The Brainstem Cavernoma Case Series: A Formula for Surgery and Surgical Technique
Abstract
:1. Introduction
2. Materials and Methods
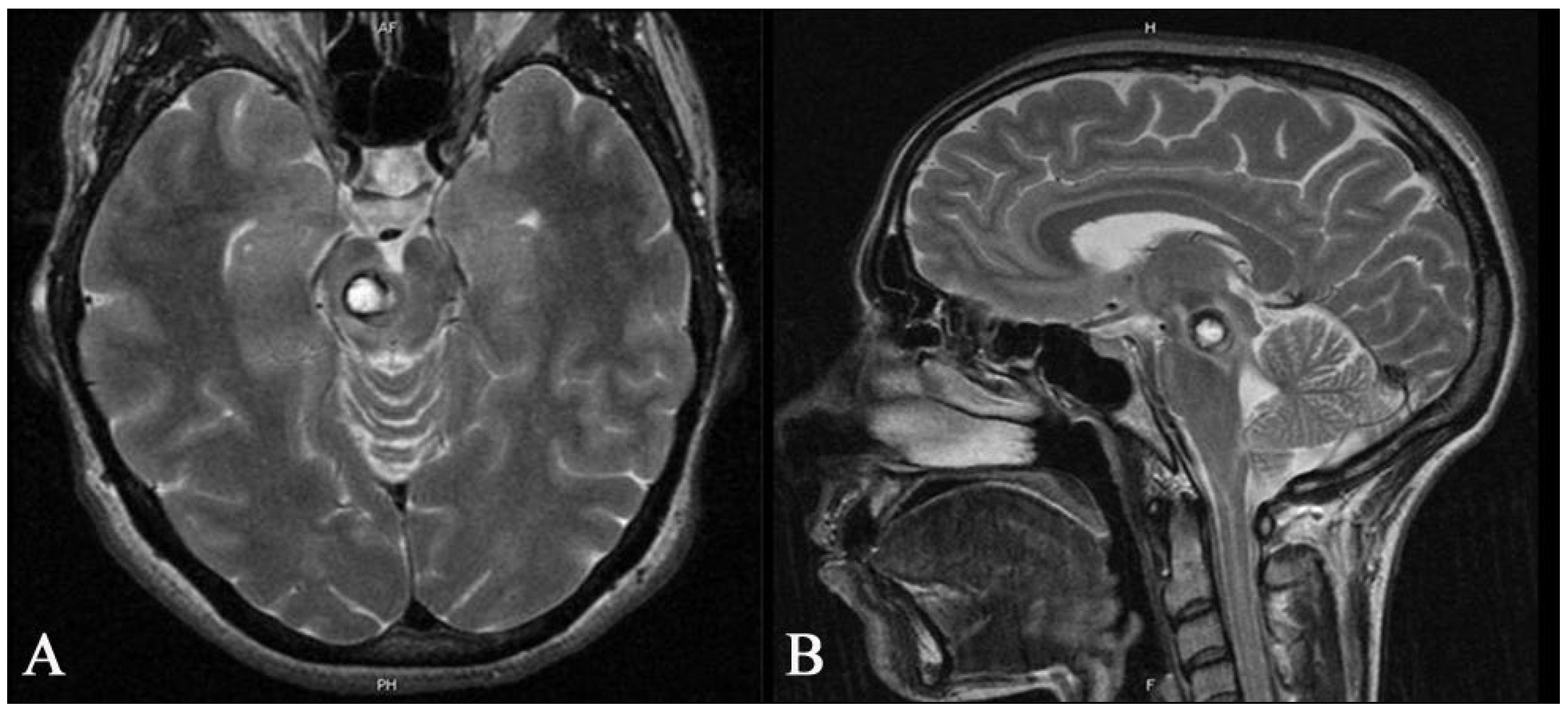
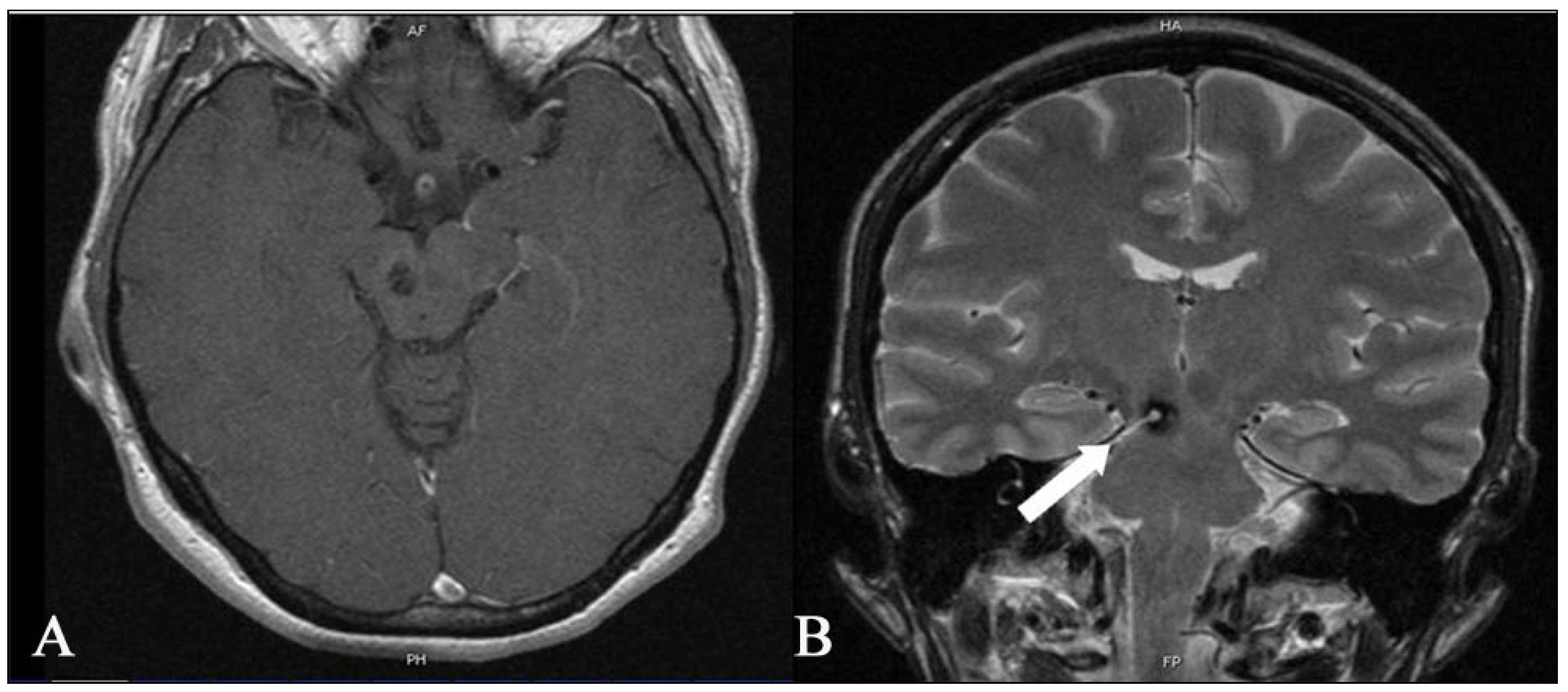
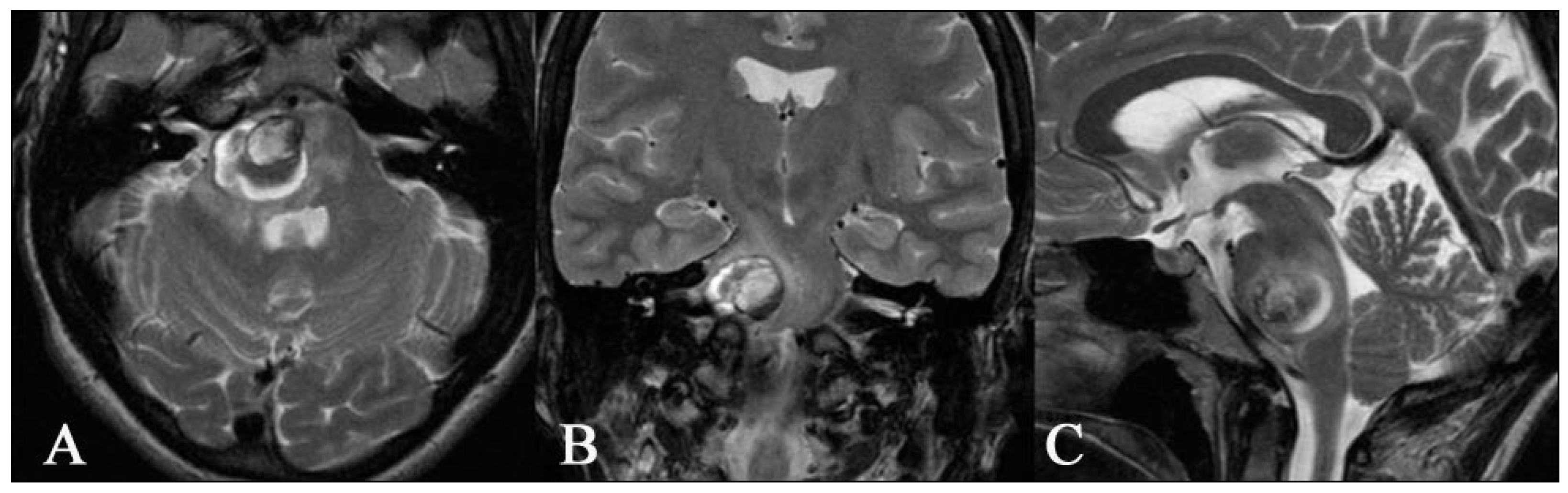
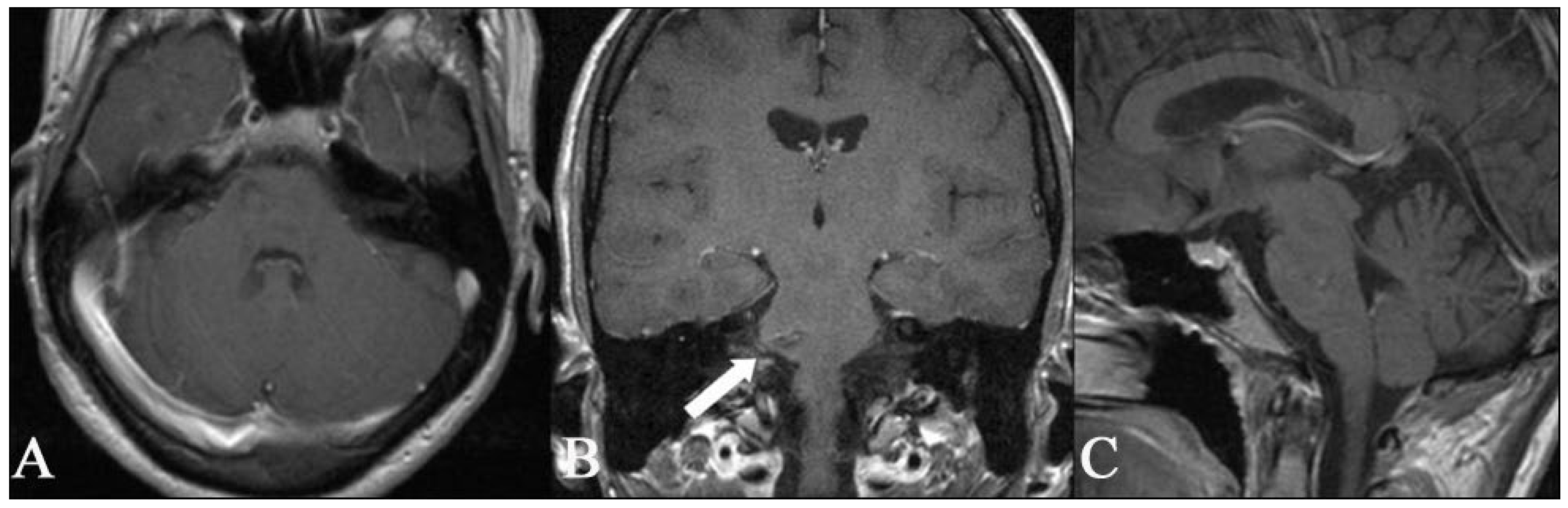
2.1. Imaging
2.2. Intraoperative Electrophysiological Recordings
2.3. Surgical Approaches
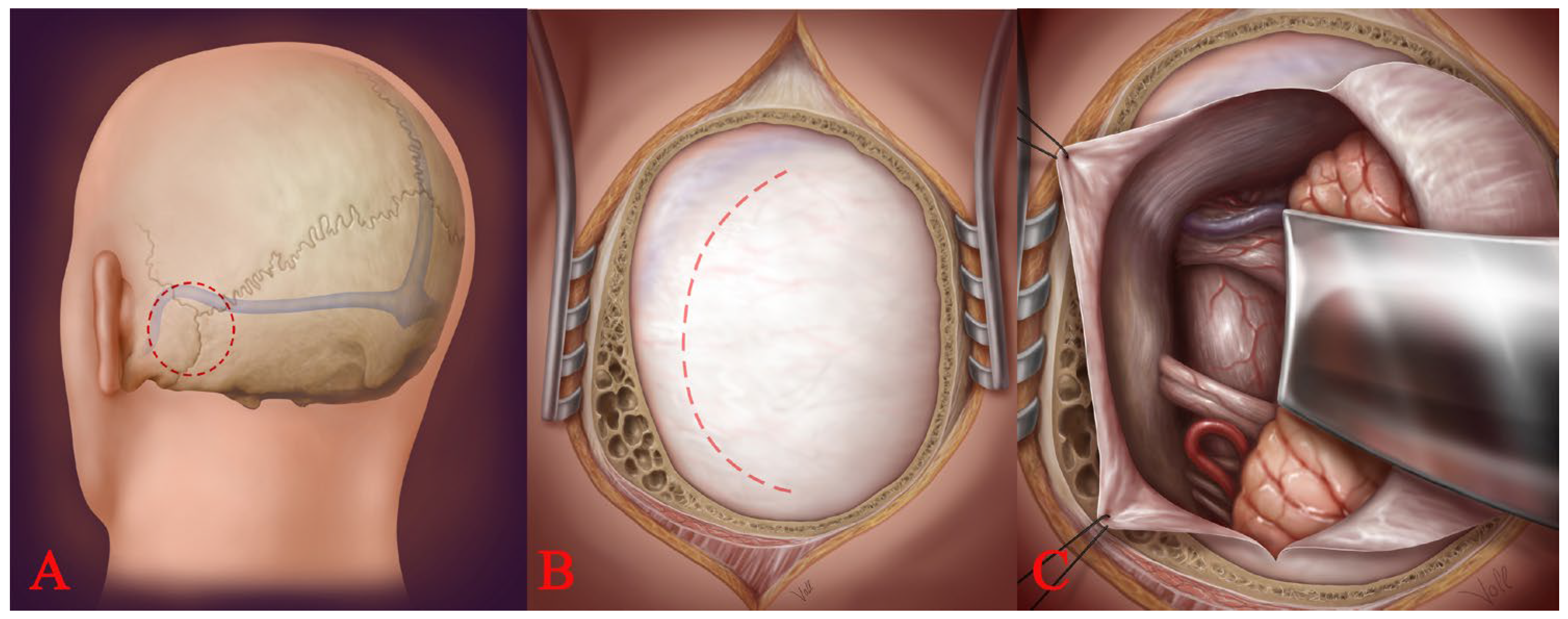
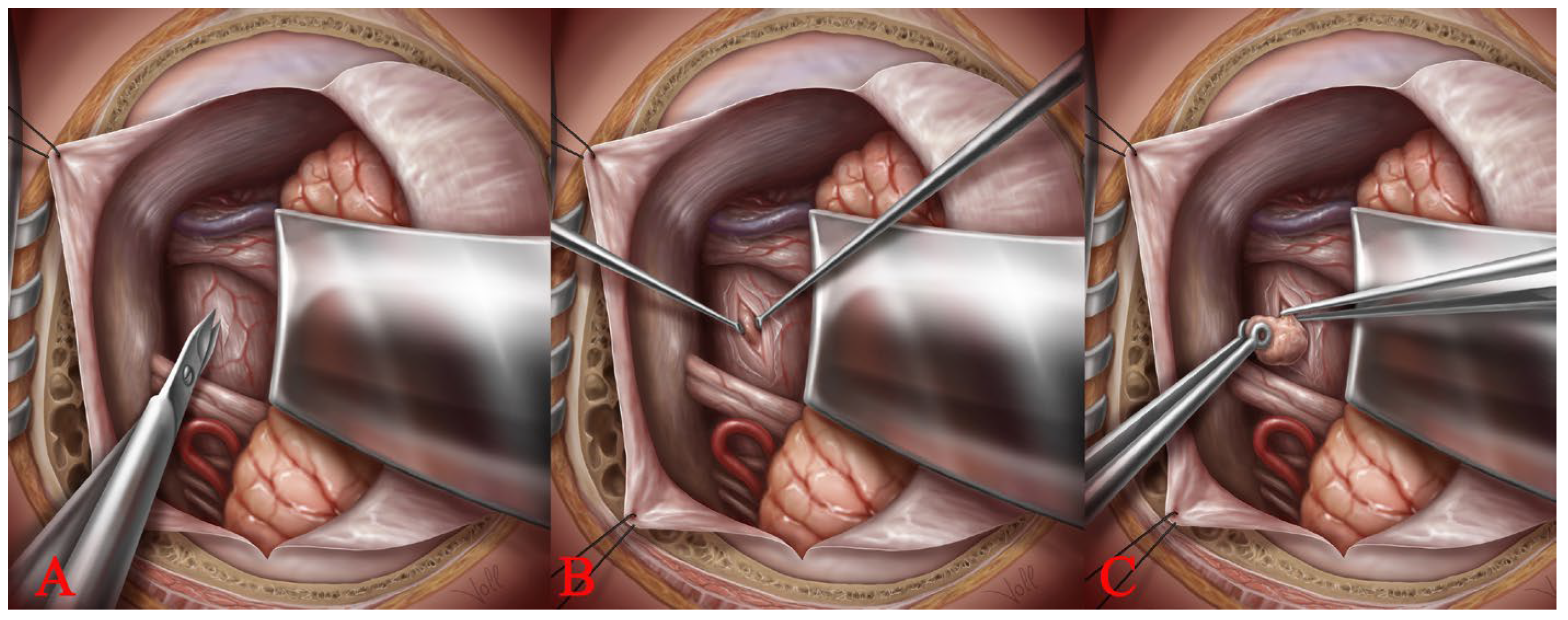
2.4. Access to the Brainstem: The Spinal Cord Dissection Technique
3. Results
3.1. Deep-Seated BS CMs (n = 14)
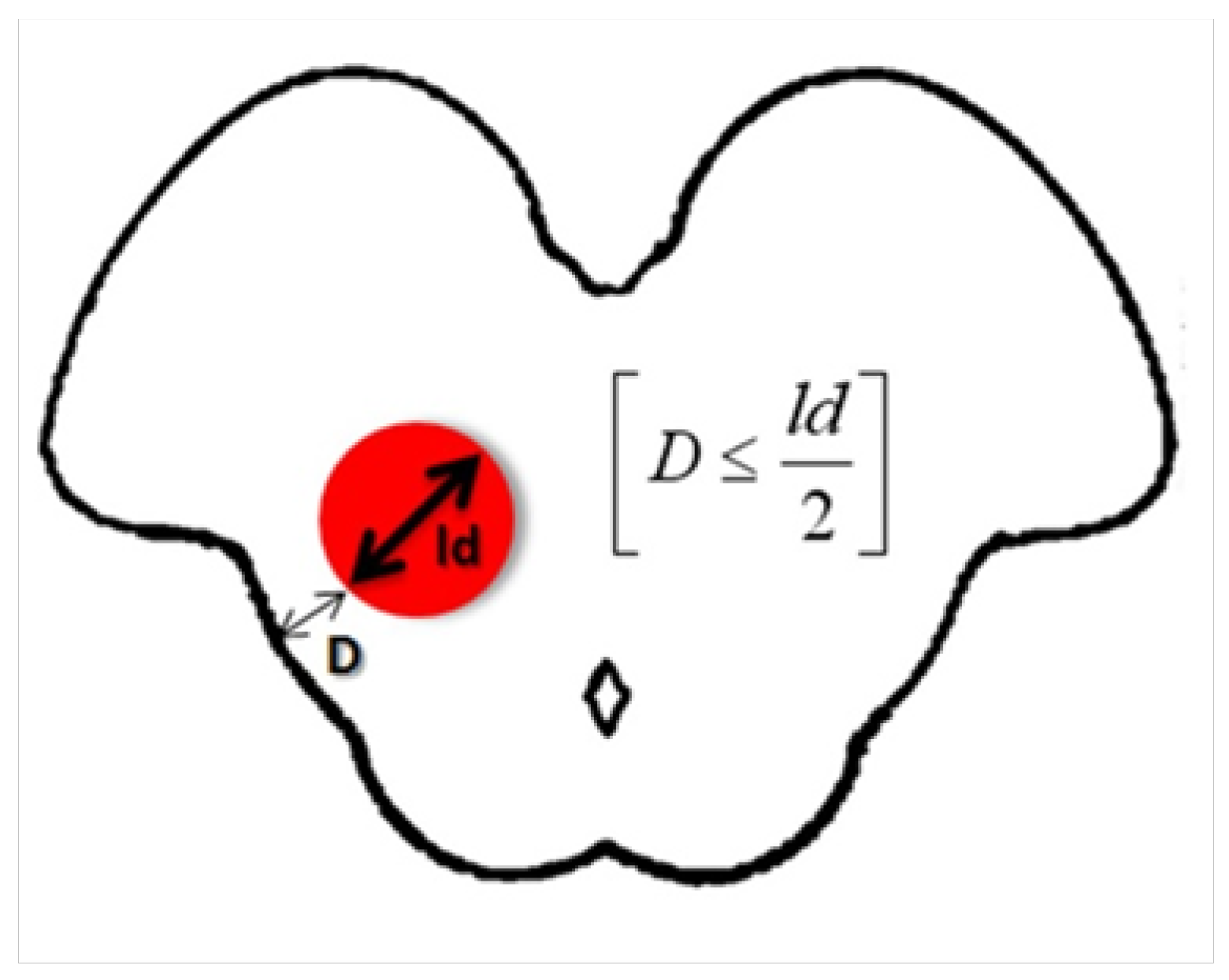
3.2. The Tuebingen Brainstem Cavernoma Equation
3.3. Superficial BS CMs (n = 20)
3.4. Neurological Deficits after the Operation
4. Discussion
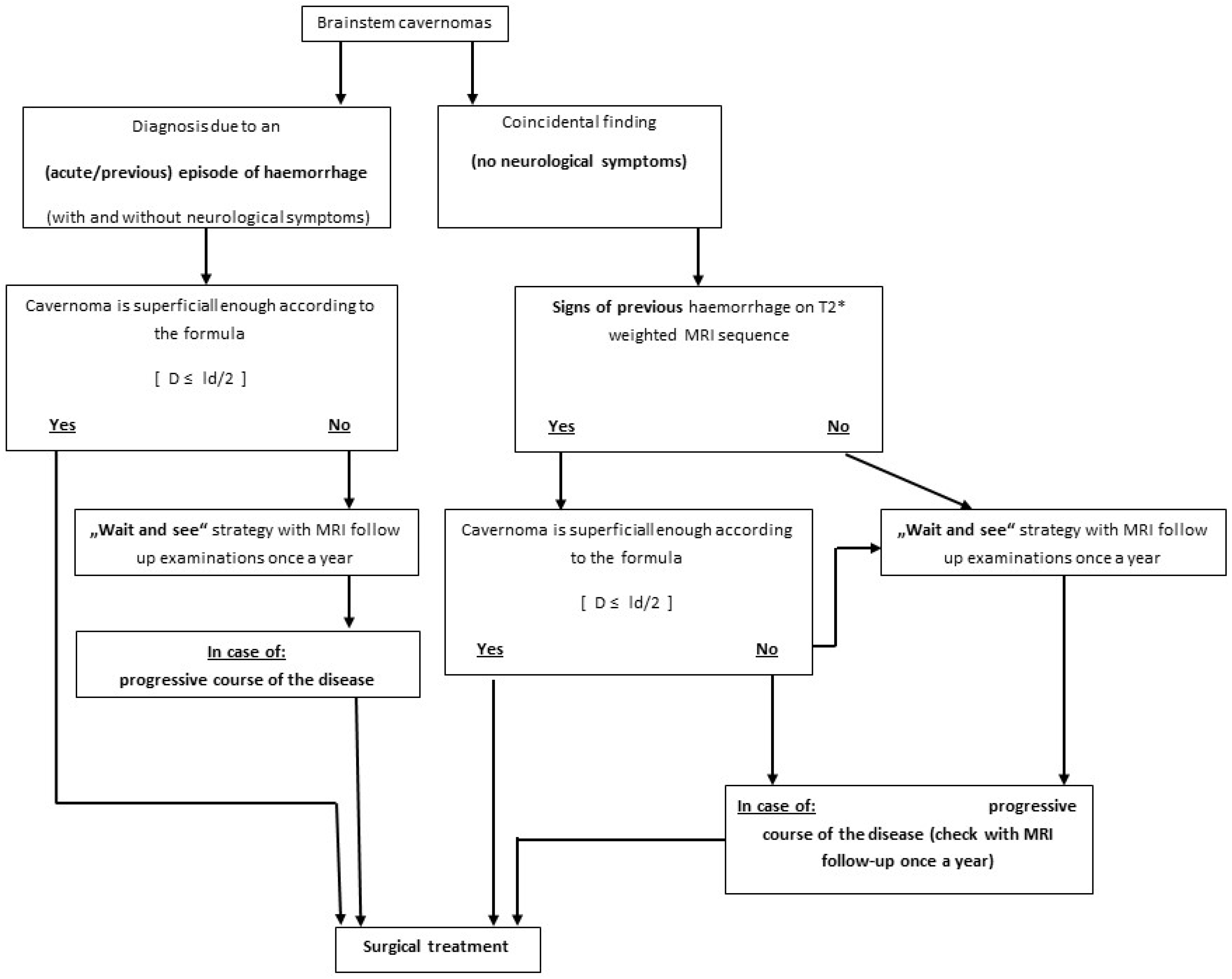
4.1. Indication and Timing for Surgical Treatment
4.2. Surgical Treatment of Deep-Seated and Superficial BS CMs
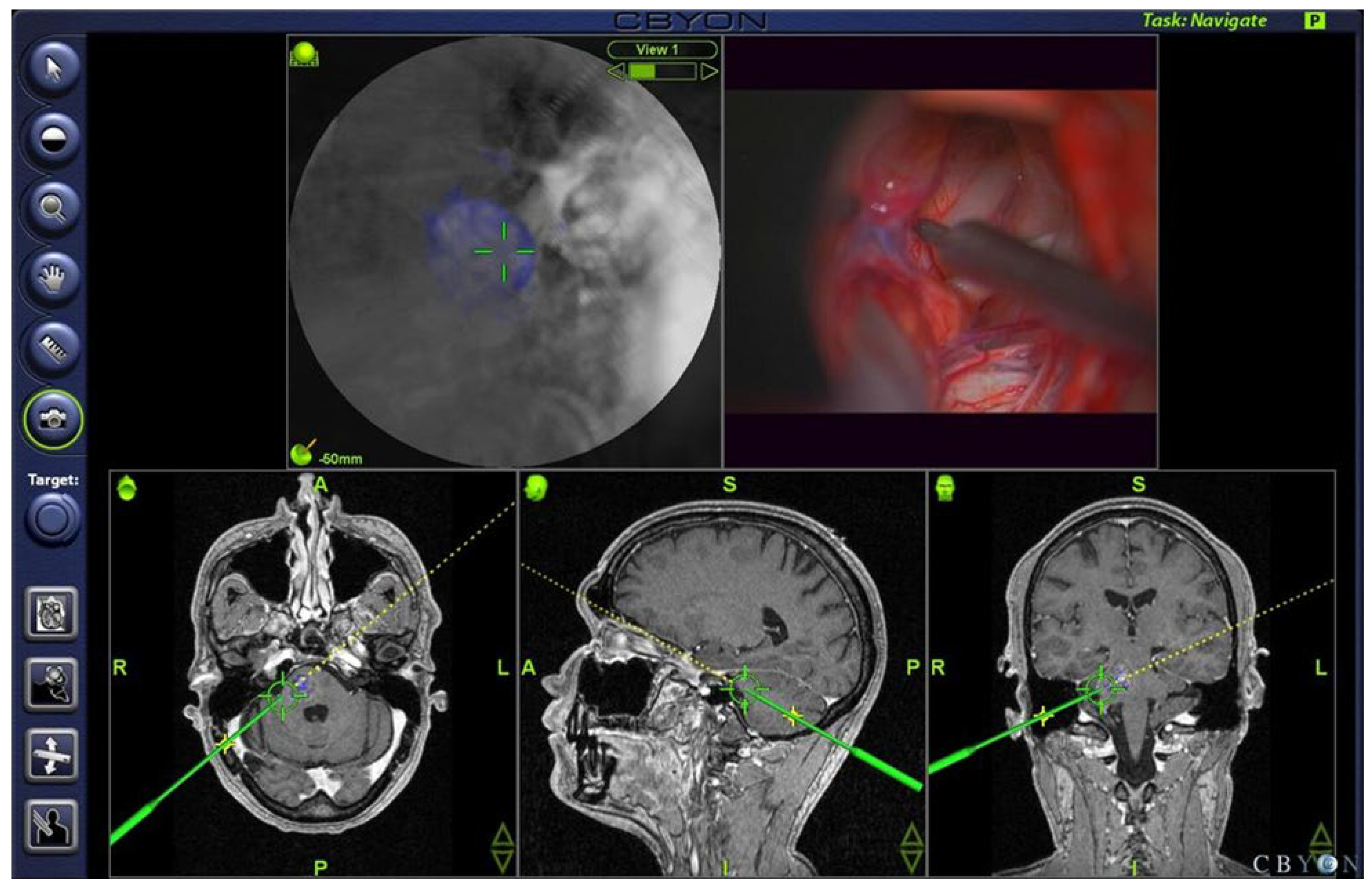
4.3. Electrophysiology, Neuronavigation, and Ultrasonography
4.4. Intraoperative Strategy
4.5. Stereotactic Radiosurgery as an Alternative Treatment Option
4.6. Possible Complications after the BS CMs Surgery
4.7. The Limitations of Our Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Washington, C.W.; McCoy, K.E.; Zipfel, G.J. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg. Focus 2010, 29, E7. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Lin, D.; Recinos, P.F.; Zhang, J.; Rigamonti, D. Cavernous malformations: Natural history, diagnosis and treatment. Nat. Rev. Neurol. 2009, 5, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.; Spetzler, R.F. Surgical treatment of brainstem cavernous malformations. Surg. Neurol. 2009, 72, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Moriarity, J.L.; Clatterbuck, R.E.; Rigamonti, D. The natural history of cavernous malformations. Neurosurg. Clin. N. Am. 1999, 10, 411–417. [Google Scholar] [CrossRef]
