Development and Validation of a Novel Risk Score for All-Cause Mortality Risk Stratification Prior to Permanent Pacemaker Implantation in Octogenarians or Older
Abstract
1. Introduction
2. Methods
2.1. Study Population and Definition
2.2. Patient Follow-Up and Main Outcome of Interest
2.3. Statistical Analysis
3. Results
3.1. Study Patients
3.2. Patient Characteristics
3.3. Major PPM Implantation-Related Complications
3.4. Causes of Death
3.5. Predictors of All-Cause Mortality Prior to PPM Implantation
3.6. Development of a Scoring System for All-Cause Mortality Risk Stratification Prior to PPM Implantation
3.7. Long-Term Mortality Risk Stratified by the MELODY Score
3.8. The Power of MELODY Scores for Predicting All-Cause Mortality 0.5, 1 and 2 Years after PPM Implantation
3.9. Validation of the MELODY Score
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019. Highlights (ST/ESA/SER.A/423). 2019. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 20 June 2023).
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019, 140, e382–e482. [Google Scholar] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Greenspon, A.J.; Patel, J.D.; Lau, E.; Ochoa, J.A.; Frisch, D.R.; Ho, R.T.; Pavri, B.B.; Kurtz, S.M. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: Increasing complexity of patients and procedures. J. Am. Coll. Cardiol. 2012, 60, 1540–1545. [Google Scholar] [CrossRef]
- De Vries, L.M.; Dijk, W.A.; Hooijschuur, C.A.; Leening, M.J.; Stricker, B.H.; van Hemel, N.M. Utilisation of cardiac pacemakers over a 20-year period: Results from a nationwide pacemaker registry. Neth. Heart J. 2017, 25, 47–55. [Google Scholar] [CrossRef]
