Treatment of Asymptomatic Bacteriuria after Kidney Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Types of Studies, Participants, and Interventions
2.2. Types of Outcome Measures
2.2.1. Primary Outcomes
2.2.2. Secondary Outcomes
2.3. Search Strategy
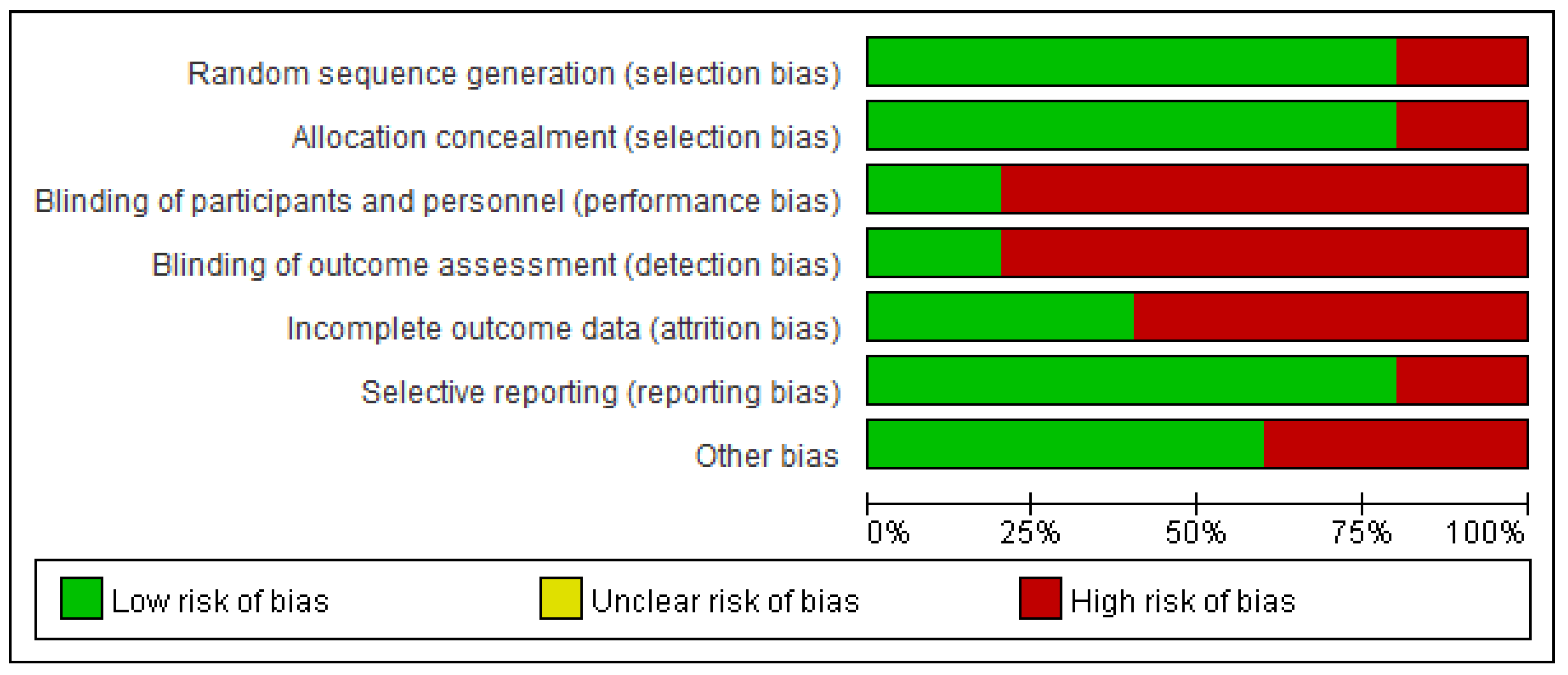
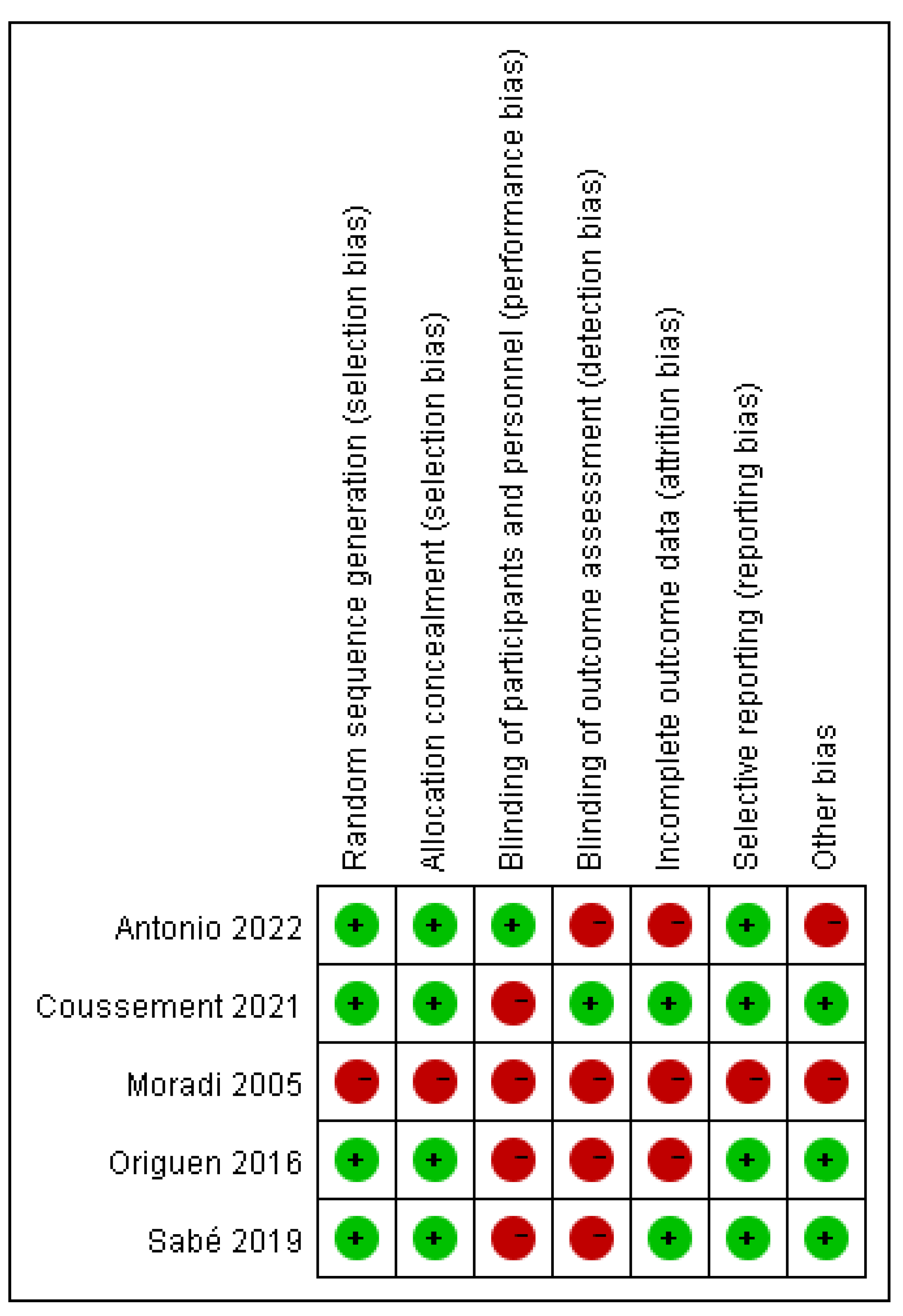
2.4. Assessment of Risk of Bias in Included Studies
2.5. Data Collection and Analysis
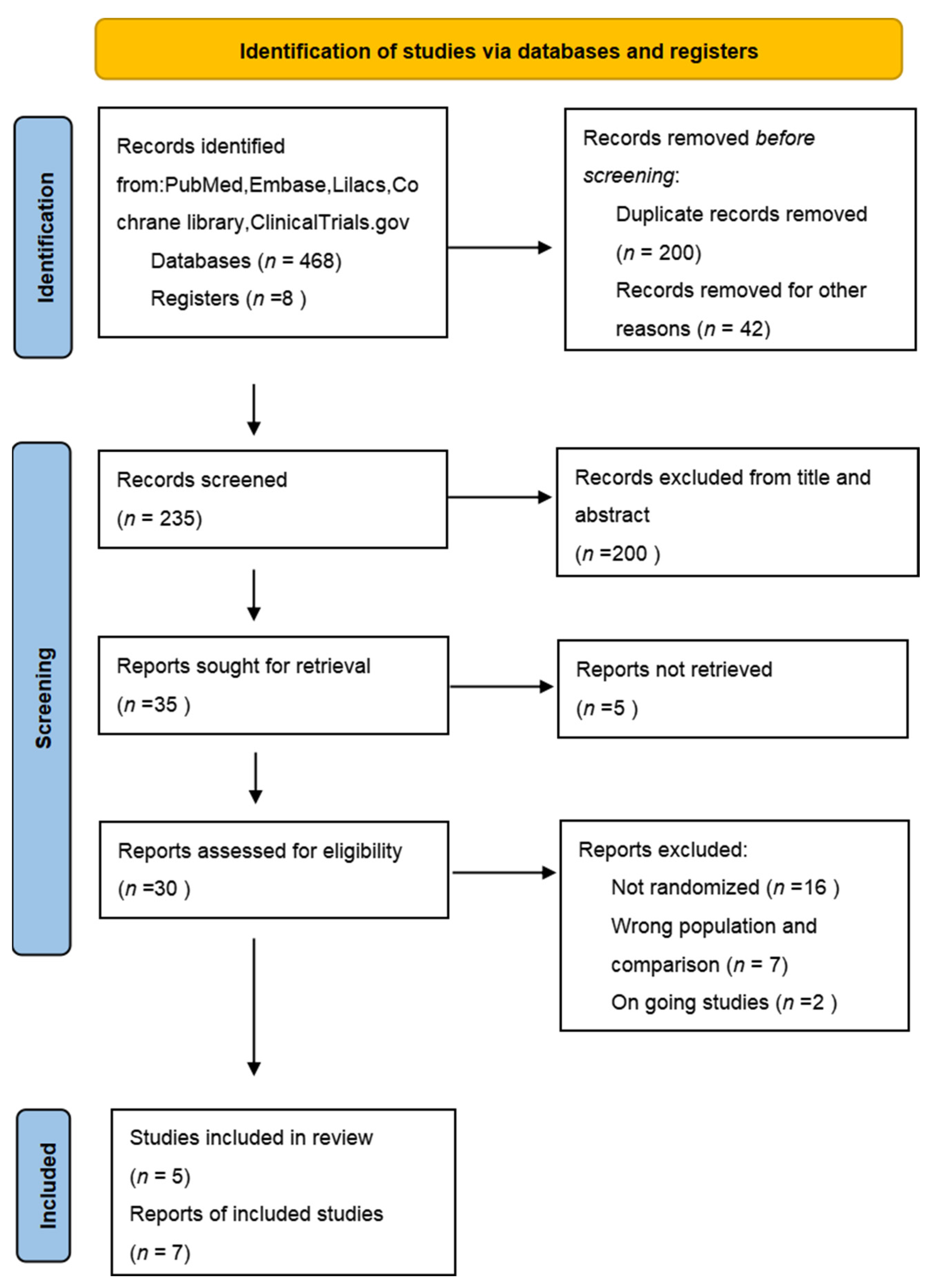
3. Results
3.1. Description of Studies
3.2. Primary Outcomes
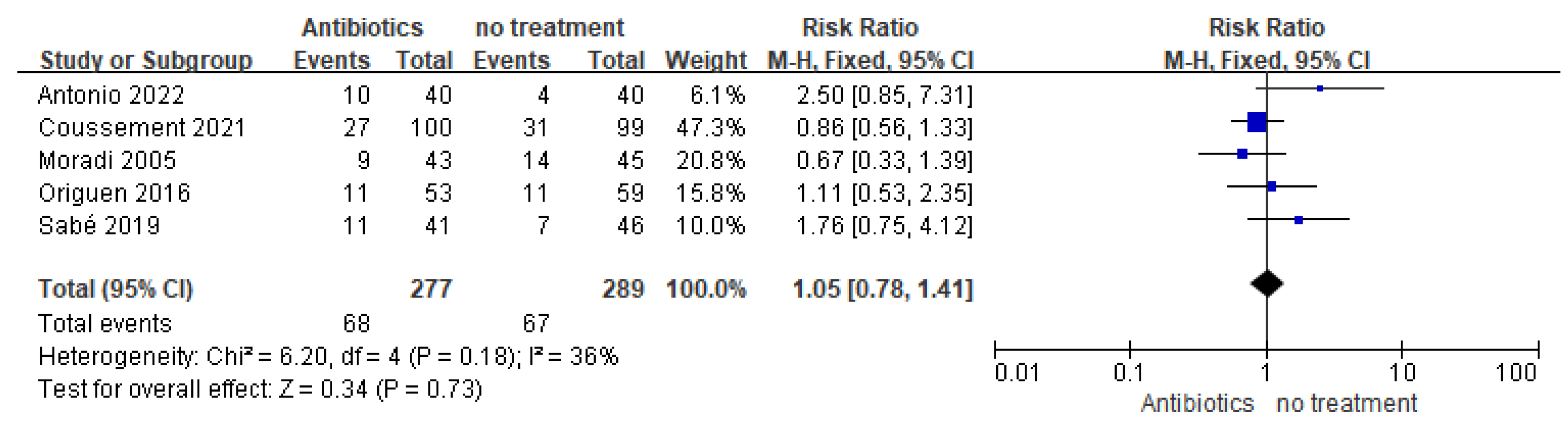
3.2.1. Symptomatic UTI
3.2.2. Antimicrobial resistance
3.3. Secondary Outcomes
3.3.1. Graft Function
3.3.2. Hospitalization due to UTI
3.3.3. Persistence or Relapse of Asymptomatic Bacteriuria
3.3.4. Other Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vidal, E.; Torre-Cisneros, J.; Blanes, M.; Montejo, M.; Cervera, C.; Aguado, J.M.; Len, O.; Carratalá, J.; Cordero, E.; Bou, G.; et al. Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transpl. Infect. Dis. 2012, 14, 595–603. [Google Scholar] [CrossRef]
