The Prognostic Utility of the Metastatic Lymph Node Ratio and the Number of Regional Lymph Nodes Removed from Patients with Small Bowel Adenocarcinomas
Abstract
:1. Introduction
2. Patients and Methods
2.1. Resected Lymph Node Number Cutoff
2.2. N Ratio Cutoff Values
3. Statistical Analyses
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Aparicio, T.; Pachev, A.; Laurent-Puig, P.; Svrcek, M. Epidemiology, Risk Factors and Diagnosis of Small Bowel Adenocarcinoma. Cancers 2022, 14, 2268. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Wayne, J.D.; Ko, C.Y.M.; Bennett, C.L.; Talamonti, M.S. Small bowel cancer in the United States: Changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 2009, 249, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Henriques, J.; Manfredi, S.; Tougeron, D.; Bouché, O.; Pezet, D.; Piessen, G.; Coriat, R.; Zaanan, A.; Legoux, J.-L.; et al. Small bowel adenocarcinoma: Results from a nationwide prospective ARCAD-NADEGE cohort study of 347 patients. Int. J. Cancer 2020, 147, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Dabaja, B.S.; Suki, D.; Pro, B.; Bonnen, M.; Ajani, J. Adenocarcinoma of the small bowel: Presentation, prognostic factors, and outcome of 217 patients. Cancer 2004, 101, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Aydin, D.; Sendur, M.A.; Kefeli, U.; Unal, O.U.; Tastekin, D.; Akyol, M.; Tanrikulu, E.; Ciltas, A.; Ustaalioglu, B.B.; Uysal, M.; et al. Evaluation of Prognostic Factors and Adjuvant Chemotherapy in Patients with Small Bowel Adenocarcinoma Who Underwent Curative