- Sindou, M.; Yada, J.; Salord, F. Functional results after microsurgical resection of brain stem cavernous malformations (retrospective study of a 12 patient series and review of the recent literature). Acta Neurochir. 2000, 142, 843–852. [Google Scholar] [CrossRef]
- Dandy, W.E. Venous abnormalities and angiomas of the brain. Arch Surg. 1928, 17, 715–793. [Google Scholar] [CrossRef]
- Bertalanffy, H.; Gilsbach, J.M.; Eggert, H.R.; Seeger, W. Microsurgery of deep-seated cavernous angiomas: Report of 26 cases. Acta Neurochir. 1991, 108, 91–99. [Google Scholar] [CrossRef]
- Ferroli, P.; Sinisi, M.; Franzini, A.; Giombini, S.; Solero, C.L.; Broggi, G. Brainstem cavernomas: Long-term results of microsurgical resection in 52 patients. Neurosurgery 2005, 56, 1203–1212. [Google Scholar] [CrossRef]
- Ohue, S.; Fukushima, T.; Kumon, Y.; Ohnishi, T.; Friedman, A.H. Surgical management of brainstem cavernomas: Selection of approaches and microsurgical techniques. Neurosurg. Rev. 2010, 33, 315–322. [Google Scholar] [CrossRef]
- Porter, R.W.; Detwiler, P.W.; Spetzler, R.F.; Lawton, M.T.; Baskin, J.J.; Derksen, P.T.; Zabramski, J.M. Cavernous malformations of the brainstem: Experience with 100 patients. J. Neurosurg. 1999, 90, 50–58. [Google Scholar] [CrossRef]
- Robinson, J.R.; Awad, I.A.; Little, J.R. Natural history of the cavernous angioma. J. Neurosurg. 1991, 75, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Samii, M.; Eghbal, R.; Carvalho, G.A.; Matthies, C. Surgical management of brainstem cavernomas. J. Neurosurg. 2001, 95, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Liu, A.; Zhang, J.T.; Sun, B.; Zhao, Y.L. Surgical management of brain-stem cavernous malformations: Report of 137 cases. Surg. Neurol. 2003, 59, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Lunsford, L.D.; Kestle, J.R. The natural history of cerebral cavernous malformations. J. Neurosurg. 1995, 83, 820–824. [Google Scholar] [CrossRef]