- Säemann, M.; Hörl, W.H. Urinary tract infection in renal transplant recipients. Eur. J. Clin. Investig. 2008, 38, S58–S65. [Google Scholar] [CrossRef]
- Valera, B.; Gentil, M.A.; Cabello, V.; Fijo, J.; Cordero, E.; Cisneros, J.M. Epidemiology of urinary infections in renal transplant recipients. Transplant. Proc. 2006, 38, 2414–2415. [Google Scholar] [CrossRef] [PubMed]
- Fiorante, S.; Fernández-Ruiz, M.; López-Medrano, F.; Lizasoain, M.; Lalueza, A.; Morales, J.M.; San-Juan, R.; Andrés, A.; Otero, J.R.; Aguado, J.M. Acute graft pyelonephritis in renal transplant recipients: Incidence, risk factors and long-term outcome. Nephrol. Dial. Transplant. 2011, 26, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Linares, L.; García-Goez, J.F.; Cervera, C.; Almela, M.; Sanclemente, G.; Cofán, F.; Ricart, M.J.; Navasa, M.; Moreno, A. Early bacteremia after solid organ transplantation. Transplant. Proc. 2009, 41, 2262–2264. [Google Scholar] [CrossRef] [PubMed]
- Silva, M., Jr.; Marra, A.R.; Pereira, C.A.; Medina-Pestana, J.O.; Camargo, L.F. Bloodstream infection after kidney transplantation: Epidemiology, microbiology, associated risk factors, and outcome. Transplantation 2010, 90, 581–587. [Google Scholar] [CrossRef]
- Chuang, P.; Parikh, C.R.; Langone, A. Urinary tract infections after renal transplantation: A retrospective review at two US transplant centers. Clin. Transplant. 2005, 19, 230. [Google Scholar] [CrossRef]
- Di Cocco, P.; Orlando, G.; Mazzotta, C.; Rizza, V.; D’Angelo, M.; Clemente, K.; Greco, S.; Famulari, A.; Pisani, F. Incidence of urinary tract infections caused by germs resistant to antibiotics commonly used after renal transplantation. Transplant. Proc. 2008, 40, 1881–1884. [Google Scholar] [CrossRef]
- Fiorante, S.; Lopez-Medrano, F.; Lizasoain, M.; Lalueza, A.; Juan, R.S.; Andrés, A.; Otero, J.R.; Morales, J.M.; Aguado, J.M. Systematic screening and treatment of asymptomatic bacteriuria in renal transplant recipients. Kidney Int. 2010, 78, 774–781. [Google Scholar] [CrossRef]
