Hyposmia in COVID-19: Temporal Recovery of Smell: A Preliminary Study
Abstract
:1. Introduction
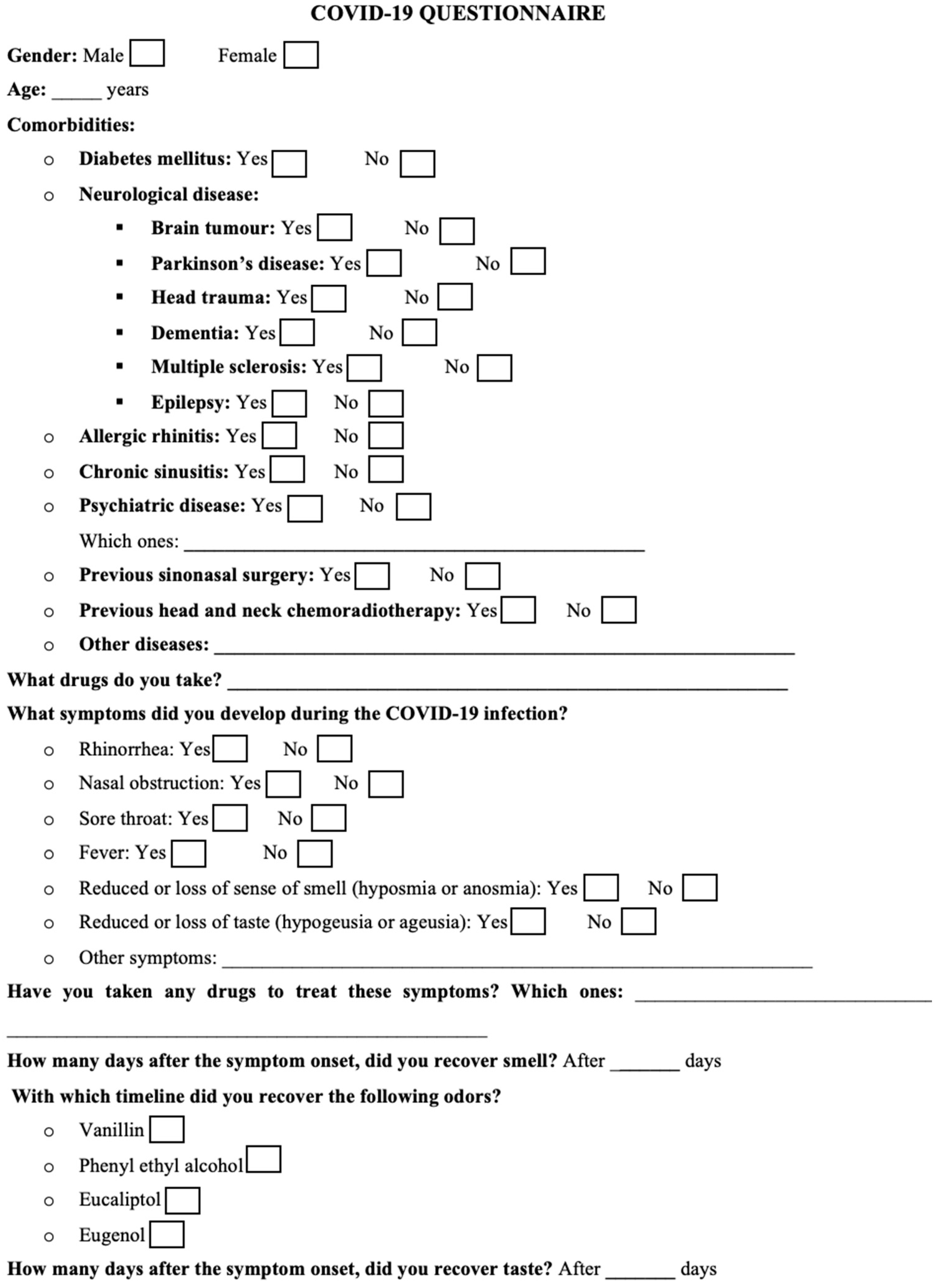
2. Materials and Methods
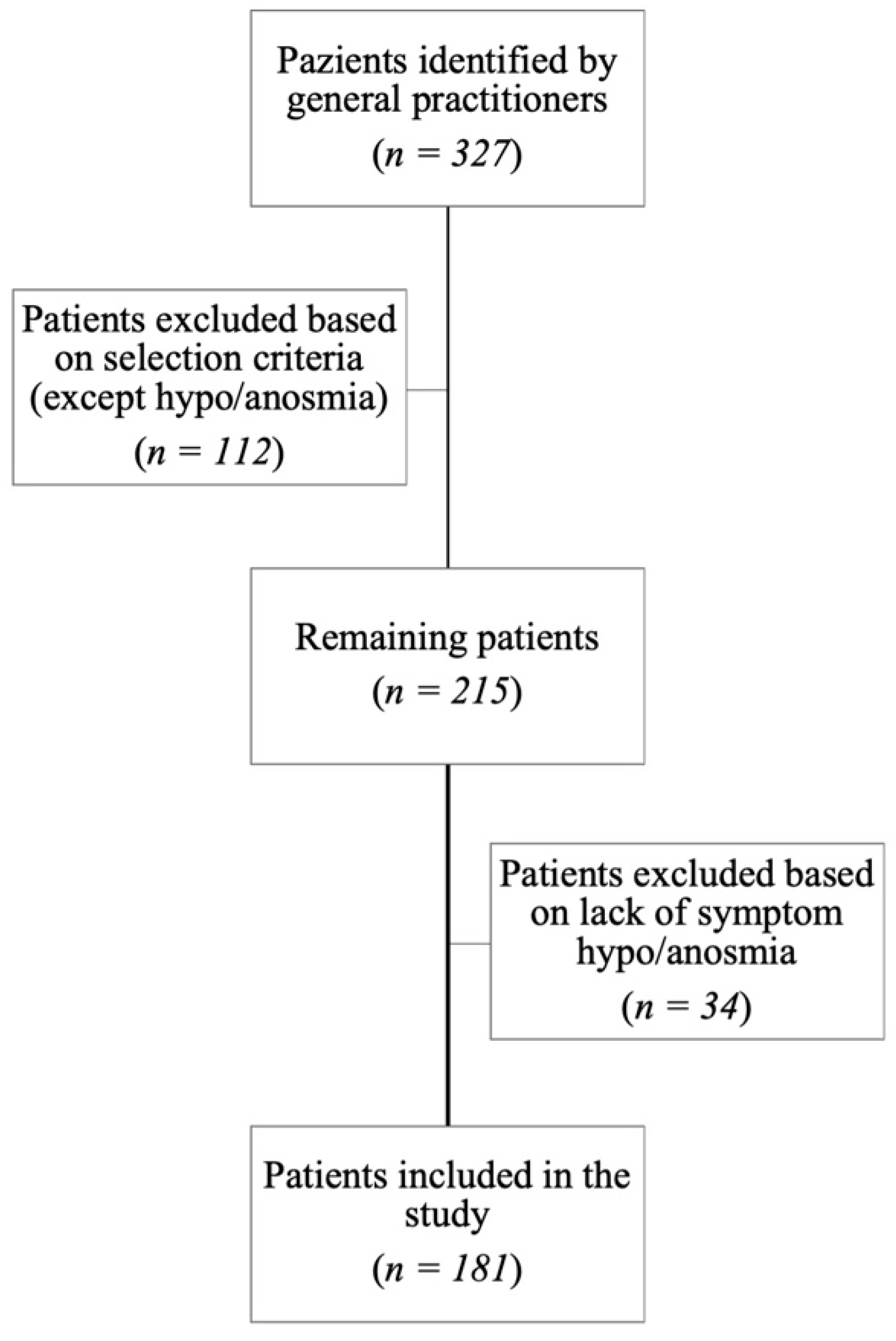
2.1. Patients’ Selection
2.2. Study Protocol
2.3. Statistical Analysis
3. Results
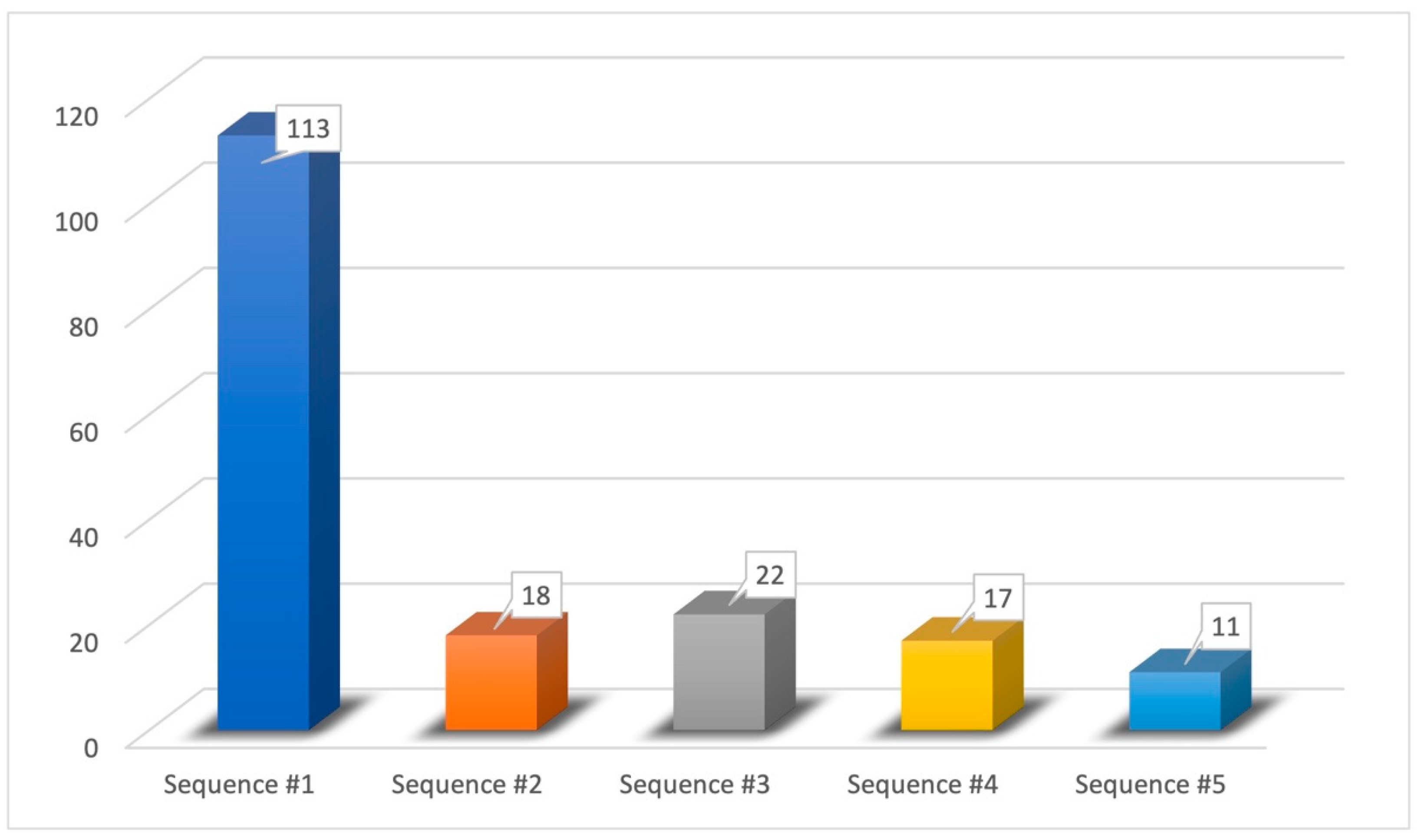
- Sequence #1 (62.43%): phenyl ethyl alcohol, eucalyptol, vanillin, eugenol;
- Sequence #2 (9.94%): phenyl ethyl alcohol, vanillin, eucalyptol, eugenol;
- Sequence #3 (12.15%): eucalyptol, phenyl ethyl alcohol, vanillin, eugenol;
- Sequence #4 (9.40%): vanillin, phenyl ethyl alcohol, eucalyptol, eugenol;
- Sequence #5 (6.08%): vanillin, eucalyptol, phenyl ethyl alcohol, eugenol.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 11 January 2021. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-january-2021 (accessed on 13 February 2021).
- Coelho, D.H.; Reiter, E.R.; Budd, S.G.; Shin, Y.; Kons, Z.A.; Costanzo, R.M. Quality of life and safety impact of COVID-19 associated smell and taste disturbances. Am. J. Otolaryngol. 2021, 42, 103001. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 13 February 2021).
- Meng, X.; Deng, Y.; Dai, Z.; Meng, Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am. J. Otolaryngol. 2020, 41, 102581. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Gane, S.B.; Kelly, C.; Hopkins, C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology 2020, 58, 299–301. [Google Scholar] [CrossRef]
- Marchese-Ragona, R.; Ottaviano, G.; Nicolai, P.; Vianello, A.; Carecchio, M. Sudden hyposmia as a prevalent symptom of COVID-19 infection. MedRxiv 2020. [Google Scholar] [CrossRef]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Symptoms of Coronavirus. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 13 February 2021).
