Abstract
Ulcerative colitis is an inflammatory disease that affects the colon, generating a crisis period associated with diarrhea and ulcerations. Stress plays a pivotal role in modulating the inflammatory response and aggravating progression. Different studies have shown that fasting reduces inflammation markers, and intermittent fasting decreases inflammatory markers such as IL-2, IL-6, and RCP. Goal: To evaluate the impact of intermittent fasting on a patient diagnosed with ulcerative colitis. A female patient underwent intermittent fasting (10/14) for eight weeks. Clinical tests were performed for blood count, RCP, biochemical profile, glycemia, and T4/TSH levels. Fecal calprotectin was determined. Clinical exams were assessed before and after intermittent fasting. Inflammation markers, such as CRP and calprotectin, were significantly reduced after eight weeks of intermittent fasting. The patient reported feeling better and was seizure-free during the following months when she continued fasting intermittently. Intermittent fasting allowed for a reduction in inflammation markers.
1. Introduction
Ulcerative colitis is an inflammatory disease that affects the colon, generating a crisis period associated with diarrhea and ulcerations. Patients have symptomatic periods (flare-ups) and asymptomatic periods (remission) [1,2]. Therapeutic management includes anti-inflammatory drugs, such as corticosteroids and 5-aminosalicylates, which have limited efficacy and cannot be used for long periods. When immune system inhibitors, such as azathioprine, mercaptopurine, and methotrexate, are used, patients experience several side effects, causing liver inflammation, nausea, and vomiting. Other drugs, such as natalizumab, vedolizumab, infliximab, adalimumab, and certolizumab, inhibit the function of immunoregulatory molecules such as IL-6 and TNF-α [3,4]. Symptoms are treated with antidiarrheals, pain relievers, vitamins, and supplements. However, the benefits of these treatments are temporary, and each is associated with risks inherent to their procedure and side effects [3,4]. Different diets, such as Atkins, Zone, Weight Watchers, Ornish, ketogenic diet, and intermittent fasting (IF), have been tested as an alternative for preventing chronic non-communicable diseases [5].
In a study carried out in mice, the effects of intermittent fasting were analyzed in the short and long term (2 weeks and 20 weeks). Results showed that both periods of time led to a significant impact on the body weight and adipose tissue content of mice, and changes were observed in the bile acid profile of feces. Fecal metabolome analysis identified several metabolites that were enriched in the intermittent fasting groups, including glucose, kynurenic acid, inosine, and 3,4-dihydroxyphenylacetic acid (3,4-DHPA). Kynurenine reduces the differentiation of T cells into highly inflammatory Th17 cells. Inosine also has anti-inflammatory effects on human monocytes and neutrophils. 3,4-DHPA can inhibit the secretion of pro-inflammatory cytokines in lipopolysaccharide-stimulated peripheral macrophages [6,7]. Differences in gut microbiota composition were also found between intermittent fasting and ad libitum feeding groups. These findings suggest that intermittent fasting might have an impact on the metabolism and composition of the gut microbiota [7]. It has been reported that during Ramadan, when the population eats in a restricted manner between 12 and 18 h a day, weight loss occurs, as well as a regulation of lipid and glucose levels. The latter is accompanied by a decrease in the levels of inflammation markers, such as IL-1 and IL-6, CRP, and TNF-α [8,9]. Furthermore, intermittent fasting in combination with physical exercise has been shown to improve LDL and HDL concentrations in plasma.
Thus, fasting reduces the risk of coronary disease [10]. Another study showed a statistically significant decrease in systolic and diastolic blood pressure along with decreased body weight in women who followed IF for six weeks [11]. Intermittent fasting has also been shown to have beneficial effects on blood glucose levels, with a very positive result in patients with C-reactive protein levels greater than 1 mg/L, which provides evidence of its effectiveness in reducing inflammatory conditions [12].