- Bradshaw, P.J.; Stobie, P.; Knuiman, M.W.; Briffa, T.G.; Hobbs, M.S. Life expectancy after implantation of a first cardiac permanent pacemaker (1995–2008): A population-based study. Int. J. Cardiol. 2015, 190, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Brunner, M.; Olschewski, M.; Hummel, C.; Faber, T.S.; Grom, A.; Giesler, U.; Bode, C.; Zehender, M. Pacemaker therapy in very elderly patients: Long-term survival and prognostic parameters. Am. Heart J. 2003, 146, 908–913. [Google Scholar] [CrossRef]
- Udo, E.O.; van Hemel, N.M.; Zuithoff, N.P.; Kelder, J.C.; Crommentuijn, H.A.; Koopman-Verhagen, A.M.; Voskuil, T.; Doevendans, P.A.; Moons, K.G. Long-term outcome of cardiac pacing in octogenarians and nonagenarians. Europace 2012, 14, 502–508. [Google Scholar] [CrossRef]
- Balla, C.; Malagu, M.; Fabbian, F.; Guarino, M.; Zaraket, F.; Brieda, A.; Smarrazzo, V.; Ferrari, R.; Bertini, M. Prognosis after pacemaker implantation in extreme elderly. Eur. J. Intern. Med. 2019, 65, 37–43. [Google Scholar] [CrossRef]
- Cheng, C.W.; Wang, C.H.; Chen, W.S.; Wang, C.C.; Cherng, W.J. Predictors of long-term survival prior to permanent pacemaker implantation in octogenarians or older. Aging Clin. Exp. Res. 2019, 31, 1001–1009. [Google Scholar] [CrossRef]
- Parshall, M.B.; Schwartzstein, R.M.; Adams, L.; Banzett, R.B.; Manning, H.L.; Bourbeau, J.; Calverley, P.M.; Gift, A.G.; Harver, A.; Lareau, S.C. An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012, 185, 435–452. [Google Scholar] [CrossRef]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef]