- Maraha, B.; Bonten, H.; van Hooff, H.; Fiolet, H.; Buiting, A.G.; Stobberingh, E.E. Infectious complications and antibiotic use in renal transplant recipients during a 1-year follow-up. Clin. Microbiol. Infect. 2001, 7, 619–625. [Google Scholar] [CrossRef]
- Veroux, M.; Giuffrida, G.; Corona, D.; Gagliano, M.; Scriffignano, V.; Vizcarra, D.; Tallarita, T.; Zerbo, D.; Virgilio, C.; Sciacca, A.; et al. Infective complications in renal allograft recipients: Epidemiology and outcome. Transplant. Proc. 2008, 40, 1873–1876. [Google Scholar] [CrossRef] [PubMed]
- Coussement, J.; Maggiore, U.; Manuel, O.; Scemla, A.; López-Medrano, F.; Nagler, E.V.; Aguado, J.M.; Abramowicz, D. Diagnosis and management of asymptomatic bacteriuria in kidney transplant recipients: A survey of current practice in Europe. Nephrol. Dial. Transplant. 2018, 33, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayyat, H.; Toussaint, N.; Holt, S.; Hughes, P. Gram-negative sepsis following biopsy of a transplant recipient with asymptomatic allograft pyelonephritis. CEN Case Rep. 2017, 6, 46–49. [Google Scholar] [CrossRef]
- Stewardson, A.J.; Gaïa, N.; François, P.; Malhotra-Kumar, S.; Delémont, C.; de Tejada, B.M.; Schrenzel, J.; Harbarth, S.; Lazarevic, V.; SATURN WP1 Study Group; et al. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: A culture-free analysis of gut microbiota. Clin. Microbiol. Infect. 2015, 21, 344.e1–344.e11. [Google Scholar] [CrossRef] [PubMed]
- Cervera, C.; Van Delden, C.; Gavaldà, J.; Welte, T.; Akova, M.; Carratalà, J.; ESCMID Study Group for Infections in Compromised Hosts (ESGICH). Multidrug-resistant bacteria in solid organ transplant recipients. Clin. Microbiol. Infect. 2014, 20, 49–73. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, e83–e110. [Google Scholar] [CrossRef]
- Goldman, J.D.; Julian, K. Urinary tract infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13507. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; [Updated February 2021]. 2021. Available online: www.training.cochrane.org/handbook (accessed on 30 April 2023).