Resection. Clin. Color. Cancer 2016, 16, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yuan, W.; Zhang, J.; Chen, Y.; Zheng, C.; Ma, J.; Jiang, Q.; Zhao, Y.; Xu, Q.; Wang, C. Clinicopathological features, surgical treatments, and survival outcomes of patients with small bowel adenocarcinoma. Medicine 2017, 96, e7713. [Google Scholar] [CrossRef]
- Hashimoto, D.; Arima, K.; Chikamoto, A.; Taki, K.; Inoue, R.; Kaida, T.; Higashi, T.; Imai, K.; Beppu, T.; Baba, H. Limited Resection of the Duodenum for Nonampullary Duodenal Tumors, with Review of the Literature. Am. Surg. 2016, 82, 1126–1132. [Google Scholar] [CrossRef]
- Locher, C.; Batumona, B.; Afchain, P.; Carrère, N.; Samalin, E.; Cellier, C.; Aparicio, T.; Becouarn, Y.; Bedenne, L.; Michel, P.; et al. Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. 2018, 50, 15–19. [Google Scholar] [CrossRef]
- Overman, M.J.; Hu, C.-Y.; Kopetz, S.; Abbruzzese, J.L.; Wolff, R.A.; Chang, G.J. A Population-Based Comparison of Adenocarcinoma of the Large and Small Intestine: Insights Into a Rare Disease. Ann. Surg. Oncol. 2011, 19, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Hu, C.-Y.; Wolff, R.A.; Chang, G.J. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: Analysis of the surveillance, epidemiology, and end results database. Cancer 2010, 116, 5374–5382. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Qadan, M.; Dua, M.M.; Norton, J.A.; Poultsides, G.A.; Visser, B.C. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery 2015, 158, 486–493. [Google Scholar] [CrossRef]