- Kupersmith, M.J.; Kalish, H.; Epstein, F.; Yu, G.; Berenstein, A.; Woo, H.; Jafar, J.; Mandel, G.; De Lara, F. Natural history of brainstem cavernous malformations. Neurosurgery 2001, 48, 47–53. [Google Scholar]
- Hasegawa, T.; McInerney, J.; Kondziolka, D.; Lee, J.Y.; Flickinger, J.C.; Lunsford, L.D. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery 2002, 50, 1190–1197. [Google Scholar]
- Kida, Y. Radiosurgery for cavernous malformations in basal ganglia, thalamus and brainstem. Prog. Neurol. Surg. 2009, 22, 31–37. [Google Scholar]
- Kim, M.S.; Pyo, S.Y.; Jeong, Y.G.; Lee, S.I.; Jung, Y.T.; Sim, J.H. Gamma knife surgery for intracranial cavernous hemangioma. J. Neurosurg. 2005, 102, 102–106. [Google Scholar] [CrossRef]
- Lindquist, C.; Guo, W.Y.; Karlsson, B.; Steiner, L. Radiosurgery for venous angiomas. J. Neurosurg. 1993, 78, 531–536. [Google Scholar] [CrossRef]
- Liscak, R.; Vladyka, V.; Simonova, G.; Vymazal, J.; Novotny, J., Jr. Gamma knife surgery of brain cavernous hemangiomas. J. Neurosurg. 2005, 102, S207–S213. [Google Scholar] [CrossRef]
- Bruneau, M.; Bijlenga, P.; Reverdin, A.; Rilliet, B.; Regli, L.; Villemure, J.G.; Porchet, F.; de Tribolet, N. Early surgery for brainstem cavernomas. Acta Neurochir. 2006, 148, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, J.A.; Reulen, H.J.; Spetzler, R.F.; Zabramski, J.M. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir. 1994, 130, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.A.; Batjer, H.H.; Awad, I.A.; Bendok, B.R. Brainstem cavernous malformations. Neurosurgery 2009, 64, E805–E818. [Google Scholar] [CrossRef]
- Kikuta, K.; Nozaki, K.; Takahashi, J.A.; Miyamoto, S.; Kikuchi, H.; Hashimoto, N. Postoperative evaluation of microsurgical resection for cavernous malformations of the brainstem. J. Neurosurg. 