In an investigation using a mouse model, intermittent fasting was found to be able to reduce intestinal inflammation, decreasing CD4+ T cells in mesenteric lymph nodes. CD4+ CD25+ regulatory T cells (Treg) play a pivotal role in the regulation of immune responses, thus preventing autoimmunity and inflammatory responses. Treg cells modulate CD8+ T cell differentiation and the effector function by regulating IL-2 homeostasis [13]. In this regard, a report has shown an increase in CD4+ CD25+ regulatory T cells in the mesenteric lymph nodes and a decrease in the infiltration of leukocytes and macrophages around the base of the crypts in the colon. These results indicate that intermittent fasting may have modulatory effects on the immune system and reduce intestinal inflammation [14].
There are few studies that have assessed the mechanisms involved in anti-inflammatory responses induced by intermittent fasting. A short-term fasting study showed decreased monocyte metabolic and inflammatory activity. This study showed that a reduction in the inflammatory response was associated with the regulation of the number of peripheral monocytes, which were dependent on glucose and protein intake. A reduction in the number of monocytes was mediated by the activation of AMPK and PPARα pathways [15]. Another study in animal models has shown that IF reduced the composition of T cells in the lamina propria of the intestine with a reduction in IL-17-producing T cells and an increase in the number of Tregs. These effects also increased the richness of gut bacteria and activated microbial metabolic pathways that modulated systemic immune responses, thus concluding that intermittent fasting has potent immunomodulatory effects that are at least partially mediated by the gut microbiome [16].
Moreover, several observational studies have shown that reducing food intake improves digestive symptoms. However, few bodies of evidence have shown how intermittent fasting can modify symptoms in patients with ulcerative colitis and how this affects markers of inflammation such as CRP and fecal calprotectin [17]. Most human studies have been carried out on the Muslim population during Ramadan. For a month, people fasted during light periods. Studies conducted on this population have shown conflicting data. An additional study on 60 patients with IBD (43 UC, 17 CD) in remission (with no history of infection, perforation, or other comorbidities) showed no significant risks to patients with mild and uncomplicated IBD [18]. Patients with UC also showed a significant decrease in the clinical colitis activity index [19].
A recent study evaluated CRP and fecal calprotectin levels before and after Ramadan in a cohort of 80 patients (60 UC and 20 CD) with no comorbidities. In detail, some of the published data showed that obese subjects, after three weeks of intermittent fasting, had reduced CRP levels of 8 to 5 mg/dL [17]. In another study, fecal CRP and fecal calprotectin levels were observed to be reduced (PCR: 5.3 v/s 5.0 mg/dL) (fecal calprotectin: 163 v/s 218 mg/kg) in patients with ulcerative colitis and Crohn’s disease that had undergone one month of intermittent fasting [18]. Similar results were observed in a group of younger subjects with a significant reduction in CRP from 5 to 2.5 mg/dL after 30 days of intermittent fasting [20]. The following section describes a case report of a 42-year-old female patient with ulcerative colitis who underwent intermittent fasting for two months. The results show a reduction in fecal, calprotectin, and PCR inflammation markers.
2. Case Report
A 42-year-old female patient was diagnosed in 2010 with ulcerative colitis in the remission period. The patient lives in the metropolitan region of Santiago, Chile. The patient was contacted through the Carlos Quintana Foundation for Crohn’s and Ulcerative Colitis. The patient had a daily consumption of mesalazine and aspirin. No allergies were declared. The patient accepted her involvement by signing the informed consent to participate in a pilot study to determine the adherence of patients with inflammatory bowel disease to intermittent fasting (add according to Helsinki regulations, etc.). Figure 1 shows the study evaluations and interventions flow. A group of psychologists evaluated the patient by applying the 5-factor test [21,22]. This test allowed the participants to be classified into adherence profiles according to their personality profiles. The patient was classified in the group with the highest expected adherence. The patient’s general condition was evaluated by means of serological tests and the detection of calprotectin as a marker of intestinal inflammation. Table 1 summarizes the most important findings of the initial and final examination. Briefly, before starting intermittent fasting, the patient showed the following values: hematocrit: 40.4%; hemoglobin 13.8 g/dL; total leukocytes: 7.4 × 103/mL; Calprotectin: 139 mg/Kg and CRP: 3.64 mg/L.
