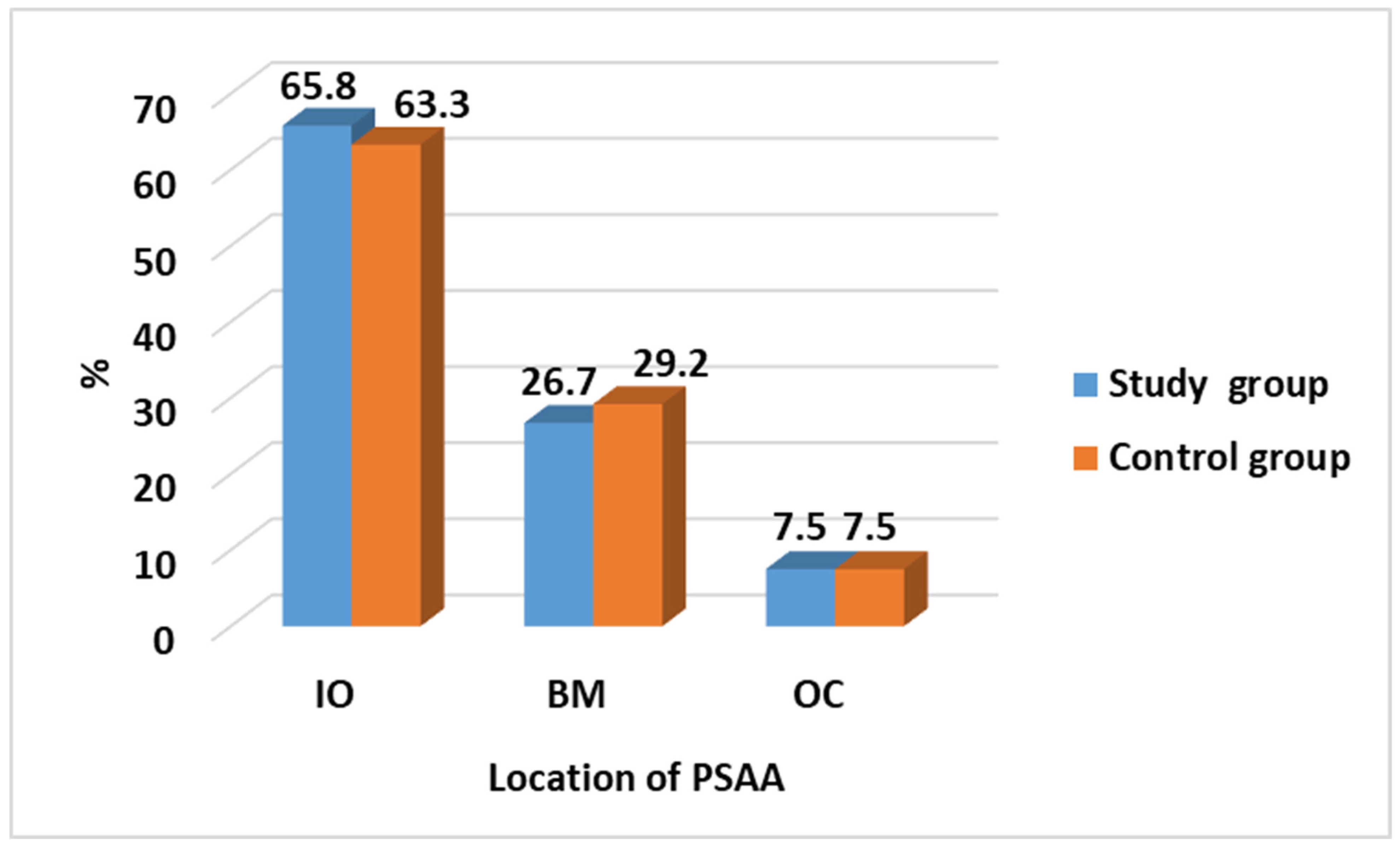
| Location of PSAA | Study Group N = 120 (%) | Control Group N = 120 (%) | Test of Significance | Significance for Every Category |
|---|---|---|---|---|
| IO | 79 (65.8) | 76 (63.3) | χ2 = 0.192 p = 0.908 | p = 0.686 |
| BM | 32 (26.7) | 35 (29.2) | p = 0.669 | |
| OC | 9 (7.5) | 9 (7.5) | p = 1.0 |
| For Study Group | Location of PSAA | |||
|---|---|---|---|---|
| Mucosal Thickening | IO | BM | OC | |
| Normal | 1 (1.3) | 0 | 6 (66.7) | MC = 114.74 p < 0.001 * |
| Mild | 9 (11.4) | 2 (6.2) | 3 (33.3) | |
| Moderate | 63 (79.7) | 10 (31.2) | 0 | |
| Severe | 6 (7.6) | 20 (62.5) | 0 | |
| For Control Group | Location of PSAA | |||
|---|---|---|---|---|
| Mucosal Thickening | IO | BM | OC | |
| Normal | 30 (39.5) | 7 (20.0) | 5 (55.6) | MC = 16.94 p = 0.009 * |
| Mild | 37 (48.7) | 13 (37.1) | 3 (33.3) | |
| Moderate | 6 (7.9) | 12 (34.3) | 1 (11.1) | |
| Severe | 3 (3.9) | 3 (8.6) | 0 | |
| Location of PSAA | Mucosal Thickening | Study Group | Control Group | Test of Significance |
|---|---|---|---|---|
| N = 120 (%) | N = 120 (%) | |||
| IO | N = 79 (%) | N = 67 (%) | MC = 92.24 p < 0.001 * | |
| Normal | 1 (1.3) | 30 (39.5) | ||
| Mild | 9 (11.4) | 37 (48.7) | ||
| Moderate | 63 (79.7) | 6 (7.9) | ||
| Severe | 6 (7.6) | 3 (3.9) | ||
| BM | N = 32 (%) | N = 35 (%) | MC = 27.74 p < 0.001 * | |
| Normal | 0 | 7 (20.0) | ||
| Mild | 2 (6.2) | 13 (37.1) | ||
| Moderate | 10 (31.2) | 12 (34.3) | ||
| Severe | 20 (62.5) | 3 (8.6) | ||
| OC | N = 9 (%) | N = 9 (%) | MC = 1.09 p = 0.580 | |