- De Vries, L.M.; Leening, M.J.G.; Dijk, W.A.; Hooijschuur, C.A.M.; Stricker, B.H.C.; van Hemel, N.M. Trends in service time of pacemakers in the Netherlands: A long-term nationwide follow-up study. Neth. Heart J. 2017, 25, 581–591. [Google Scholar] [CrossRef]
- Stevenson, R.T.; Lugg, D.; Gray, R.; Hollis, D.; Stoner, M.; Williams, J.L. Pacemaker implantation in the extreme elderly. J. Interv. Card. Electrophysiol. 2012, 33, 51–58. [Google Scholar] [CrossRef]
- Schoenenberger, A.W.; Russi, I.; Berte, B.; Weberndörfer, V.; Schoenenberger-Berzins, R.; Chodup, P.; Beeler, R.; Cuculi, F.; Toggweiler, S.; Kobza, R. Evaluation of comprehensive geriatric assessment in older patients undergoing pacemaker implantation. BMC Geriatr. 2020, 20, 287. [Google Scholar] [CrossRef]
- Montesanto, A.; De Rango, F.; Berardelli, M.; Mari, V.; Lattanzio, F.; Passarino, G.; Corsonello, A. Glomerular filtration rate in the elderly and in the oldest old: Correlation with frailty and mortality. Age 2014, 36, 9641. [Google Scholar] [CrossRef]
- Mandelli, S.; Riva, E.; Tettamanti, M.; Detoma, P.; Giacomin, A.; Lucca, U. Mortality Prediction in the Oldest Old with Five Different Equations to Estimate Glomerular Filtration Rate: The Health and Anemia Population-based Study. PLoS ONE 2015, 10, e0136039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hegendörfer, E.; Vaes, B.; Matheï, C.; Van Pottelbergh, G.; Degryse, J.M. Correlates of dyspnoea and its association with adverse outcomes in a cohort of adults aged 80 and over. Age Ageing 2017, 46, 994–1000. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Rosendael, A.R.; Bax, A.M.; van den Hoogen, I.J.; Smit, J.M.; Al’Aref, S.J.; Achenbach, S.; Al-Mallah, M.; Andreini, D.; Berman, D.S.; Budoff, M.J.; et al. Associations between dyspnoea, coronary atherosclerosis, and cardiovascular outcomes: Results from the long-term follow-up CONFIRM registry. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yi, S.W.; Sull, J.W.; Hong, J.S.; Jee, S.H.; Ohrr, H. Body mass index and mortality among Korean elderly in rural communities: Kangwha Cohort Study. PLoS ONE 2015, 10, e0117731. [Google Scholar] [CrossRef][Green Version]
- Ng, T.P.; Jin, A.; Chow, K.Y.; Feng, L.; Nyunt, M.S.Z.; Yap, K.B. Age-dependent relationships between body mass index and mortality: Singapore longitudinal ageing study. PLoS ONE 2017, 12, e0180818. [Google Scholar] [CrossRef]
- Trivedi, V.; Bleeker, H.; Kantor, N.; Visintini, S.; McIsaac, D.I.; McDonald, B. Survival, Quality of Life, and Functional Status Following Prolonged ICU Stay in Cardiac Surgical Patients: A Systematic Review. Crit. Care Med. 2019, 47, e52–e63. [Google Scholar] [CrossRef] [PubMed]
- Hiura, G.; Lebwohl, B.; Seres, D.S. Malnutrition Diagnosis in Critically Ill Patients Using 2012 Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition Standardized Diagnostic Characteristics Is Associated With Longer Hospital and Intensive Care Unit Length of Stay and Increased In-Hospital Mortality. J. Parenter. Enter. Nutr. 2020, 44, 256–264. [Google Scholar]
- Bertelli, M.; Toniolo, S.; Ziacchi, M.; Gasperetti, A.; Schiavone, M.; Arosio, R.; Capobianco, C.; Mitacchione, G.; Statuto, G.; Angeletti, A.; et al. Is Less Always More? A Prospective Two-Centre Study Addressing Clinical Outcomes in Leadless versus Transvenous Single-Chamber Pacemaker Recipients. J. Clin. Med. 2022, 11, 6071. [Google Scholar] [CrossRef] [PubMed]