2004, 101, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kyoshima, K.; Kobayashi, S.; Gibo, H.; Kuroyanagi, T. A study of safe entry zones via the floor of the fourth ventricle for brain-stem lesions. Report of three cases. J. Neurosurg. 1993, 78, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, T.; Edner, G.; Kihlstrom, L. Deep and brainstem cavernomas: A consecutive 8-year series. J. Neurosurg. 2003, 99, 31–37. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Chang, S.D.; Gewirtz, R.J.; Lopez, J.R. Microsurgical resection of brainstem, thalamic, and basal ganglia angiographically occult vascular malformations. Neurosurgery 2000, 46, 260–270. [Google Scholar] [CrossRef]
- Strauss, C.; Romstock, J.; Nimsky, C.; Fahlbusch, R. Intraoperative identification of motor areas of the rhomboid fossa using direct stimulation. J. Neurosurg. 1993, 79, 393–399. [Google Scholar] [CrossRef]
- Nagy, G.; Razak, A.; Rowe, J.G.; Hodgson, T.J.; Coley, S.C.; Radatz, M.W.; Patel, U.J.; Kemeny, A.A. Stereotactic radiosurgery for deep-seated cavernous malformations: A move toward more active, early intervention. J. Neurosurg. 2010, 113, 691–699. [Google Scholar] [CrossRef]
- Ramina, K.; Ebner, F.H.; Ernemann, U.; Tatagiba, M. Surgery of Cavernous Hemangioma of the Optic Nerve: Case Report and Review. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2013, 74, 265–270. [Google Scholar]
- UK-TIA STUDY GROUP. United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: Interim results. Br. Med. J. 1988, 296, 316–320. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, Y.G.; Choi, J.U.; Chung, S.S.; Lee, K.C. An analysis of the natural history of cavernous malformations. Surg. Neurol. 1997, 48, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bozinov, O.; Hatano, T.; Sarnthein, J.; Burkhardt, J.K.; Bertalanffy, H. Current clinical management of brainstem cavernomas. Swiss. Med. Wkly. 2010, 140, w13120. [Google Scholar] [CrossRef]
- Gross, B.A.; Batjer, H.H.; Awad, I.A.; Bendok, B.R. Cavernous malformations of the basal ganglia and thalamus. Neurosurgery 2009, 65, 7–18. [Google Scholar] [CrossRef]