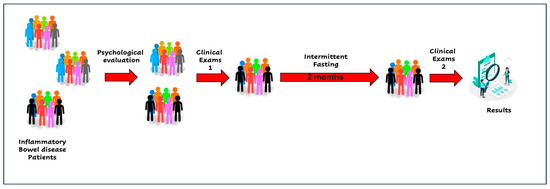
Figure 1.
Intermittent fasting intervention diagram.

Table 1.
Results of clinical evaluation before (Pre-T) and after two months (post-T) of intermittent fasting.
A nutriologist evaluated the patient by authorizing her participation in the study. The intervention consisted of eight weeks of intermittent fasting for 10/14. The day was divided into 10 h for food and 14 h for fasting. The adherence to fasting was determined by a weekly self-report that the patient answered. Patient-reported adherence to intermittent fasting was greater than 96%. The nutritional recommendations given to the patient are shown in Table 1. After eight weeks of intervention, the serological markers and fecal calprotectin were evaluated again. The main findings are shown in Table 2. Briefly, after eight weeks of intermittent fasting, the patient showed the following values: hematocrit: 42.1%; hemoglobin 13.8 g/dL; total leukocytes: 8.6 × 103/mL; Calprotectin: 51 mg/Kg and CRP: 1.57 mg/L. The patient reported decreased inflammation and the absence of other gastrointestinal symptoms. Currently, the patient continues intermittent fasting at 10/14 and is controlled by medical staff.

Table 2.
Meal plan recommendations for intermittent fasting.
3. Discussion
Inflammatory bowel disease (IBD) is described as a chronic inflammation of the gastrointestinal tract, leading to tissue damage, malabsorption, and systemic complications. Ulcerative colitis (UC) is determined by superficial and continuous colon ulceration with rectal complications [23]. IBD patients avoid dietary ingestion to minimize gastrointestinal complications. Psychological stress can predispose patients to disease episodes [24]. Inflammatory bowel disease is also associated with dysbiosis [25]. UC represents a risk for mental health problems, including depression and anxiety-like behaviors [22,25]. Dysbiosis plays a pivotal role in the deregulation of the Brain-GIT axe [25,26]. IF is a group of periodic energy restriction dietary patterns, including alternate-day fasting (ADF), time-restricted fasting (TRF), and intermittent energy restriction (IER) [27].
Previous research has reported that IF has beneficial effects on the compositions of gut microbes in animal models and human trials [28].
The results published by Hu et al. have shown that intermittent fasting generates changes in the microbiota by increasing the number of bacteroides and parabacteroides, which are known to be involved in beneficial effects on health [29]. In addition, enrichment of GABA-producing P. distasonis and B. thetaiotaomicron was observed. This neurotransmitter has been associated with an anti-inflammatory response [30].
Khan’s et al. previous results have shown that IF increases the number of Actinobacteria [31]. Bifidobacteria, which belong to this bacterial phylum, have been described for their importance and their anti-inflammatory response [32]. Supplementation with bifidobacteria has been shown to reduce TNF-α expression in mice, leading to a reduction in inflammatory responses [33]. Bifidobacteria release acetate, which is used by other fermenting bacteria to synthesize butyrate and propionate. These short-chain fatty acids have been shown to have the ability to reduce inflammatory response by promoting and regulating the regulatory T population of the colon [34]. The activity of dendritic cells and T lymphocytes was modulated [35].
Publications have shown, both in animal models and in clinical trials, that intermittent fasting can lead to symptom reduction in young IBD patients [10,11,12,15]. However, few studies have looked at the impact of intermittent fasting in patients with ulcerative colitis and how this affects inflammatory markers, such as the C-reactive protein and fecal calprotectin [28]. Studies have reported that Ramadan fasts are associated with significantly lower concentrations of inflammatory markers, such as CRP, IL-6, and TNF- α [19,20]. This small number of participants limits the results in relation to Ramadan; however, they showed that, in the case of UC, there is a duality of effects. It seems to be more beneficial in younger people when compared to an older population [14,18,36].