- Mitacchione, G.; Schiavone, M.; Gasperetti, A.; Arabia, G.; Breitenstein, A.; Cerini, M.; Palmisano, P.; Montemerlo, E.; Ziacchi, M.; Gulletta, S.; et al. Outcomes of leadless pacemaker implantation following transvenous lead extraction in high-volume referral centers: Real-world data from a large international registry. Heart Rhythm 2023, 20, 395–404. [Google Scholar] [CrossRef]
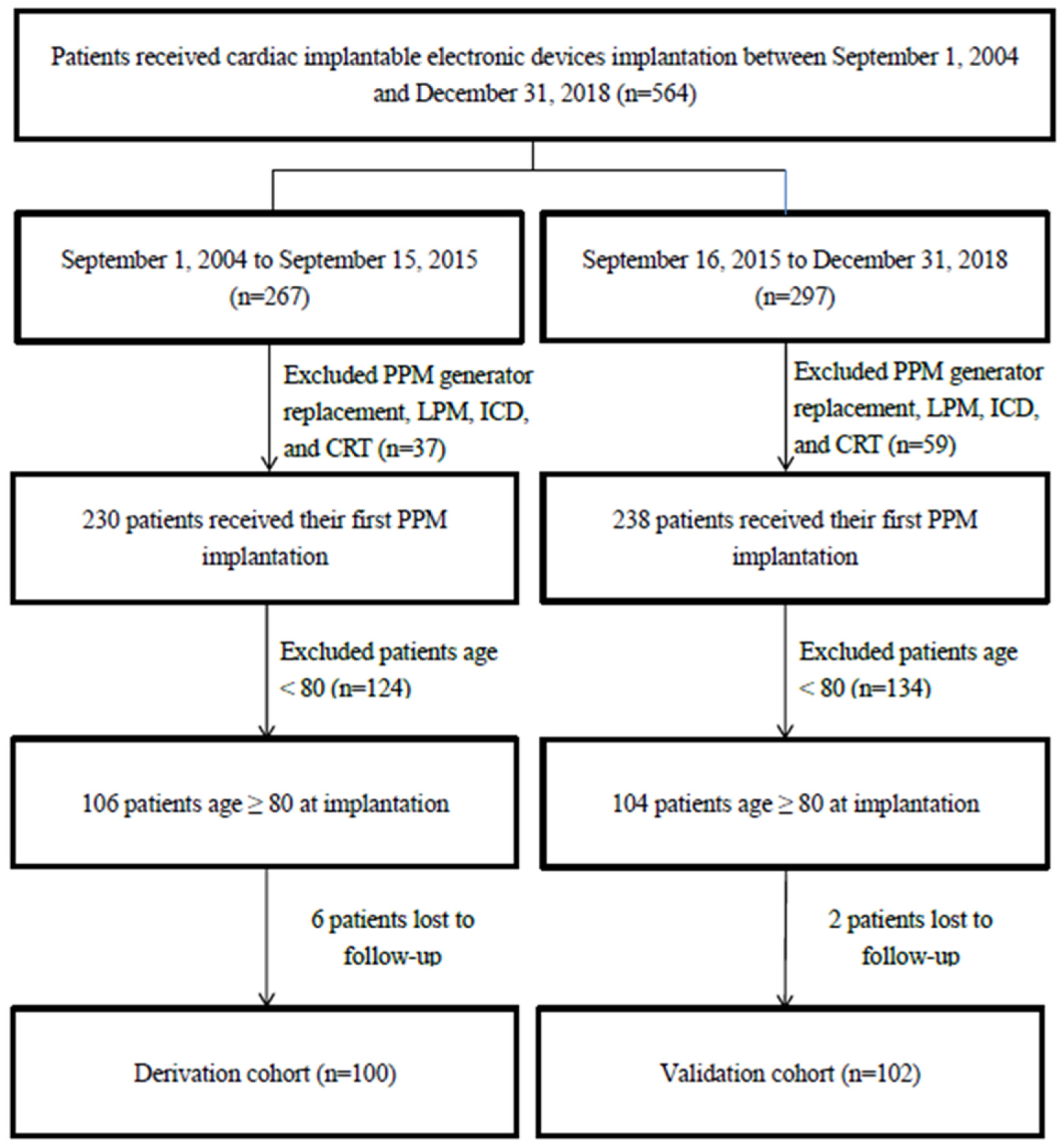
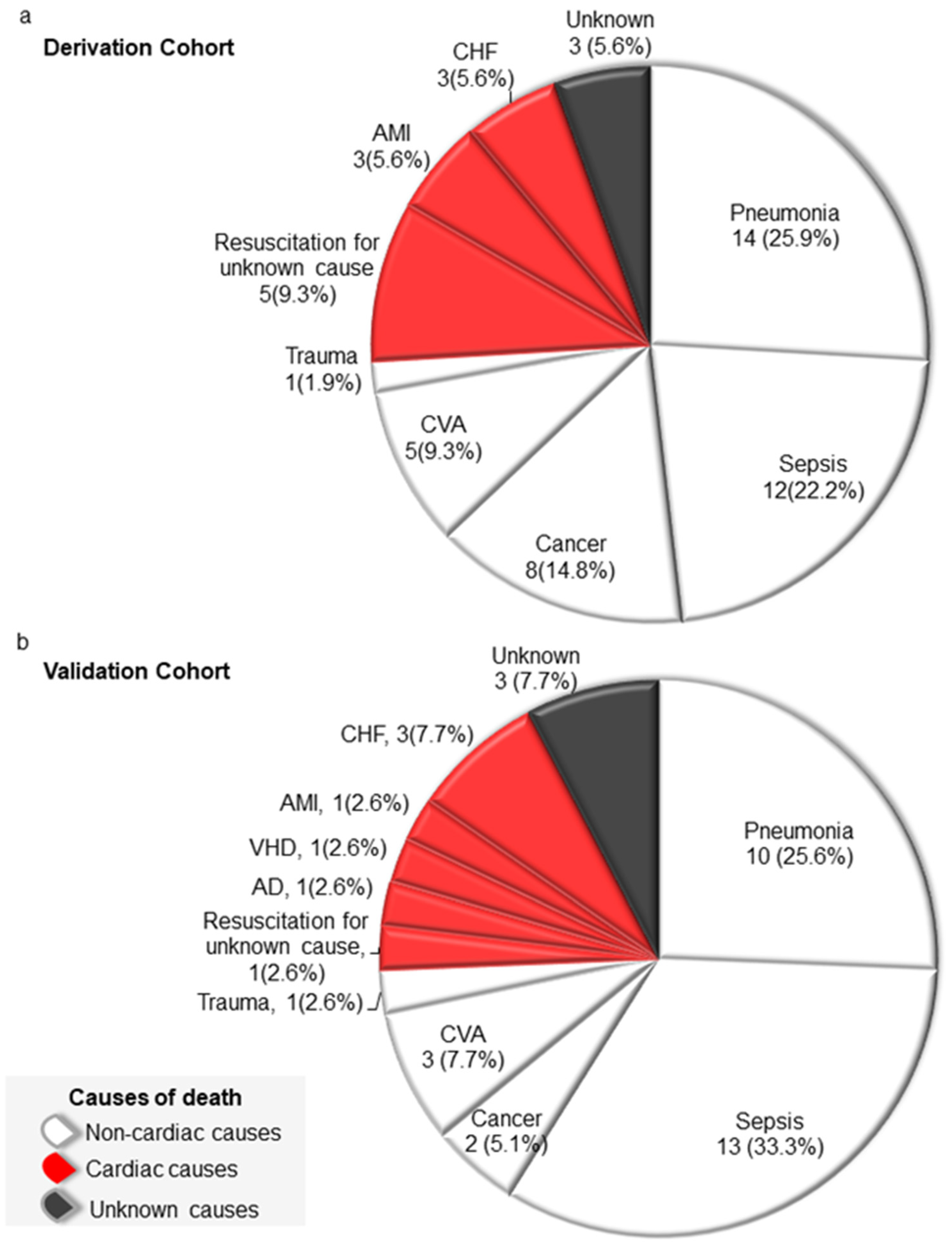
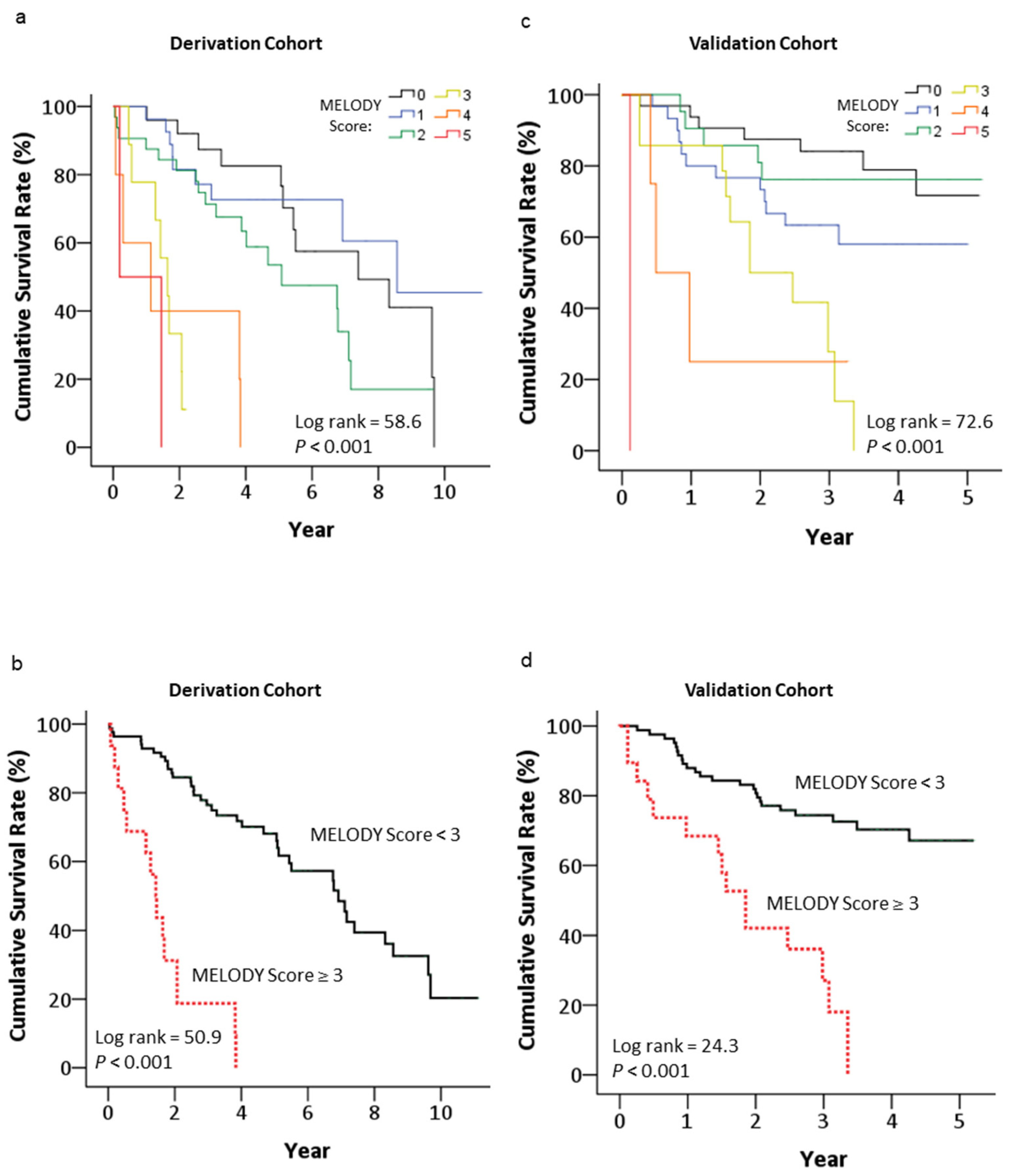
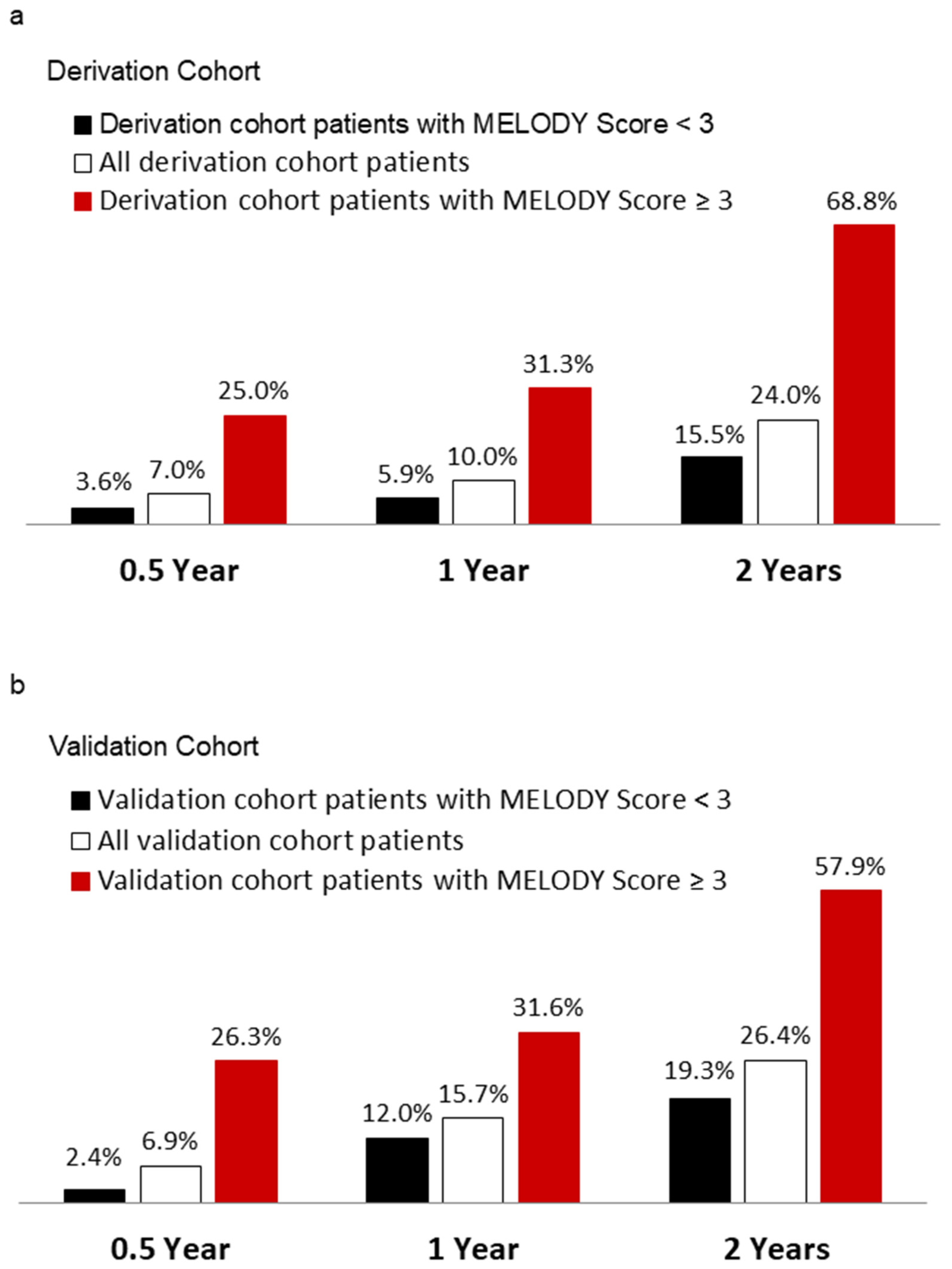
| Characteristics | Derivation Cohortvb ‡ | Validation Cohort | p Value |
|---|---|---|---|
| (n = 100) | (n = 102) | ||
| Age at implantation (years) * | 84.5 (81.3–88.0) | 85.0 (82.0–88.3) | 0.73 |
| Male gender, n (%) | 52 (52.0) | 38 (37.3) | 0.04 |
| Admitted from ED, n (%) | 72 (72.0) | 61 (59.8) | 0.07 |
| Left ventricle ejection fraction (%) * | 68 (62; 73) | 69 (62; 76) | 0.47 |
| Main symptoms at presentation | <0.001 | ||
| Syncope, near syncope, n (%) | 24 (24.0) | 17 (16.7) | |
| Dizziness, n (%) | 26 (26.0) | 52 (51.0) | |
| Dyspnea, n (%) | 50 (50.0) | 33 (32.4) | |
| Comorbidities | |||
| Hypertension, n (%) | 88 (88.0) | 77 (75.5) | 0.02 |
| Diabetes mellitus, n (%) | 33 (33.0) | 38 (37.3) | 0.46 |
| Coronary artery disease, n (%) | 25 (25.0) | 31 (30.4) | 0.39 |
| Valvular heart disease, n (%) | 24 (24.0) | 30 (29.4) | 0.39 |
| Cerebral vascular accident, n (%) | 23 (23.0) | 14 (13.7) | 0.08 |
| COPD, n (%) | 19 (19.0) | 17 (16.7) | 0.67 |
| Atrial arrhythmia, n (%) | 39 (39.0) | 54 (52.9) | 0.04 |