- Zyck, S.; Gould, G.C. Cavernous Venous Malformation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Atwal, G.S.; Sarris, C.E.; Spetzler, R.F. Brainstem and cerebellar cavernous malformations. Handb. Clin. Neurol. 2017, 143, 291–295. [Google Scholar] [PubMed]
- Li, Z.; Ma, L.; Quan, K.; Liu, P.; Shi, Y.; Liu, Y.; Zhu, W. Rehemorrhage of brainstem cavernous malformations: A benchmark approach to individualized risk and severity assessment. J. Neurosurg. 2022, 139, 94–105. [Google Scholar] [CrossRef]
- Kong, L.; Ma, X.J.; Xu, X.Y.; Liu, P.P.; Wu, Z.Y.; Zhang, L.W.; Zhang, J.T.; Wu, Z.; Wang, L.; Li, D. Five-year symptomatic hemorrhage risk of untreated brainstem cavernous malformations in a prospective cohort. Neurosurg. Rev. 2022, 45, 2961–2973. [Google Scholar] [CrossRef]
- Geraldo, A.F.; Alves, C.A.P.F.; Luis, A.; Tortora, D.; Guimarães, J.; Abreu, D.; Reimão, S.; Pavanello, M.; de Marco, P.; Scala, M.; et al. Natural history of familial cerebral cavernous malformation syndrome in children: A multicenter cohort study. Neuroradiology 2023, 65, 401–414. [Google Scholar] [CrossRef]
- Menon, G.; Gopalakrishnan, C.V.; Rao, B.R.; Nair, S.; Sudhir, J.; Sharma, M. A single institution series of cavernomas of the brainstem. J. Clin. Neurosci. 2011, 18, 1210–1214. [Google Scholar] [CrossRef]
- Nataf, F.; Roux, F.X.; Devaux, B.; Page, P.; Turak, B.; Dezamis, E.; Abi, L.G. Brainstem cavernomas: Surgical experience at the CH Sainte-Anne general hospital. Neurochirurgie 2007, 53, 192–201. [Google Scholar] [CrossRef]
- Morota, N.; Deletis, V.; Epstein, F.J.; Kofler, M.; Abbott, R.; Lee, M.; Ruskin, K. Brain stem mapping: Neurophysiological localization of motor nuclei on the floor of the fourth ventricle. Neurosurgery 1995, 37, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Morota, N.; Deletis, V. The importance of brainstem mapping in brainstem surgical anatomy before the fourth ventricle and implication for intraoperative neurophysiological mapping. Acta Neurochir. 