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration. 2011. Available online: www.cochrane-handbook.org (accessed on 30 April 2023).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Moradi, M.; Abbasi, M.; Moradi, A.; Boskabadi, A.; Jalali, A. Effect of antibiotic therapy on asymptomatic bacteriuria in kidney transplant recipients. Urol. J. 2005, 2, 32–35. [Google Scholar]
- Origüen, J.; López-Medrano, F.; Fernández-Ruiz, M.; Polanco, N.; Gutiérrez, E.; González, E.; Mérida, E.; Ruiz-Merlo, T.; Morales-Cartagena, A.; Asín, M.P.J.; et al. Should asymptomatic bacteriuria be systematically treated in kidney transplant recipients? Results from a randomized controlled trial. Am. J. Transplant. 2016, 16, 2943–2953. [Google Scholar] [CrossRef]
- Sabé, N.; Oriol, I.; Melilli, E.; Manonelles, A.; Bestard, O.; Polo, C.; Los Arcos, I.; Perelló, M.; Garcia, D.; Riera, L.; et al. Antibiotic treatment versus no treatment for asymptomatic bacteriuria in kidney transplant recipients: A multicenter randomized trial. open forum. Infect. Dis. 2019, 6, ofz243. [Google Scholar] [CrossRef]
- Coussement, J.; Kamar, N.; Matignon, M.; Weekers, L.; Scemla, A.; Giral, M.; Racapé, J.; Alamartine, É.; Mesnard, L.; Kianda, M.; et al. Antibiotics versus no therapy in kidney transplant recipients with asymptomatic bacteriuria (BiRT): A pragmatic, multicentre, randomized, controlled trial. Clin. Microbiol. Infect. 2021, 27, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.E.E.; Cassandra, B.G.C.; Emiliano, R.J.D.; Guadalupe, O.L.M.; Lilian, R.E.A.; Teresa, T.G.M.; Mario, G.G.; Ivan, R.C.G.; Mercedes, R.V.; Alfredo, C.W.; et al. Treatment of asymptomatic bacteriuria in the first 2 months after kidney transplant: A controlled clinical trial. Transpl. Infect. Dis. 2022, 24, e13934. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.S. Antibiotic Treatment versus No Therapy in Kidney Transplant Recipients with Asymptomatic Bacteriuria. Available online: www.clinicaltrials.gov/ct2/show/NCT01771432 (accessed on 27 April 2023).
- Rahamimov, R. The Impact of Antimicrobial Treatment for Asymptomatic Bacteriuria in Renal Transplant Patients. Available online: www.clinicaltrials.gov/ct2/show/NCT02113774 (accessed on 27 April 2023).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- European Association of Urology EAU. Guidelines on Urological Infections. Limited Update March 2018. Available online: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urological-Infections-2018-large-text.pdf (accessed on 20 April 2023).