| Normal | 6 (66.7) | 5 (55.6) | ||
| Mild | 3 (33.3) | 3 (33.3) | ||
| Moderate | 0 | 1 (11.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboelmaaty, W.; Alfadley, A.; Awawdeh, M.; Sapri, A.S.; Awawdeh, L.; Mira, E.S. Utilizing a Novel AI Tool to Detect the Posterior Superior Alveolar Artery’s Location’s Impact on Maxillary Sinus Mucosal Thickening in the Presence of Periapical Lesions. Medicina 2024, 60, 140. https://doi.org/10.3390/medicina60010140
Aboelmaaty W, Alfadley A, Awawdeh M, Sapri AS, Awawdeh L, Mira ES. Utilizing a Novel AI Tool to Detect the Posterior Superior Alveolar Artery’s Location’s Impact on Maxillary Sinus Mucosal Thickening in the Presence of Periapical Lesions. Medicina. 2024; 60(1):140. https://doi.org/10.3390/medicina60010140
Chicago/Turabian StyleAboelmaaty, Wael, Abdulmohsen Alfadley, Mohammed Awawdeh, Ahmed Saaduddin Sapri, Lama Awawdeh, and Eman Shawky Mira. 2024. "Utilizing a Novel AI Tool to Detect the Posterior Superior Alveolar Artery’s Location’s Impact on Maxillary Sinus Mucosal Thickening in the Presence of Periapical Lesions" Medicina 60, no. 1: 140. https://doi.org/10.3390/medicina60010140
APA StyleAboelmaaty, W., Alfadley, A., Awawdeh, M., Sapri, A. S., Awawdeh, L., & Mira, E. S. (2024). Utilizing a Novel AI Tool to Detect the Posterior Superior Alveolar Artery’s Location’s Impact on Maxillary Sinus Mucosal Thickening in the Presence of Periapical Lesions. Medicina, 60(1), 140. https://doi.org/10.3390/medicina60010140