- Wilhelm, A.; Müller, S.A.; Steffen, T.; Schmied, B.M.; Beutner, U.; Warschkow, R. Patients with Adenocarcinoma of the Small Intestine with 9 or More Regional Lymph Nodes Retrieved Have a Higher Rate of Positive Lymph Nodes and Improved Survival. J. Gastrointest. Surg. 2015, 20, 401–410. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J.-N.; Zhang, Q.-W.; Tang, C.-T.; Zhang, X.-T.; Tang, M.-Y.; Li, X.-B.; Ge, Z.-Z. A New Metastatic Lymph Node Classification-based Survival Predicting Model in Patients With Small Bowel Adenocarcinoma: A Derivation and Validation Study. Ebiomedicine 2018, 32, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; McWilliams, R.R.; Donohue, J.H.; Quevedo, J.F. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am. J. Surg. 2010, 199, 797–803. [Google Scholar] [CrossRef]
- Hatzaras, I.; Palesty, J.A.; Abir, F.; Sullivan, P.; Kozol, R.A.; Dudrick, S.J.; Longo, W.E. Small-bowel tumors: Epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch. Surg. 2007, 142, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-J.; Yeh, C.-N.; Chao, T.-C.; Jan, Y.-Y.; Chen, M.-F. Prognostic Factors of Primary Small Bowel Adenocarcinoma: Univariate and Multivariate Analysis. World J. Surg. 2006, 30, 391–398 discussion 399. [Google Scholar] [CrossRef]
- Brücher, B.; Stein, H.J.; Roder, J.D.; Busch, R.; Fink, U.; Werner, M.; Siewert, J.R. New aspects of prognostic factors in adenocarcinomas of the small bowel. Hepato-Gastroenterology 2001, 48, 727–732. [Google Scholar]