| eGFR (ml/min/1.73 m2) * | 56.3 (32.6; 75.1) | 51.0 (31.5; 72.4) | 0.29 |
| <30, n (%) | 20 (20.0) | 25 (24.5) | 0.44 |
| BMI (kg/m2) † | 24.0 ± 4.1 | 23.5 ± 4.6 | 0.39 |
| <21, n (%) | 17 (17.0) | 31 (30.4) | 0.03 |
| AVCD, n (%) | 70 (70.0) | 43 (42.2) | <0.001 |
| LOS-B (days) † | 6 (3;11) | 3 (1;7) | 0.001 |
| Dual chamber pacemaker, n (%) | 80 (80.0) | 93 (91.2) | 0.02 |
| Follow-up duration (years) † | 4.0 ± 2.7 | 2.8 ± 1.3 | <0.001 |
| Variables | Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | Beta (SE) | HR (95% CI) | p Value | |
| Age at implantation * | 1.05 (0.99–1.11) | 0.10 | |||
| Male gender | 0.73 (0.43–1.24) | 0.25 | |||
| Admitted from ED | 1.22 (0.67–2.25) | 0.52 | |||
| Ejection fraction (%) † | 0.99 (0.97–1.02) | 0.66 | |||
| Dyspnea | 1.73 (1.01–2.98) | 0.04 | 0.64 (0.31) | 1.90 (1.03–3.50) | 0.03 |
| Hypertension | 1.03 (0.50–2.14) | 0.93 | |||
| Diabetes mellitus | 1.37 (0.78–2.42) | 0.28 | |||
| Coronary artery disease | 1.29 (0.69–2.43) | 0.43 | |||
| Valvular heart disease | 1.47 (0.81–2.67) | 0.21 | |||
| Cerebral vascular accident | 1.34 (0.74–2.44) | 0.34 | |||
| COPD | 1.07 (0.52–2.19) | 0.87 | |||
| Atrial arrhythmia | 0.79 (0.45–1.39) | 0.42 | |||
| eGFR < 30 mL/min/1.73 m2 † | 2.57 (1.40–4.72) | 0.002 | 1.21 (0.32) | 3.35 (1.77–6.35) | <0.001 |
| BMI < 21 kg/m2 | 2.27 (1.14–4.49) | 0.01 | 0.79 (0.37) | 2.21 (1.06–4.61) | 0.03 |
| AVCD | 2.07 (1.07–4.04) | 0.03 | |||
| LOS-B > 7 days | 2.32 (1.33–4.03) | 0.003 | 0.63 (0.31) | 1.87 (1.02–3.43) | 0.04 |
| Characteristics | Point |
|---|---|
| BMI < 21 kg/m2 | 1 |
| eGFR < 30 mL/min/1.73 m2 | 2 |