2006, 148, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Lanteri, P.; Bricolo, A. Motor evoked potential monitoring for spinal cord and brain stem surgery. Adv. Tech. Stand. Neurosurg. 2004, 29, 133–169. [Google Scholar]
- Abla, A.A.; Lekovic, G.P.; Turner, J.; de Oliveira, J.G.; Porter, R.; Spetzler, R.F. Advances in the Treatment and Outcome of Brain Stem Cavernous Malformation Surgery: A Case Series of 300 Surgically Treated Patients. Neurosurgery 2010, 68, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Thompson, B.G.; Spetzler, R.F. The two-pointmethod: Evaluating brain stem lesions. BNI Q. 1996, 12, 20–24. [Google Scholar]
- Steiner, L.; Karlsson, B.; Yen, C.P.; Torner, J.C.; Lindquist, C.; Schlesinger, D. Radiosurgery in cavernous malformations: Anatomy of a controversy. J. Neurosurg. 2010, 113, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Gradišnik, L.; Bošnjak, R.; Bunc, G.; Ravnik, J.; Maver, T.; Velnar, T. Neurosurgical Approaches to Brain Tissue Harvesting for the Establishment of Cell Cultures in Neural Experimental Cell Models. Materials 2021, 14, 6857. [Google Scholar] [CrossRef]
- Anetsberger, S.; Mellal, A.; Garvayo, M.; Diezi, M.; Perez, M.H.; Beck Popovic, M.; Renella, R.; Cossu, G.; Daniel, R.T.; Starnoni, D.; et al. Predictive Factors for the Occurrence of Perioperative Complications in Pediatric Posterior Fossa Tumors. World Neurosurg. 2023, 172, e508–e516. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, T.; Izzo, G.; Alexandre, A.; Botto, A.; Di Lella, G.M.; Gaudino, S.; Caldarelli, M.; Colosimo, C. MRI findings of olivary degeneration after surgery for posterior fossa tumours in children: Incidence, time course and correlation with tumour grading. Radiol. Med. 2015, 120, 474–482. [Google Scholar] [CrossRef]