- Schwarz, R.E.; Smith, D.D. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann. Surg. Oncol. 2006, 14, 317–328. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.-K.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Lykke, J.; Roikjaer, O.; Jess, P.; The Danish Colorectal Cancer Group. The relation between lymph node status and survival in Stage I-III colon cancer: Results from a prospective nationwide cohort study. Color. Dis. 2012, 15, 559–565. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Palis, B.; Stewart, A.K.; Bentrem, D.J.; Freel, A.C.; Sigurdson, E.R.; Talamonti, M.S.; Ko, C.Y. Impact of Tumor Location on Nodal Evaluation for Colon Cancer. Dis. Colon Rectum 2008, 51, 154–161. [Google Scholar] [CrossRef]
- Meijer, L.L.; Alberga, A.J.; de Bakker, J.K.; van der Vliet, H.J.; Le Large, T.Y.S.; van Grieken, N.C.T.; de Vries, R.; Daams, F.; Zonderhuis, B.M.; Kazemier, G. Outcomes and Treatment Options for Duodenal Adenocarcinoma: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2018, 25, 2681–2692. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Norton, J.A.; Visser, B.C.; Poultsides, G.A. Does the Extent of Resection Impact Survival for Duodenal Adenocarcinoma? Analysis of 1,611 Cases. Ann. Surg. Oncol. 2014, 22, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Burasakarn, P.; Yamamoto, M.; Nunobe, S.N.S.; Kanaji, S.K.S.; Eguchi, H.E.H.; Okada, K.O.K.-I.; Fujii, T.F.T.; Nagakawa, Y.N.Y.; Kanetaka, K.K.K.; Yamashita, H.Y.H.; et al. Limited resection vs. pancreaticoduodenectomy for primary duodenal adenocarcinoma: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2021, 26, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Kong, S.; Hannouf, M.B.; Cheung, W.Y. A