In studies in which the CRP and fecal Calprotectin levels have been determined, contradictory results have been shown. Negm et al. analyzed CRP and fecal calprotectin levels in patients with ulcerative colitis. The results showed that after 30 days of intermittent fasting, the mean CRP value increased, on average, from 1.17 to 1.85 mg/L for 60 patients (UC). Fecal calprotectin also increased, on average, from 176 to 218 mg/Kg. Despite the fact that their results were not shown to be statistically significant, it was reported that many patients had to drop out due to increased symptoms [18].
In our clinical case, we observed a significant reduction in CRP from 3.64 to 1.57 mg/L and in fecal calprotectin from 139 to 51 mg/Kg. The patient always showed a reduction in symptoms while performing intermittent fasting for two months with very high adherence (which was determined by weekly self-reports).
El Mountassir et al. observed that fasting was tolerated in 94% of cases. No negative symptoms associated with fasting were reported [37]. Aksunger et al. carried out a study and showed that, after 30 days of intermittent fasting, CRP levels were significantly reduced from 5 to 2.5 mg/L. The authors did not determine the levels of fecal calprotectin [20]. Unalacak et al. showed similar results in obese patients, in which CRP levels were shown to decrease from 2.83 to 2.7 mg/L after 30 days of intermittent fasting [38]. Widhani et al. also showed a significant reduction in CRP in HIV patients during two weeks of intermittent fasting [39].
Interestingly, this case report is one of the few interventional studies performed in humans. It shows how a 42-year-old female patient with ulcerative colitis, in the remission period and without comorbidities, underwent intermittent fasting (10/14) for two months. Clinical markers, including complete blood count, lipid profile, liver profile, C-reactive protein, and fecal calprotectin, showed a reduction in inflammatory processes. These clinical findings correlate with a reduction in the patient’s symptoms. The patient has continued to perform intermittent fasting and is regularly monitored by a medical doctor.
4. Conclusions
In the present study, a 42-year-old patient with UC in remission underwent IF (10/14) for eight weeks. The patient had no problems adhering to intermittent fasting. After two months of fasting, no appearance of gastrointestinal symptoms was reported. A significant reduction in CRP and fecal calprotectin was observed, as previously published by other authors. There was no evidence of an alteration in blood markers as well as alteration in the lipid and hepatic profile.
Although this research has limitations, such as the follow-up time that restricted the ability to observe long-term changes in health markers, lipid, and liver profiles, several aspects are presented that can be considered novel. One of these precisely includes the evaluation of multiple clinical variables with a comparison before and after the period of intermittent fasting, accompanied by the intake recommendations based on easily accessible foods that ensured sources of vitamins and minerals, as well as elements with anti-inflammatory properties. Another contribution of this research is related to the fact that it is a study in a specific population of which there is little information regarding the effectiveness of intermittent fasting, such as Chilean women. The Latin American demographic group, especially the Chilean population, has not been widely studied in terms of the effectiveness of intermittent fasting; therefore, this research is a relevant contribution as a source of background information regarding this demographic group.
In general terms, it can be noted that these findings suggest that intermittent fasting may be a safe and viable strategy to improve health in Chilean women since no adverse effects on liver function or lipid metabolism were found. However, further studies are needed. In this regard, additional long-term randomized controlled studies are required to confirm these findings and assess the effects of intermittent fasting diets in different populations and in combination with other interventions and dietary recommendations. Research is also required to understand the underlying mechanisms of how the intermittent fasting diet affects health and lipid and liver profiles in relation to the diet of the Latin American population and especially the Chilean population.
Author Contributions
Conceptualization: C.V.-A. and Á.R.-V.; clinical evaluation, D.M.-P.; development and application of psychological assessment: T.C.-A.; data curation, C.V.-A. and C.P.-A.; writing—original draft preparation, C.V.-A., C.P.-A., Á.R.-V. and M.B.-O.; writing—proofreading and editing, C.V.-A., C.P.-A. and Á.R.-V.; supervision, C.V.-A.; fund acquisition, Á.R.-V. and M.B.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Regular Research Project Contest”, grant number “DI-04/23”, entitled “Prevalence of polymorphisms rs1861868-FTO and rs7975232-VDR in Chilean women and their association with BMI, anthropometry, and cardiovascular risk factors according to the consumption of estrogen-based contraceptives”, awarded by the Research Department of the Universidad de las Américas, Providencia, Chile.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethical Committee of the Central Area of Integramedica (Prot n° 168; 20 September 2021).