- Speth, M.M.; Singer-Cornelius, T.; Oberle, M.; Gengler, I.; Brockmeier, S.J.; Sedaghat, A.R. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol. Head Neck Surg. 2020, 163, 114–120. [Google Scholar] [CrossRef]
- Brandão Neto, D.; Fornazieri, M.A.; Dib, C.; Di Francesco, R.C.; Doty, R.L.; Voegels, R.L.; Pinna, F.R. Chemosensory Dysfunction in COVID-19: Prevalences, Recovery Rates, and Clinical Associations on a Large Brazilian Sample. Otolaryngol. Head Neck Surg. 2021, 164, 512–518. [Google Scholar] [CrossRef]
- Kaye, R.; Chang, C.W.D.; Kazahaya, K.; Brereton, J.; Denneny, J.C., 3rd. COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngol. Head Neck Surg. 2020, 163, 132–134. [Google Scholar] [CrossRef]
- Wu, D.; Wang, V.Y.; Chen, Y.H.; Ku, C.H.; Wang, P.C. The prevalence of olfactory and gustatory dysfunction in COVID-19—A systematic review. Auris Nasus Larynx 2022, 49, 165–175. [Google Scholar] [CrossRef]
- Sayin, İ.; Yaşar, K.K.; Yazici, Z.M. Taste and Smell Impairment in COVID-19: An AAO-HNS Anosmia Reporting Tool-Based Comparative Study. Otolaryngol. Head Neck Surg. 2020, 163, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zen, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, T.; Kadiane-Oussou, N.J.; Toko, L.; Royer, P.Y.; Lepiller, Q.; Gendrin, V.; Zayet, S. Features of anosmia in COVID-19. Med. Mal. Infect. 2020, 50, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, J.; Charbonneau, G.; Collignon, O.; Lepore, F. Odor Localization and Sniffing. Chem. Senses 2009, 34, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, J.; La Buissonnière Ariza, V.; Collignon, O.; Lepore, F. Localisation of unilateral nasal stimuli across sensory systems. Neurosci. Lett. 2010, 478, 102–106. [Google Scholar] [CrossRef]
- Rocke, J.; Hopkins, C.; Philpott, C.; Kumar, N. Is loss of sense of smell a diagnostic marker in COVID-19: A systematic review and meta-analysis. Clin. Otolaryngol. 2020, 45, 914–922. [Google Scholar] [CrossRef]
- Agyeman, A.A.; Chin, K.L.; Landersdorfer, C.B.; Liew, D.; Ofori-Asenso, R. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2020, 95, 1621–1631. [Google Scholar] [CrossRef]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G.; et al. Self-reported Olfactory and Taste Disorders in Patients with Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin. Infect. Dis. 2020, 71, 889–890. [Google Scholar] [CrossRef]
- Vaira, L.A.; Salzano, G.; Petrocelli, M.; Deiana, G.; Salzano, F.A.; De Riu, G. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck 2020, 42, 1570–1576. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B.; Momen-Heravi, M. An Insight into the Sex Differences in COVID-19 Patients: What are the Possible Causes? Prehosp. Disaster Med. 2020, 35, 438–441. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Place, S.; Van Laethem, Y.; Cabaraux, P.; Mat, Q.; Huet, K.; Plzak, J.; Horoi, M.; Hans, S.; et al. COVID-19 Task Force of YO-IFOS. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J. Intern. Med. 2020, 288, 335–344. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, J.; Li, N.; Nisenbaum, E.; Sun, Q.; Chen, B.; Casiano, R.; Weed, D.; Telischi, F.; Denneny, J.C., III; et al. Otolaryngology Providers Must Be Alert for Patients with Mild and Asymptomatic COVID-19. Otolaryngol. Head Neck Surg. 2020, 162, 809–810. [Google Scholar] [CrossRef]