Population-Based Study to Evaluate the Associations of Nodal Stage, Lymph Node Ratio and Log Odds of Positive Lymph Nodes with Survival in Patients with Small Bowel Adenocarcinoma. Curr. Oncol. 2022, 29, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; McMillan, M.T.; Datta, J.; Mamtani, R.; Giantonio, B.J.; Dempsey, D.T.; Fraker, D.L.; Drebin, J.A.; Karakousis, G.C.; Roses, R.E. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: A propensity score-matched analysis. Cancer 2015, 122, 693–701. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, G.; Chen, H.; Li, Y. Correction: Meta-analysis of postoperative adjuvant therapy for small bowel adenocarcinoma. PLoS ONE 2018, 13, e0207816. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin As Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef]
| Variable | Med (IQR) | p |
|---|---|---|
| Gender | 0.800 1 | |
| Female n = 41 | 16.6 (0–43.5) | |
| Male n = 56 | 8.3 (0–38.2) | |
| Age at diagnosis (in categories) | 0.476 1 | |
| <60 n = 55 | 8.3 (0–38.4) | |
| ≥60 n = 42 | 13.7 (0–42.1) | |
| Histopathology | NA | |
| Adenocarcinoma n = 87 | 8.3 (0–38.9) | |
| Signet ring cell n = 7 | 33.3 (0–42.8) | |
| Mucinous n = 3 | 25 (na) | |
| Tumor Differentiation | 0.686 2 | |
| Well differentiated n = 21 | 11.1 (0–45.1) | |
| Moderately differentiated n = 56 | 7.7 (0.34.4) | |
| Poorly differentiated n = 14 | 32.4 (6.1–62.3) | |
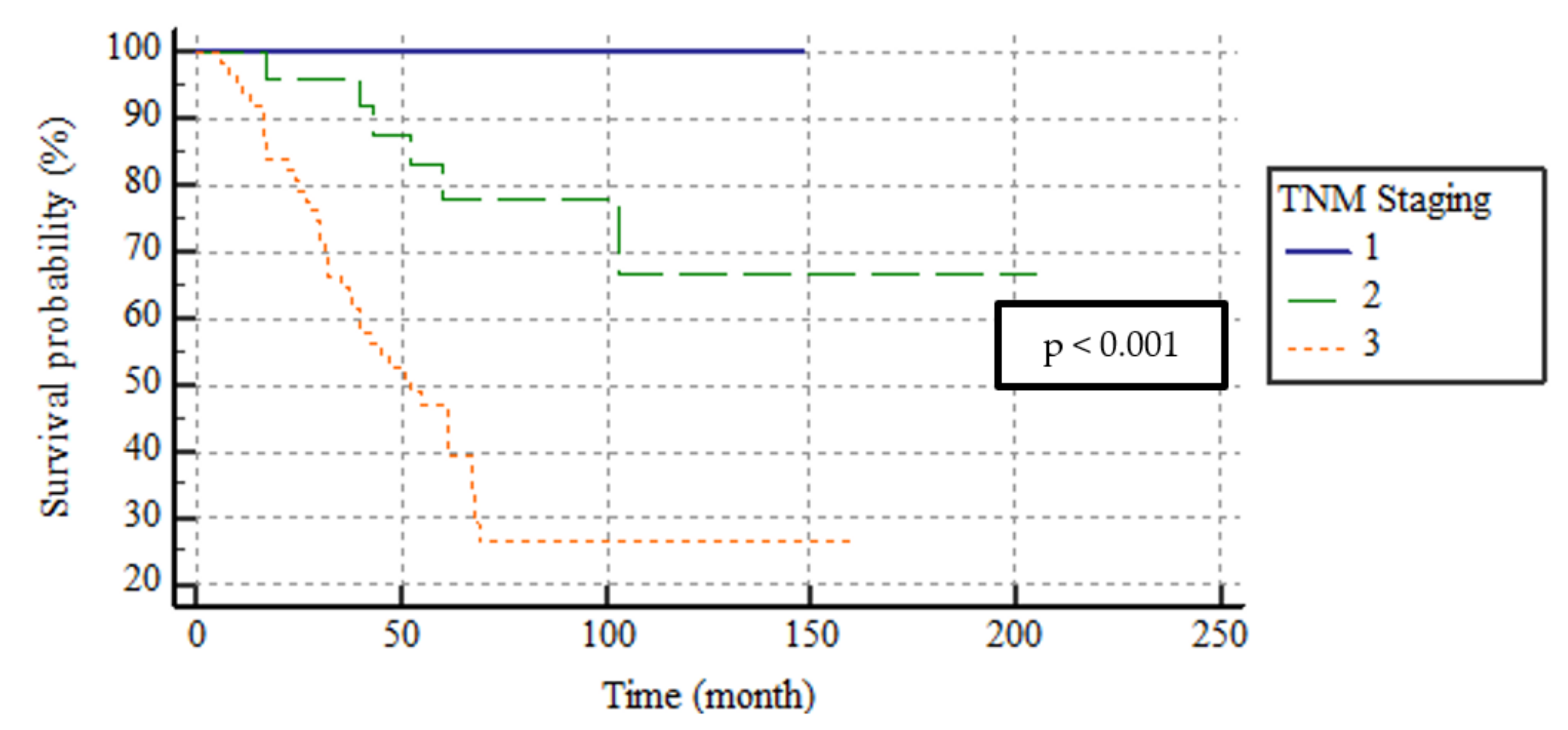
| TNM Stage | <0.001 2 | |
| Stage I n = 8 | 0 (0–0) | |
| Stage II n = 25 | 0 (0–0) | |