| LOS-B > 7 days | 1 |
| Dyspnea as the main symptom at presentation | 1 |
| Cohort | 0.5 Year | 1 Year | 2 Years | |||
|---|---|---|---|---|---|---|
| AUC | p Value | AUC | p Value | AUC | p Value | |
| Derivation cohort | 0.86 | 0.002 | 0.81 | 0.001 | 0.74 | <0.001 |
| Validation cohort | 0.78 | 0.02 | 0.71 | 0.01 | 0.72 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-C.; Hung, M.-J.; Wang, C.-H.; Chen, T.-H.; Chen, W.-S.; Cheng, C.-W. Development and Validation of a Novel Risk Score for All-Cause Mortality Risk Stratification Prior to Permanent Pacemaker Implantation in Octogenarians or Older. Medicina 2023, 59, 1499. https://doi.org/10.3390/medicina59081499
Lin H-C, Hung M-J, Wang C-H, Chen T-H, Chen W-S, Cheng C-W. Development and Validation of a Novel Risk Score for All-Cause Mortality Risk Stratification Prior to Permanent Pacemaker Implantation in Octogenarians or Older. Medicina. 2023; 59(8):1499. https://doi.org/10.3390/medicina59081499
Chicago/Turabian StyleLin, Hsuan-Ching, Ming-Jui Hung, Chao-Hung Wang, Tien-Hsing Chen, Wei-Siang Chen, and Chi-Wen Cheng. 2023. "Development and Validation of a Novel Risk Score for All-Cause Mortality Risk Stratification Prior to Permanent Pacemaker Implantation in Octogenarians or Older" Medicina 59, no. 8: 1499. https://doi.org/10.3390/medicina59081499
APA StyleLin, H.-C., Hung, M.-J., Wang, C.-H., Chen, T.-H., Chen, W.-S., & Cheng, C.-W. (2023). Development and Validation of a Novel Risk Score for All-Cause Mortality Risk Stratification Prior to Permanent Pacemaker Implantation in Octogenarians or Older. Medicina, 59(8), 1499. https://doi.org/10.3390/medicina59081499