- Najafloo, R.; Majidi, J.; Asghari, A.; Aleemardani, M.; Kamrava, S.K.; Simorgh, S.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Mechanism of Anosmia Caused by Symptoms of COVID-19 and Emerging Treatments. ACS Chem. Neurosci. 2021, 12, 3795–3805. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Brechbühl, J.; Lopes, A.C.; Wood, D.; Bouteiller, S.; de Vallière, A.; Verdumo, C.; Broillet, M.C. Age-dependent appearance of SARS-CoV-2 entry sites in mouse chemosensory systems reflects COVID-19 anosmia-ageusia symptoms. Commun. Biol. 2021, 4, 880. [Google Scholar] [CrossRef]
- Ohla, K.; Veldhuizen, M.G.; Green, T.; Hannum, M.E.; Bakke, A.J.; Moein, S.T.; Tognetti, A.; Postma, E.M.; Pellegrino, R.; Hwang, D.L.D.; et al. A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology 2022, 60, 207–217. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Müller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef]
- Lovato, A.; de Filippis, C.; Marioni, G. Upper airway symptoms in coronavirus disease 2019 (COVID-19). Am. J. Otolaryngol. 2020, 41, 102474. [Google Scholar] [CrossRef] [PubMed]
- Luers, J.C.; Rokohl, A.C.; Loreck, N.; Wawer Matos, P.A.; Augustin, M.; Dewald, F.; Klein, F.; Lehmann, C.; Heindl, L.M. Olfactory and Gustatory Dysfunction in Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2262–2264. [Google Scholar] [CrossRef]
- Gupta, K.; Mohanty, S.K.; Mittal, A.; Kalra, S.; Kumar, S.; Mishra, T.; Ahuja, J.; Sengupta, D.; Ahuja, G. The Cellular basis of the loss of smell in 2019-nCoV-infected individuals. Brief Bioinform. 2021, 22, 873–881. [Google Scholar] [CrossRef]
- Park, J.W.; Wang, X.; Xu, R.H. Revealing the mystery of persistent smell loss in Long COVID patients. Int. J. Biol. Sci. 2022, 18, 4795–4808. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, A.; Bonato, M.; Cinque, P. Smell and taste disorders in COVID-19: From pathogenesis to clinical features and outcomes. Neurosci. Lett. 2021, 748, 135694. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.-J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef] [PubMed]
- Karamali, K.; Elliott, M.; Hopkins, C. COVID-19 related olfactory dysfunction. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 19–25. [Google Scholar] [CrossRef]
- Bryche, B.; St Albin, A.; Murri, S.; Lacôte, S.; Pulido, C.; Ar Gouilh, M.; Lesselier, S.; Servat, A.; Wasniewski, M.; Picard Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020, 89, 579–586. [Google Scholar] [CrossRef]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef]
- Tremblay, C.; Frasnelli, J. Olfactory and Trigeminal Systems Interact in the Periphery. Chem. Senses 2018, 43, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Surda, P.; Whitehead, E.; Kumar, B.N. Early recovery following new onset anosmia during the COVID-19 pandemic—An observational cohort study. J. Otolaryngol. Head Neck Surg. 2020, 49, 26. [Google Scholar] [CrossRef]
- Xu, W.; Sunavala-Dossabhoy, G.; Spielman, A.I. Chemosensory loss in COVID-19. Oral Dis. 2022, 28 (Suppl. 2), 2337–2346. [Google Scholar] [CrossRef]
- Vinayachandran, D.; Balasubramanian, S. Is gustatory impairment the first report of an oral manifestation in COVID-19? Oral Dis. 2021, 27 (Suppl 3), 748–749. [Google Scholar] [CrossRef]
- Obiefuna, S.; Donohoe, C. Neuroanatomy, Nucleus Gustatory. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: https://www.ncbi.nlm.nih.gov/books/NBK554522/ (accessed on 20 March 2021).
- American Academy of Otolaryngology-Head and Neck Surgery (AAO-NHS). Anosmia Reporting Tool. Published March 2020. Available online: https://www.entnet.org/content/reporting-tool-patients-anosmia-related-covid-19 (accessed on 20 April 2020).