| Stage III n = 64 | 32.4 (8.8–46.5) | |
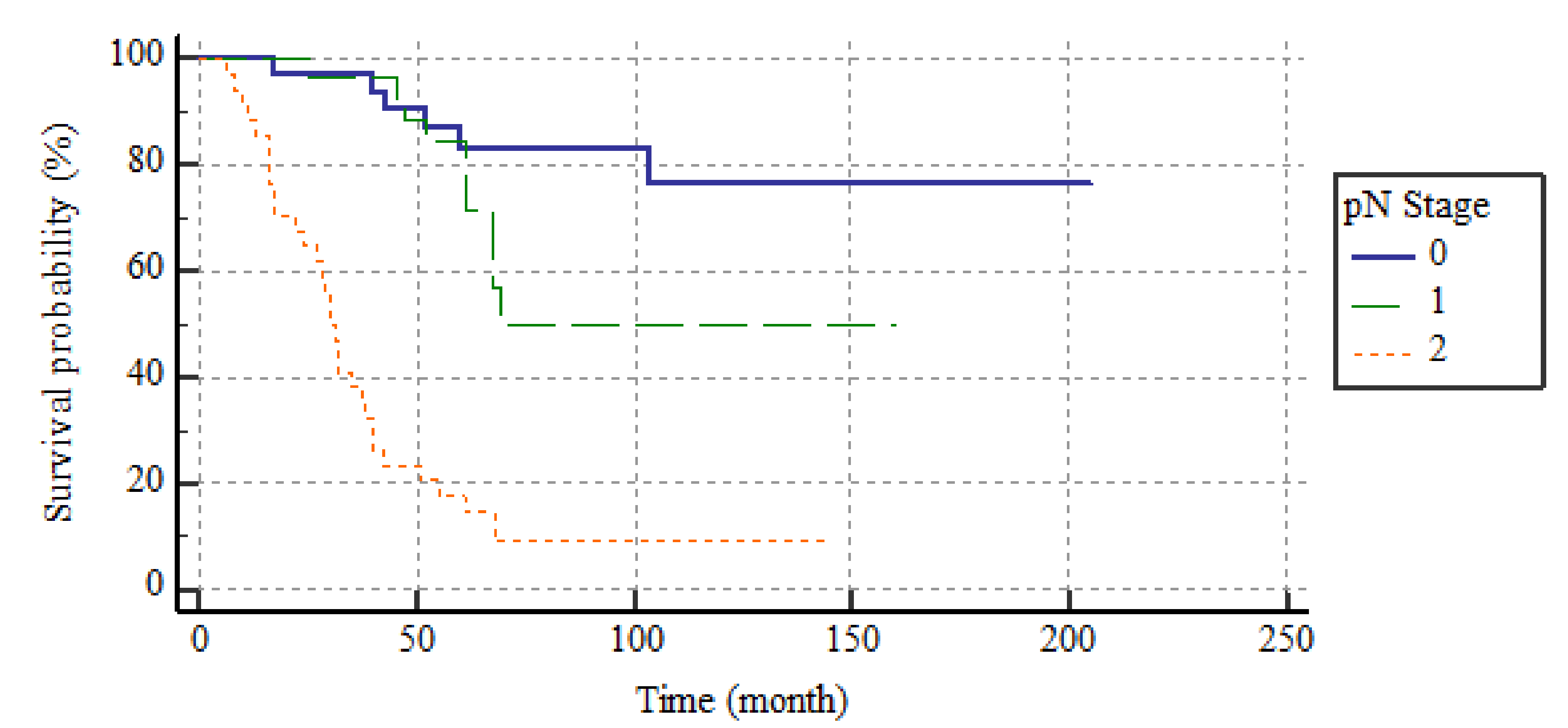
| pN Stage | <0.001 2 | |
| pN0 n = 33 | 0 (0–0) | |
| pN1 n = 29 | 8.3 (6.9–18.2) | |
| pN2 n = 35 | 44 (37.5–62.5) | |
| pT Stage | 0.119 2 | |
| 1 n = 2 | 0 (0–0) | |
| 2 n = 10 | 0 (0–23.6) | |
| 3 n = 60 | 8.9 (0–44.1) | |
| 4 n = 25 | 16.6 (0–42.7) | |
| Vascular Invasion | <0.001 1 | |
| (−) n = 43 | 0 (0–0) | |
| (+) n = 53 | 36.3 (8.7–47) | |
| Perineural Invasion | <0.001 1 | |
| (−) n = 39 | 0 (0–8.3) | |
| (+) n = 58 | 33.3 (7.6–46.2) | |
| Lymph nodes Invasion | <0.001 1 | |
| (−) n = 33 | 0 (0–0) | |
| (+) n = 64 | 32.4 (8.8–46.5) | |
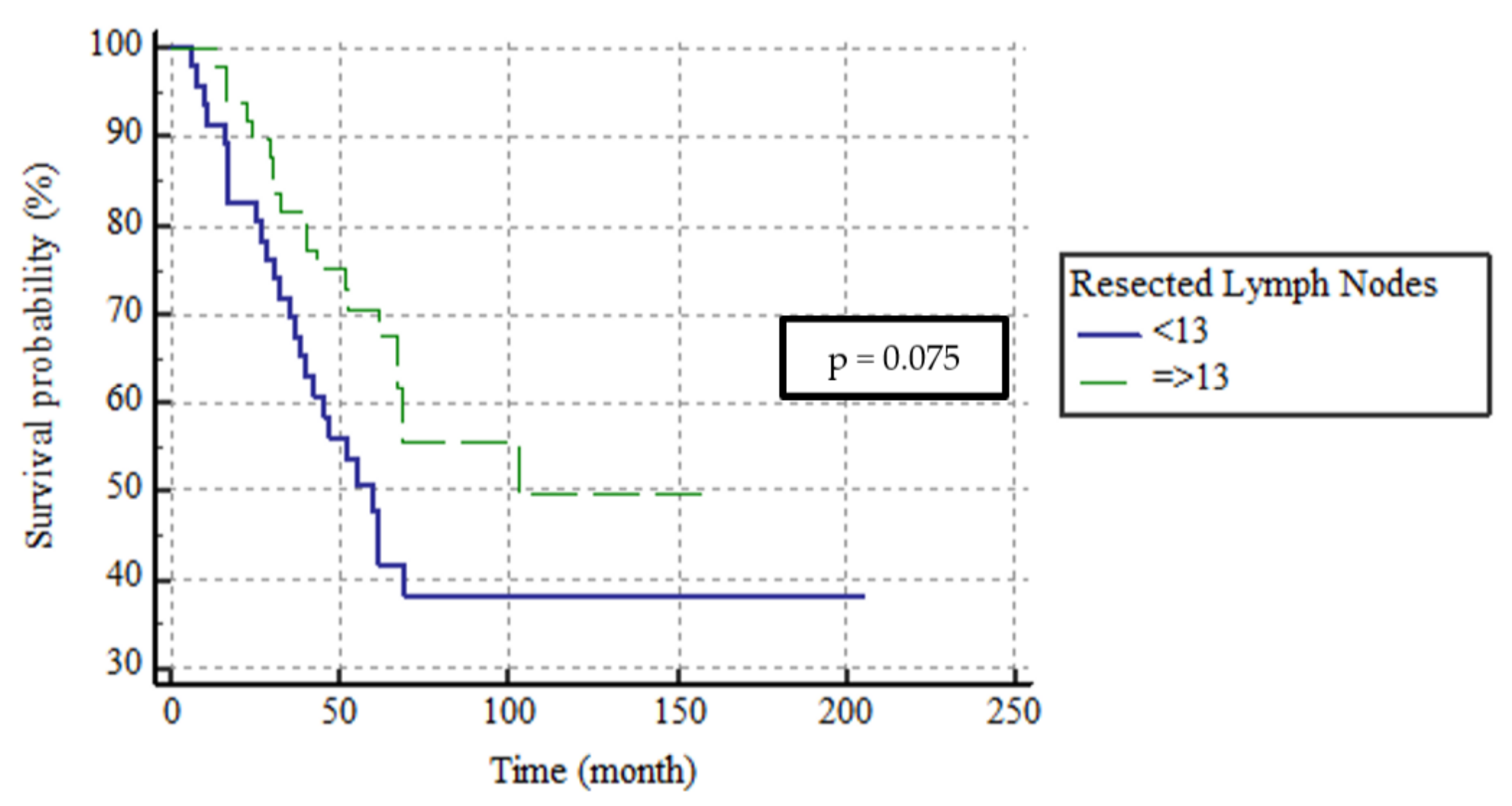
| Category of resected lymph nodes | 0.432 1 | |
| <13 n = 46 | 17.4 (0–46.6) | |
| ≥13 n = 51 | 7.7 (0–38.4) |
| Factor | No. of Patients (%) | Median DSS Time (Months) | 95% CI | p | |
|---|---|---|---|---|---|
| All | 97 | 68 | 25–111 | - | |
| Gender | 0.145 | ||||
| Male | 41 (42.3) | 125.2 | 101.6–148.8 | ||
| Female | 56 (57.7) | 78.3 | 60.5–96.3 | ||
| Age (year) | 0.883 | ||||
| <60 | 55 (56.7) | 69 | 22.4–115.6 | ||
| ≥60 | 42 (43.3) | 68 | - | ||
| Tumor Location | 0.667 | ||||
| Duodenum | 57 (58.8) | 109.4 | 86.7–132.2 | ||
| Jejenum | 24 (24.7) | 98.8 | 75.3–122.2 | ||
| Ileum | 16 (16.5) | 91.0 | 59.9–122.2 | ||
| Surgery Type | 0.998 | ||||
| Segmental resection | 71 (73.2) | 69 | 23.6–114.4 | ||
| Pancreaticoduodenectomy | 26 (26.8) | 67 | - | ||
| Histopathology | NA | ||||
| Adenocarcinoma | 87 (89.7) | 68 | 25.5–110.5 | ||
| Signet ring cell | 7 (7.2) | 47 | 21.3–72.7 | ||