- Onen, M.R.; Moore, K.; Cikla, U.; Ucer, M.; Schmidt, B.; Field, A.S.; Baskaya, M.K. Hypertrophic Olivary Degeneration: Neurosurgical Perspective and Literature Review. World Neurosurg. 2018, 112, e763–e771. [Google Scholar] [CrossRef]
- Ogut, E.; Armagan, K.; Tufekci, D. The Guillain-Mollaret triangle: A key player in motor coordination and control with implications for neurological disorders. Neurosurg. Rev. 2023, 46, 181. [Google Scholar] [CrossRef]
| Location of Lesion | Approach | Craniotomy | Patient’s Position |
|---|---|---|---|
| Anterior Midbrain | Trans-Sylvian | Pterional | Supine |
| Lateral Midbrain | Supracerebellar infratentorial lateral | Retrosigmoid | Semi-Sitting |
| Medial Midbrain | Supracerebellar infratentorial medial | Medial suboccipital | Semi-Sitting |
| Anterolateral Pons | Cerebellopontine angle | Retrosigmoid | Semi-Sitting |
| Medial Pons | Transventricular (4th ventricle) | Medial suboccipital | Prone |
| Lateral Medulla | Trans-vellum medullare | Medial suboccipital | Prone |
| Medial Medulla | Subtonsilar Transventricular (4th ventricle) | Medial suboccipital | Prone |
| Symptoms (n = 34) | Headaches | Hypaesthesia | Gait Disturbance | Diplopia (CN VI) | Facial Palsy (CN VII) | Hearing Loss (CN VIII) | Difficulty Swallowing CN (IX–X) | |
|---|---|---|---|---|---|---|---|---|
| Cavernoma Location | ||||||||
| Deep-seated BSCs preoperative | 5.8% (n = 2) | 14.7% (n = 5) | 14.7% (n = 5) | 14.7% (n = 5) | 8.8% (n = 3) | 0 | 8.8% (n = 3) | |
| Deep-seated BSCs postoperative | 0 | 11.7% (n = 4) | 8.8% (n = 3) | 11.7% (n = 4) | 11.7% (n = 4) | 0 | 11.7% (n = 4) | |
| Superficial BSCs preoperative | 17.6% (n = 6) | 23.5% (n = 8) | 23.5% (n = 8) | 8.8% (n = 3) | 11.7% (n = 4) | 0 | 8.8% (n = 3) | |
| Superficial BSCs postoperative | 2.9% (n = 1) | 14.7% (n = 5) | 14.7% (n = 5) | 14.7% (n = 5) | 14.7% (n = 5) | 2.9% (n = 1) | 5.8% (n = 2) | |
| New neurological deficit after surgery | ||||||||