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Ostrander, B.T.; DeConde, A.S. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Chary, E.; Carsuzaa, F.; Trijolet, J.P.; Capitaine, A.L.; Roncato-Saberan, M.; Fouet, K.; Cazenave-Roblot, F.; Catroux, M.; Allix-Beguec, C.; Dufour, X. Prevalence and Recovery from Olfactory and Gustatory Dysfunctions in COVID-19 Infection: A Prospective Multicenter Study. Am. J. Rhinol. Allergy 2020, 34, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Suardi, L.R.; Busoni, M.; Roberts, A.T.; Fortini, A. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: Sex differences and recovery time in real-life. Eur. Arch. Otorhinolaryngol. 2020, 277, 3519–3523. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Abdel-Aziz, N.M.; Abdel-Aziz, D.M.; Azab, N. Subjective Smell Assessment as An Office-based Rapid Procedure In COVID-19 Era. J. Craniofac. Surg. 2021, 32, e439–e441. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Domínguez, A.; Rojas-Lechuga, M.J.; Chiesa-Estomba, C.; Calvo-Henríquez, C.; Ninchritz-Becerra, E.; Soriano-Reixach, M.; Poletti-Serafini, D.; Villarreal, I.M.; Maza-Solano, J.M.; Moreno-Luna, R.; et al. Smell and Taste Dysfunction in COVID-19 Is Associated with Younger Age in Ambulatory Settings: A Multicenter Cross-Sectional Study. J. Investig. Allergol. Clin. Immunol. 2020, 30, 346–357. [Google Scholar] [CrossRef]
- Husain, Q.; Kokinakos, K.; Kuo, Y.H.; Zaidi, F.; Houston, S.; Shargorodsky, J. Characteristics of COVID-19 smell and taste dysfunction in hospitalized patients. Am. J. Otolaryngol. 2021, 42, 103068. [Google Scholar] [CrossRef]
| Types of Olfactory Substances | |
|---|---|
| Pure olfactory substances | Phenyl ethyl alcohol |
| Mixed olfactory/trigeminal substances | Eucalyptol |