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Veauthier, B.; Hornecker, J.R. Crohn’s Disease: Diagnosis and Management. Am. Fam. Physician 2018, 98, 661–669. [Google Scholar] [PubMed]
- Pagnini, C.; Pizarro, T.T.; Cominelli, F. Novel Pharmacological Therapy in Inflammatory Bowel Diseases: Beyond Anti-Tumor Necrosis Factor. Front. Pharmacol. 2019, 10, 671. [Google Scholar] [CrossRef]
- Alfredsson, J.; Wick, M.J. Mechanism of fibrosis and stricture formation in Crohn’s disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef] [PubMed]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mandi, Y.; Stone, T.W.; Guillemin, G.J.; Vecsei, L.; Williams, R.O. Editorial: Multiple Implications of the Kynurenine Pathway in Inflammatory Diseases: Diagnostic and Therapeutic Applications. Front. Immunol. 2022, 13, 860867. [Google Scholar] [CrossRef]
- Wu, J.; Man, D.; Shi, D.; Wu, W.; Wang, S.; Wang, K.; Li, Y.; Yang, L.; Bian, X.; Wang, Q.; et al. Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome. Nutrients 2022, 14, 5311. [Google Scholar] [CrossRef]
- Mushtaq, R.; Akram, A.; Mushtaq, R.; Khwaja, S.; Ahmed, S. The role of inflammatory markers following Ramadan Fasting. Pak. J. Med. Sci. 2019, 35, 77–81. [Google Scholar] [CrossRef]
- Rahbar, A.R.; Safavi, E.; Rooholamini, M.; Jaafari, F.; Darvishi, S.; Rahbar, A. Effects of intermittent fasting during ramadan on insulin-like growth factor-1, interleukin 2, and lipid profile in healthy muslims. Int. J. Prev. Med. 2019, 10, 7. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Eshghinia, S.; Mohammadzadeh, F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J. Diabetes Metab. Disord. 2013, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; Hill, G.R.; Sparwasser, T.; Thomas, R.; Steptoe, R.J. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc. Natl. Acad. Sci. USA 2011, 108, 7529–7534. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, L.; Bai, M.; Wang, S.; Ye, X.; Lin, Y.; Luo, X.; Li, Z.; Zhang, L.; Zhu, X.; et al. Time-restricted feeding ameliorates dextran sulfate sodium-induced colitis via reducing intestinal inflammation. Front. Nutr. 2022, 9, 1043783. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114.e17. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Mi-crobiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535, Erratum in Redox Biol. 2021, 44, 101955. [Google Scholar] [CrossRef] [PubMed]
- Negm, M.; Bahaa, A.; Farrag, A.; Lithy, R.M.; Badary, H.A.; Essam, M.; Kamel, S.; Sakr, M.A.B.D.; El Aaty, W.; Shamkh, M.; et al. Effect of Ramadan intermittent fasting on inflammatory markers, disease severity, depression, and quality of life in patients with inflammatory bowel diseases: A prospective cohort study. BMC Gastroenterol. 2022, 22, 203. [Google Scholar] [CrossRef]
- Tavakkoli, H.; Haghdani, S.; Emami, M.H.; Adilipour, H.; Tavakkoli, M.; Tavakkoli, M. Ramadan fasting and inflammatory bowel disease. Indian. J. Gastroenterol. 2008, 27, 239–241. [Google Scholar]
- Aksungar, F.B.; Topkaya, A.E.; Akyildiz, M. Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting. Ann. Nutr. Metab. 2007, 51, 88–95. [Google Scholar] [CrossRef]
- Calabrese, W.R.; Rudick, M.M.; Simms, L.J.; Clark, L.A. Development and validation of Big Four personality scales for the Schedule for Nonadaptive and Adaptive Personality—Second Edition (SNAP-2). Psychol. Assess. 2012, 24, 751–763. [Google Scholar] [CrossRef]