| Deep-seated BSCs | 0 | 0 | 2.9% (n = 1) | 2.9% (n = 1) | 2.9% (n = 1) | 2.9% (n = 1) | ||
| Superficial BSCs | 5.8% (n = 2) | 5.8% (n = 2) | 2.9% (n = 1) | 2.9% (n = 1) | ||||
| MRS (n = 34) | MRS (Median) | MRS 0 | MRS 1 | MRS 2 | MRS 3 | MRS 4 | MRS 5 | |
|---|---|---|---|---|---|---|---|---|
| Cavernoma Location | ||||||||
| deep-seated BSCs preoperative | 1 | 0 | 8 (23.5%) | 4 (11.7%) | 0 | 1 (2.9%) | 0 | |
| deep-seated BSCs postoperative | 2 | 3 (8.8%) | 7 (20.5%) | 3 (8.8%) | 0 | 0 | 0 | |
| superficial BSCs preoperative | 2 | 1 (2.9%) | 6 (17.6) | 2 (5.8%) | 2 (5.8%) | 3 (8.8%) | 0 | |
| superficial BSCs postoperative | 3 | 2 (5.8%) | 5 (14.7%) | 3 (8.8%) | 3 (8.8%) | 1(2.9%) | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatagiba, M.; Lepski, G.; Kullmann, M.; Krischek, B.; Danz, S.; Bornemann, A.; Klein, J.; Fahrig, A.; Velnar, T.; Feigl, G.C. The Brainstem Cavernoma Case Series: A Formula for Surgery and Surgical Technique. Medicina 2023, 59, 1601. https://doi.org/10.3390/medicina59091601
Tatagiba M, Lepski G, Kullmann M, Krischek B, Danz S, Bornemann A, Klein J, Fahrig A, Velnar T, Feigl GC. The Brainstem Cavernoma Case Series: A Formula for Surgery and Surgical Technique. Medicina. 2023; 59(9):1601. https://doi.org/10.3390/medicina59091601
Chicago/Turabian StyleTatagiba, Marcos, Guilherme Lepski, Marcel Kullmann, Boris Krischek, Soeren Danz, Antje Bornemann, Jan Klein, Antje Fahrig, Tomaz Velnar, and Guenther C. Feigl. 2023. "The Brainstem Cavernoma Case Series: A Formula for Surgery and Surgical Technique" Medicina 59, no. 9: 1601. https://doi.org/10.3390/medicina59091601
APA StyleTatagiba, M., Lepski, G., Kullmann, M., Krischek, B., Danz, S., Bornemann, A., Klein, J., Fahrig, A., Velnar, T., & Feigl, G. C. (2023). The Brainstem Cavernoma Case Series: A Formula for Surgery and Surgical Technique. Medicina, 59(9), 1601. https://doi.org/10.3390/medicina59091601