| Mixed olfactory/gustatory substances | Vanillin |
| Mixed olfactory/trigeminal/gustatory substances | Eugenol |
| Parameters | N° Patients (%) |
|---|---|
| Sex | |
| Female | 108 (59.67) |
| Male | 73 (40.33) |
| Age | |
| Mean age | 32.34 (±11.09) |
| Minimum | 18 |
| Maximum | 66 |
| COVID-19 ENT symptoms | |
| Rhinorrhea | 112 (61.88) |
| Nasal obstruction | 79 (43.64) |
| Sore throat | 45 (26.86) |
| Fever | 119 (65.74) |
| Hypo/ageusia | 170 (93.92) |
| Drugs taken for COVID-19 infection | |
| None | 171 (94.47) |
| Nasal decongestants | 10 (5.53) |
| Oral corticosteroids | 0 |
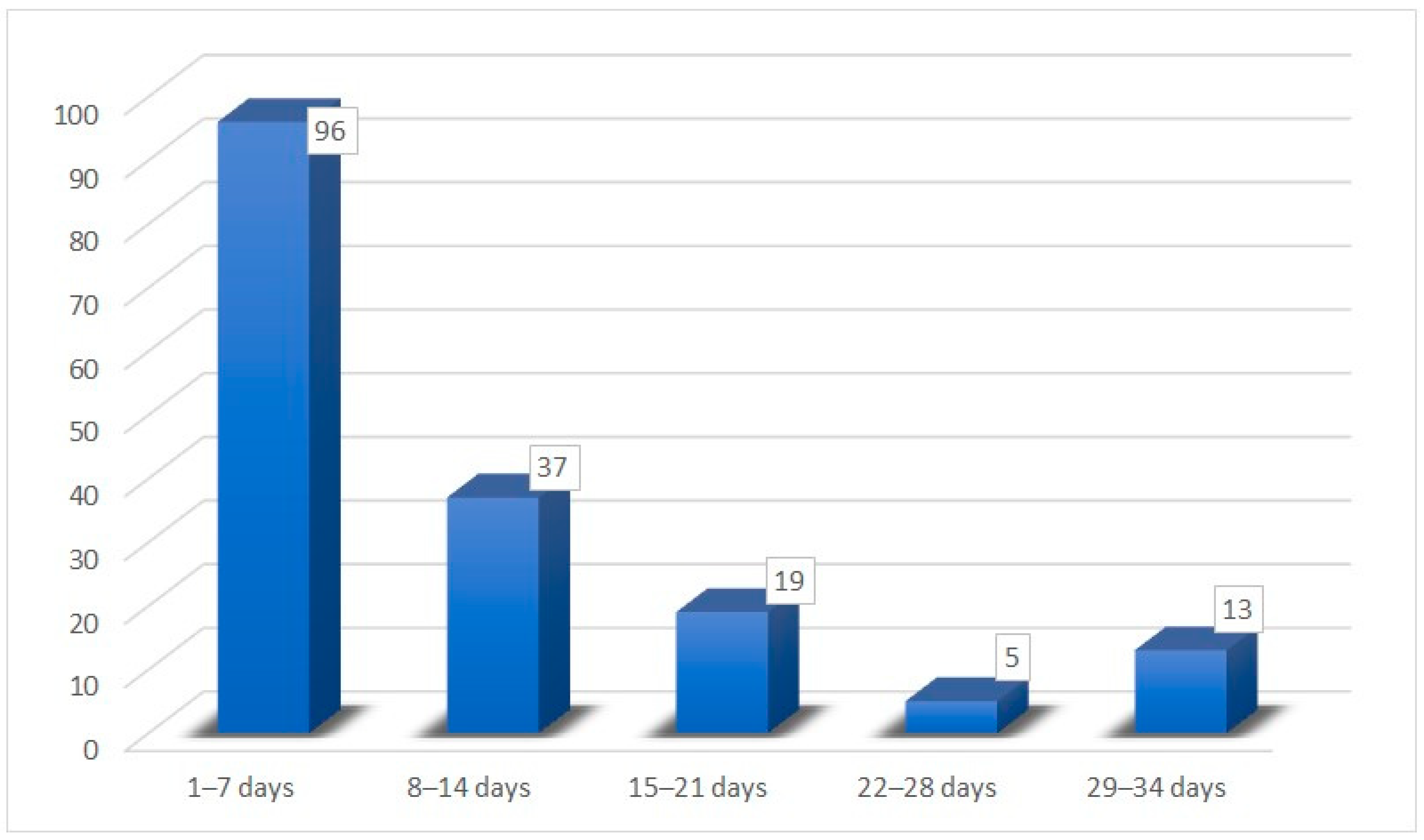
| Recovery time for hypo/anosmia | |
| Mean time | 12.8 (±8.80) days |
| Minimum time | 3 days |
| Maximum time | 30 days |
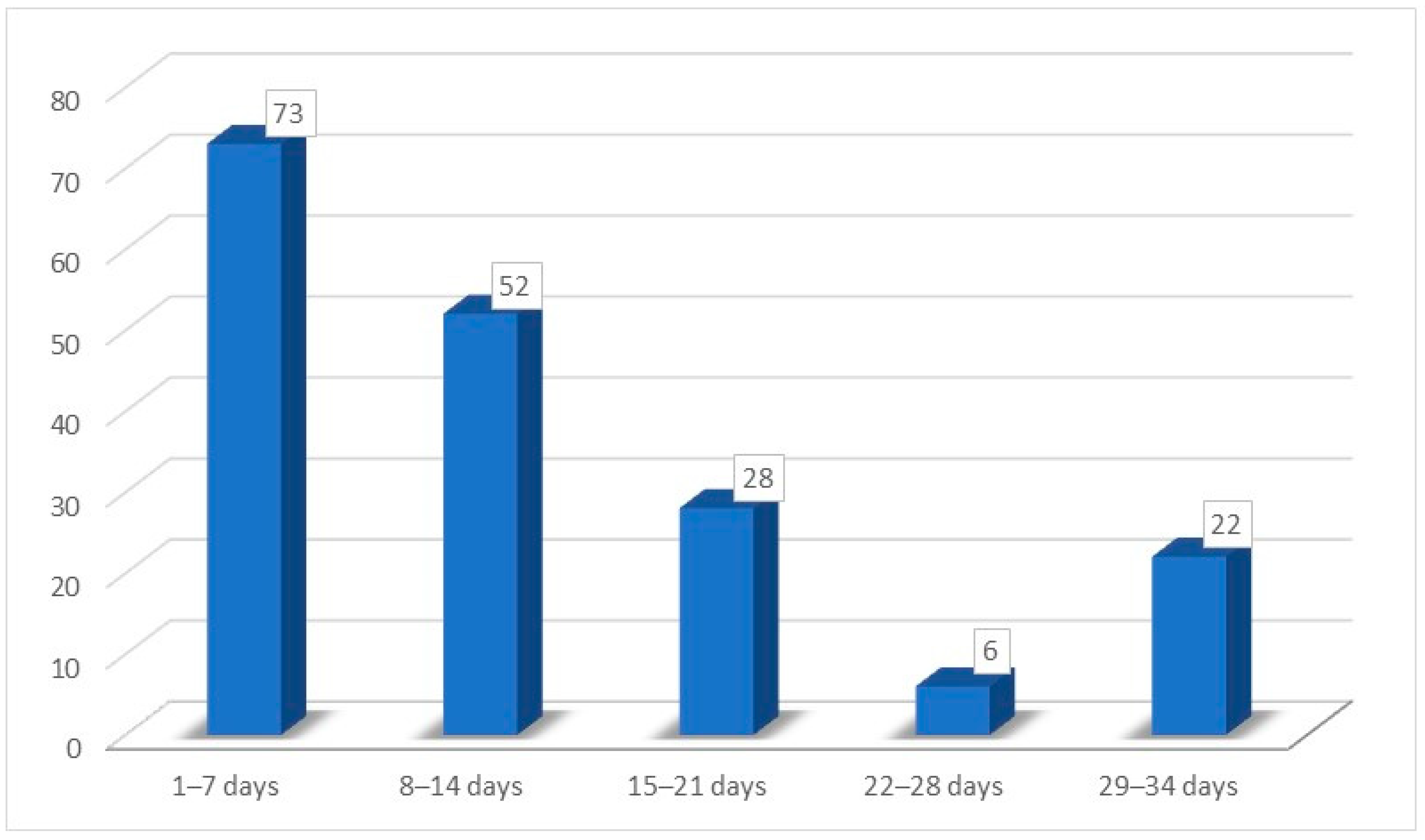
| Recovery time for hypo/ageusia | |
| Mean time | 10.25 (±8.26) days |
| Minimum time | 2 days |
| Maximum time | 30 days |
| Total | 181 (100) |
| Combination | Sequence of Smells Perception Recovery | N° Patients (%) |
|---|---|---|
| 1 | Phenyl ethyl alcohol → eucalyptol → vanillin → eugenol | 113 (62.43) |
| 2 | Phenyl ethyl alcohol → vanillin → eucalyptol → eugenol | 18 (9.94) |
| 3 | Eucalyptol → phenyl ethyl alcohol → vanillin → eugenol | 22 (12.15) |
| 4 | Vanillin → phenyl ethyl alcohol → eucalyptol → eugenol | 17 (9.40) |
| 5 | Vanillin → eucalyptol → phenyl ethyl alcohol → eugenol | 11 (6.08) |
| 6 | Phenyl ethyl alcohol → eucalyptol → eugenol s → vanillin | 0 |
| 7 | Phenyl ethyl alcohol → vanillin → eugenol → eucalyptol | 0 |
| 8 | Phenyl ethyl alcohol → eugenol → eucalyptol → vanillin | 0 |
| 9 | Phenyl ethyl alcohol → eugenol → vanillin → eucalyptol | 0 |
| 10 | Eucalyptol → phenyl ethyl alcohol → eugenol → vanillin | 0 |