- Smaill, F.M.; Vazquez, J.C. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst. Rev. 2019, 11, CD000490. [Google Scholar] [CrossRef] [PubMed]
- Coussement, J.; Scemla, A.; Abramowicz, D.; Nagler, E.V.; Webster, A.C. Antibiotics for asymptomatic bacteriuria in kidney transplant recipients. Cochrane Database Syst. Rev. 2018, 2, CD011357. [Google Scholar] [CrossRef]
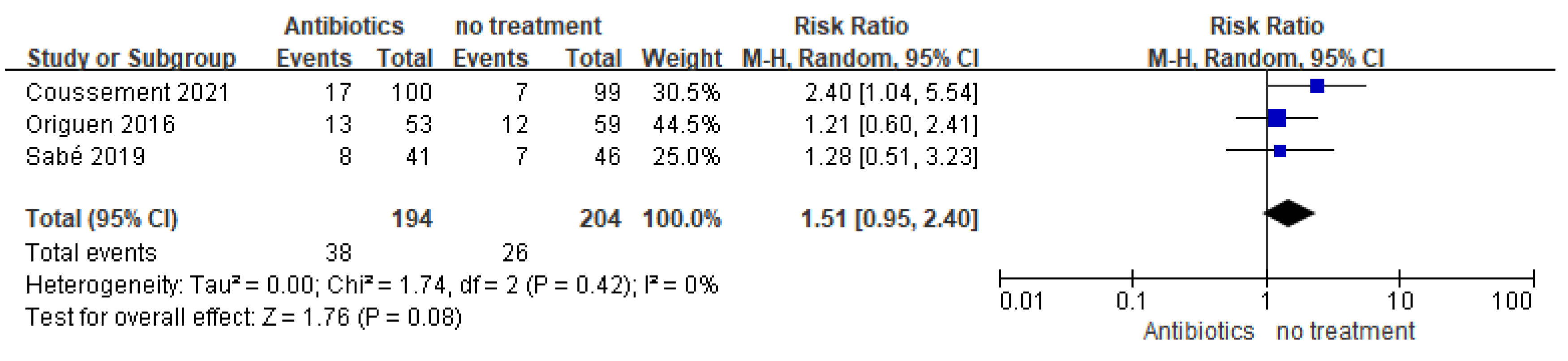
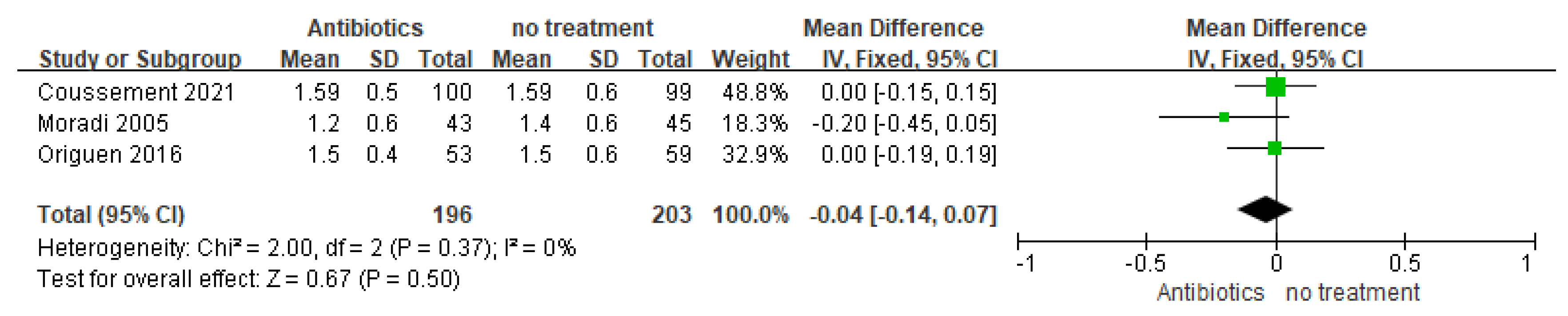

| Study | Study Type | Location | Course of Antibiotics in Treatment Group | Number of Patients (Antibiotics vs. No Treatment) | Enrollment Time (Post-Transplantation) | Follow-Up Duration | Outcome: Symptomatic Urinary Tract Infection (Antibiotics vs. no Treatment) |
|---|---|---|---|---|---|---|---|
| Moradi 2005 [22] | quasi-RCT | Iran | 10 days | 88 (43 vs. 45) | >1 year | 12 months | 20.9% vs. 30.4% |
| Origuen 2016 [23] | RCT | Spain | 3–7 days | 112 (53 vs. 59) | >2 months | 24 months | 20.8% vs. 18.6% |
| Sabé 2019 [24] | RCT | Spain | 5–7 days | 87 (41 vs. 46) | Post-operation after urinary catheter removal | 12 months | 26.8% vs. 15.2% |
| Coussement 2021 [25] | RCT | France and Belgium | 10 days | 199 (100 vs. 99) | >2 months | 12 months | 27% vs. 31.3% |
| Antonio 2022 [26] | RCT | México | 5 days | 80 (40 vs. 40) | Post-operation after urinary catheter removal | 63 days | 25% vs. 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, Z.; Wang, Z.; Tang, M.; Shen, L.; Zhang, K. Treatment of Asymptomatic Bacteriuria after Kidney Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2023, 59, 1600. https://doi.org/10.3390/medicina59091600
Rao Z, Wang Z, Tang M, Shen L, Zhang K. Treatment of Asymptomatic Bacteriuria after Kidney Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina. 2023; 59(9):1600. https://doi.org/10.3390/medicina59091600
Chicago/Turabian StyleRao, Zhengsheng, Zhiling Wang, Ming Tang, Linguo Shen, and Keqin Zhang. 2023. "Treatment of Asymptomatic Bacteriuria after Kidney Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Medicina 59, no. 9: 1600. https://doi.org/10.3390/medicina59091600
APA StyleRao, Z., Wang, Z., Tang, M., Shen, L., & Zhang, K. (2023). Treatment of Asymptomatic Bacteriuria after Kidney Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina, 59(9), 1600. https://doi.org/10.3390/medicina59091600