- Olivares-Tirado, P.; Leyton, G.; Salazar, E. Personality factors and self-perceived health in Chilean elderly population. Health 2013, 5, 86–96. [Google Scholar] [CrossRef]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.M.; Lewis, J.D.; Mayer, E.A.; Plevy, S.E.; Chuang, E.; Rappaport, S.M.; Croitoru, K.; Korzenik, J.R.; Krischer, J.; Hyams, J.S.; et al. Challenges in IBD research: Environmental triggers. Inflamm. Bowel Dis. 2019, 25, S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Navabi, V.S.; Gorrepati, S.; Yadav, J.; Chintanaboina, S.; Maher, P.; Demuth, B.; Stern, A.; Stuart, A.; Tinsley, K.; Clarke, E.D.; et al. Coates, Influences and impact of anxiety and depression in the setting of inflammatory bowel disease. Inflamm. Bowel Dis. 2018, 24, 2303–2308. [Google Scholar] [CrossRef]
- Neuendorf, A.; Harding, N.; Stello, D.; Hanes, H. Wahbeh, Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J. Psychosom. Res. 2016, 87, 70–80. [Google Scholar] [CrossRef]
- Szebeni, G.B.; Veres, A.; Dezsõfi, K.; Rusai, A. Arató,IncreasedexpressionofToll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin. Exp. Immunol. 2008, 151, 34–41. [Google Scholar] [CrossRef]
- Hu, X.; Xia, K.; Dai, M.; Duan, S. Intermittent fasting modulates the intestinal microbiota and improves obesity and host energy metabolism. Npj Biofilms Microbiomes 2023, 9, 19. [Google Scholar] [CrossRef]
- Bhandage, A.K.; Jin, Z.; Korol, S.V.; Shen, Q.; Pei, Y.; Deng, Q.; Espes, D.; Carlsson, P.O.; Kamali-Moghaddam, M.; Birnir, B. GABA Regulates Release of Inflammatory Cytokines from Peripheral Blood Mononuclear Cells and CD4+ T Cells and Is Immunosuppressive in Type 1 Diabetes. EBioMedicine 2018, 30, 283–294. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, S.I.; Rana, M.I.; Ayyaz, A.; Khan, M.Y.; Imran, M. Intermittent fasting positively modulates human gut microbial diversity and ameliorates blood lipid profile. Front. Microbiol. 2022, 13, 922727. [Google Scholar] [CrossRef]
- de Souza, C.H.B.; Gioielli, L.A.; Saad, S.M.I. Inulin increases Bifidobacterium animalis Bb-12 in vitro gastrointestinal resistance in margarine. LWT Food Sci. Technol. 2017, 79, 205–212. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, K.E.; Kim, J.K.; Kim, D.H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019, 9, 11814. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Alexander, Y.R. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.A.; Kacimi, S.; Al-Kurd, R.A.; Yasser, K.B. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr. Res. 2012, 32, 947–955. [Google Scholar] [CrossRef] [PubMed]
- El Mountassir, M.; Benelbarhdadi, I.; Borahma, M.; Ajana, F. Impact of Ramadan on Crohn’s disease. Gastroenterol. Hepatol. Endosc. 2021, 6, 1000217. [Google Scholar] [CrossRef]
- Unalacak, M.; Kara, I.H.; Baltaci, D.; Erdem, O.; Bucaktepe, P.G. Effects of Ramadan fasting on biochemical and hematological parameters and cytokines in healthy and obese individuals. Metab. Syndr. Relat. Disord. 2011, 9, 157–161. [Google Scholar] [CrossRef]
- Widhani, A.; Yunihastuti, E.; Setiati, S.; Witjaksono, F.; Karjadi, T.H. Ramadan fasting reduces high-sensitivity C-reactive protein among HIV-infected patients receiving antiretroviral therapy. Front. Nutr. 2023, 9, 964797. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).