| 11 | Eucalyptol → vanillin → phenyl ethyl alcohol → eugenol | 0 |
| 12 | Eucalyptol → vanillin → eugenol → phenyl ethyl alcohol | 0 |
| 13 | Eucalyptol → eugenol → vanillin → phenyl ethyl alcohol | 0 |
| 14 | Eucalyptol → eugenol → phenyl ethyl alcohol → vanillin | 0 |
| 15 | Vanillin → phenyl ethyl alcohol → eugenol → eucalyptol | 0 |
| 16 | Vanillin → eucalyptol → eugenol → phenyl ethyl alcohol | 0 |
| 17 | Vanillin → eugenol → eucalyptol → phenyl ethyl alcohol | 0 |
| 18 | Vanillin → eugenol → phenyl ethyl alcohol → eucalyptol | 0 |
| 19 | Eugenol → phenyl ethyl alcohol → eucalyptol → vanillin | 0 |
| 20 | Eugenol → phenyl ethyl alcohol → vanillin → eucalyptol | 0 |
| 21 | Eugenol → vanillin → phenyl ethyl alcohol → eucalyptol | 0 |
| 22 | Eugenol → vanillin → eucalyptol → phenyl ethyl alcohol | 0 |
| 23 | Eugenol → eucalyptol → vanillin → phenyl ethyl alcohol | 0 |
| 24 | Eugenol → eucalyptol → phenyl ethyl alcohol → vanillin | 0 |
| Total | 181 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verro, B.; Vivoli, G.; Saraniti, C. Hyposmia in COVID-19: Temporal Recovery of Smell: A Preliminary Study. Medicina 2023, 59, 1511. https://doi.org/10.3390/medicina59091511
Verro B, Vivoli G, Saraniti C. Hyposmia in COVID-19: Temporal Recovery of Smell: A Preliminary Study. Medicina. 2023; 59(9):1511. https://doi.org/10.3390/medicina59091511
Chicago/Turabian StyleVerro, Barbara, Giulia Vivoli, and Carmelo Saraniti. 2023. "Hyposmia in COVID-19: Temporal Recovery of Smell: A Preliminary Study" Medicina 59, no. 9: 1511. https://doi.org/10.3390/medicina59091511
APA StyleVerro, B., Vivoli, G., & Saraniti, C. (2023). Hyposmia in COVID-19: Temporal Recovery of Smell: A Preliminary Study. Medicina, 59(9), 1511. https://doi.org/10.3390/medicina59091511