| Mucinous | 3 (3.1) | - | - | ||
| Tumor Differentiation | 0.331 | ||||
| Well differentiated | 21 (23.1) | 78.4 | 54–102.9 | ||
| Moderately differentiated | 56 (61.5) | 128 | 103.7–152.3 | ||
| Poorly differentiated | 14 (15.4) | 86 | 52.6–119.4 | ||
| Vascular Invasion | <0.001 | ||||
| Present | 53 (55.2) | 72.0 | 51.7–92.3 | ||
| Absent | 43 (44.8) | 128.5 | 115.3–141.7 | ||
| Perineural Invasion | <0.001 | ||||
| Present | 58 (59.8) | 67.1 | 52.1–82 | ||
| Absent | 39 (40.2) | 175.0 | 153.2–197 | ||
| pT Stage | 0.133 | ||||
| 1 | 2 (2.1) | 85 | 85–85 | ||
| 2 | 10 (10.3) | 115.2 | 83.6–146.9 | ||
| 3 | 60 (61.9) | 116.4 | 93.2–139.6 | ||
| 4 | 25 (25.8) | 70.4 | 45.5–95.3 | ||
| pN Stage | <0.001 | ||||
| pN0 | 33 (34) | 170.9 | 146.4–195.4 | ||
| pN1 | 29 (29.9) | 109.2 | 84.4–133.9 | ||
| pN2 | 35 (36.1) | 40.8 | 28.6–52.9 | ||
| TNM Stage | <0.001 | ||||
| Stage I | 8 (8.2) | 148 | 148–148 | ||
| Stage II | 25 (25.8) | 157.9 | 125.2–190.6 | ||
| Stage III | 64 (66) | 71.5 | 56.1–86.9 | ||
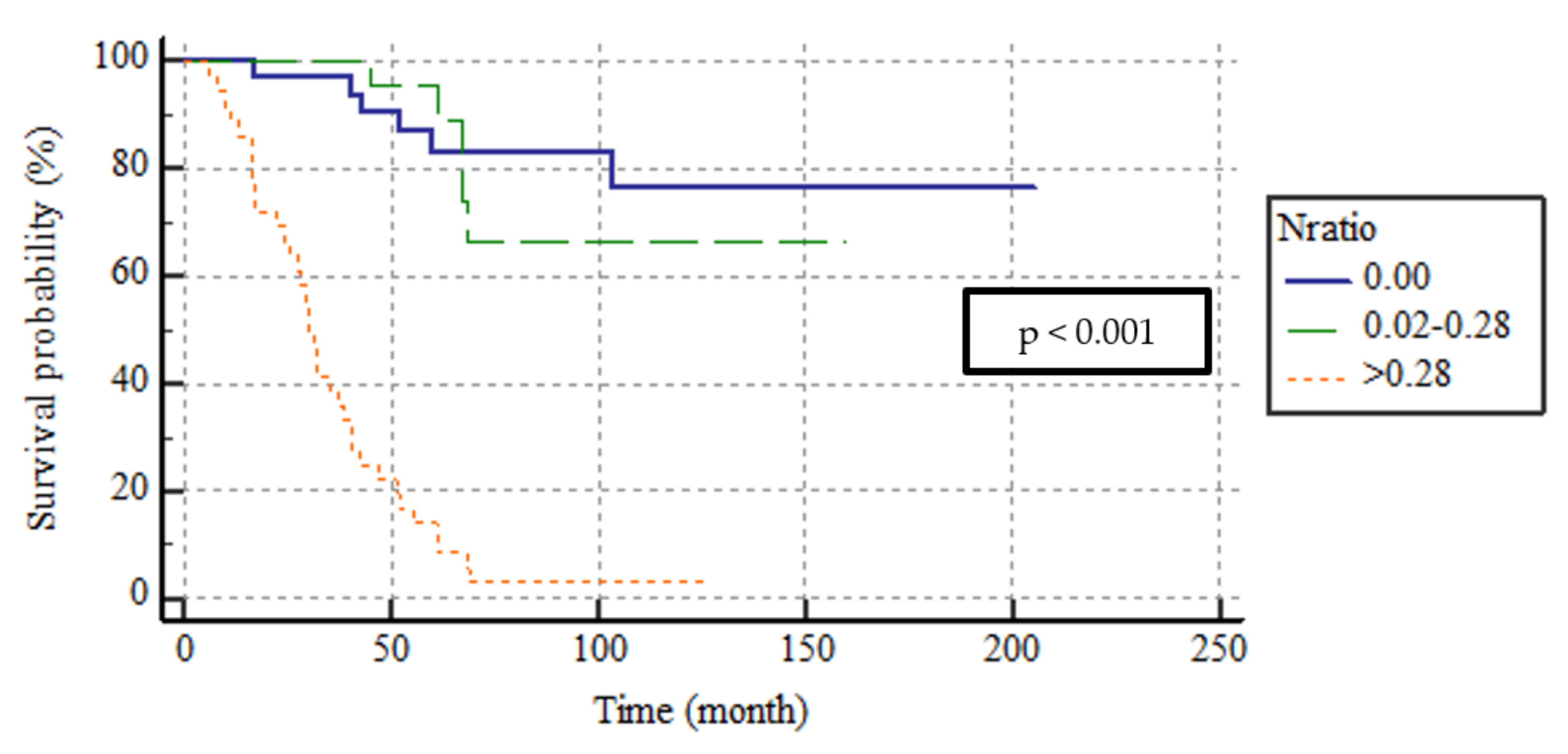
| N Ratio | <0.001 | ||||
| N ratio 0 | (0.00) | 33 (34) | 170.8 | 146.3–195.4 | |
| N ratio 1 | (0.02–0.28) | 29 (29.9) | 127.7 | 104.1–151.2 | |
| N ratio 2 | (>0.28) | 35 (36.1) | 35.1 | 27.6–42.6 | |
| Resected lymph nodes | 0.075 | ||||
| <13 | 46 (47.4) | 60 | 45.1–74.9 | ||
| ≥13 | 51 (52.6) | 103 | - | ||
| Factors | Wald | p | HR | 95% CI |
|---|---|---|---|---|
| Vascular invasion | 0.103 | 0.748 | 0.812 | 0.23–2.89 |
| Perineural Invasion | 7.534 | 0.006 | 4.21 | 1.50–11.78 |
| N ratio | 27.54 | <0.001 | ||
| 0.00 vs. 0.02–0.28 | 0.381 | 0.537 | 0.664 | 0.18–2.44 |
| 0.00 vs. >0.28 | 12.28 | <0.001 | 9.75 | 2.72–34.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, D.; Kefeli, U.; Ozcelik, M.; Erdem, G.U.; Sendur, M.A.; Yildirim, M.E.; Oven, B.B.; Bilici, A.; Gumus, M. The Prognostic Utility of the Metastatic Lymph Node Ratio and the Number of Regional Lymph Nodes Removed from Patients with Small Bowel Adenocarcinomas. Medicina 2023, 59, 1472. https://doi.org/10.3390/medicina59081472
Aydin D, Kefeli U, Ozcelik M, Erdem GU, Sendur MA, Yildirim ME, Oven BB, Bilici A, Gumus M. The Prognostic Utility of the Metastatic Lymph Node Ratio and the Number of Regional Lymph Nodes Removed from Patients with Small Bowel Adenocarcinomas. Medicina. 2023; 59(8):1472. https://doi.org/10.3390/medicina59081472
Chicago/Turabian StyleAydin, Dincer, Umut Kefeli, Melike Ozcelik, Gokmen Umut Erdem, Mehmet Ali Sendur, Mahmut Emre Yildirim, Basak Bala Oven, Ahmet Bilici, and Mahmut Gumus. 2023. "The Prognostic Utility of the Metastatic Lymph Node Ratio and the Number of Regional Lymph Nodes Removed from Patients with Small Bowel Adenocarcinomas" Medicina 59, no. 8: 1472. https://doi.org/10.3390/medicina59081472
APA StyleAydin, D., Kefeli, U., Ozcelik, M., Erdem, G. U., Sendur, M. A., Yildirim, M. E., Oven, B. B., Bilici, A., & Gumus, M. (2023). The Prognostic Utility of the Metastatic Lymph Node Ratio and the Number of Regional Lymph Nodes Removed from Patients with Small Bowel Adenocarcinomas. Medicina, 59(8), 1472. https://doi.org/10.3390/medicina59081472





