Epidemiology and Antimicrobial Resistance Patterns of Urinary Tract Infections: A Cross-Sectional Study from Southwestern Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size, Inclusion Criteria, and Exclusion Criteria
2.2. Data Collection
2.3. Urine Collection and Analysis
2.4. Antibiotic Susceptibility Test
2.5. Ethical Approval
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alrasheedy, M.; Abousada, H.J.; Abdulhaq, M.M.; Alsayed, R.A.; Alghamdi, K.A.; Alghamdi, F.D.; Al Muaibid, A.F.; Ajjaj, R.G.; Almohammadi, S.S.; Almohammadi, S.S.; et al. Prevalence of Urinary Tract Infection in Children in the Kingdom of Saudi Arabia. Arch. Ital. Urol. Androl. 2021, 93, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.A.; Sadoma, H.H.M.; Mathew, S.; Alghamdi, S.; Malik, J.A.; Anwar, S. Retrospective Analysis of Antimicrobial Susceptibility of Uropathogens Isolated from Pediatric Patients in Tertiary Hospital at Al-Baha Region, Saudi Arabia. Healthcare 2021, 9, 1564. [Google Scholar] [CrossRef] [PubMed]
- Alqasim, A.; Abu Jaffal, A.; Alyousef, A.A. Prevalence of Multidrug Resistance and Extended-Spectrum β-Lactamase Carriage of Clinical Uropathogenic Escherichia coli Isolates in Riyadh, Saudi Arabia. Int. J. Microbiol. 2018, 2018, 3026851. [Google Scholar] [CrossRef] [PubMed]
- Target Product Profiles for Oral Therapy of Urinary Tract Infections. Available online: https://www.who.int/publications/i/item/9789240003873 (accessed on 28 July 2023).
- CDC Urinary Tract Infection. Available online: https://www.cdc.gov/antibiotic-use/uti.html (accessed on 28 July 2023).
- Choe, H.S.; Lee, S.J.; Cho, Y.H.; Çek, M.; Tandoğdu, Z.; Wagenlehner, F.; Bjerklund-Johansen, T.E.; Naber, K.; GPIU Asian Investigators. Aspects of urinary tract infections and antimicrobial resistance in hospitalized urology patients in Asia: 10-Year results of the Global Prevalence Study of Infections in Urology (GPIU). J. Infect. Chemother. 2018, 24, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Belete, M.A.; Saravanan, M. A Systematic Review on Drug Resistant Urinary Tract Infection Among Pregnant Women in Developing Countries in Africa and Asia; 2005–2016. Infect. Drug Resist. 2020, 13, 1465–1477. [Google Scholar] [CrossRef]
- Abalkhail, A.; AlYami, A.S.; Alrashedi, S.F.; Almushayqih, K.M.; Alslamah, T.; Alsalamah, Y.A.; Elbehiry, A. The Prevalence of Multidrug-Resistant Escherichia coli Producing ESBL among Male and Female Patients with Urinary Tract Infections in Riyadh Region, Saudi Arabia. Healthcare 2022, 10, 1778. [Google Scholar] [CrossRef]
- Sula, I.; Alreshidi, M.A.; Alnasr, N.; Hassaneen, A.M.; Saquib, N. Urinary Tract Infections in the Kingdom of Saudi Arabia, a Review. Microorganisms 2023, 11, 952. [Google Scholar] [CrossRef]
- Alasmary, M.Y. Antimicrobial Resistance Patterns and ESBL of Uropathogens Isolated from Adult Females in Najran Region of Saudi Arabia. Clin. Pract. 2021, 11, 650–658. [Google Scholar] [CrossRef]
- Balkhi, B.; Mansy, W.; AlGhadeer, S.; Alnuaim, A.; Alshehri, A.; Somily, A. Antimicrobial Susceptibility of Microorganisms Causing Urinary Tract Infections in Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 220–227. [Google Scholar] [CrossRef]
- Alanazi, M.Q. An Evaluation of Community-Acquired Urinary Tract Infection and Appropriateness of Treatment in an Emergency Department in Saudi Arabia. Ther. Clin. Risk Manag. 2018, 14, 2363–2373. [Google Scholar] [CrossRef]
- Alhomayani, F.K.; Alazwari, N.M.; Alshhrani, M.S.; Alkhudaydi, A.S.; Basaba, A.S.; Alharthi, T.M.; Alghamdi, M.M.; Aljuaid, A.S.; Alosimi, N.M.; Alqethami, A.M. The Prevalence of Multiple Drug Resistant Urinary Tract Infections: A Single-Centered, Observational Retrospective Study in King Abdulaziz Specialized Hospital, Taif, Saudi Arabia: A Single-Centered, Observational Retrospective Study in King Abdulaziz Specialized Hospital, Taif, Saudi Arabia. Saudi Med. J. 2022, 43, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A.; Abuallut, I.; Alwadani, I.; Haddad, M.; Ageeli, B.; Majrabi, H.; Muslihi, I.; AlAli, L.; Homadi, H.; Madkhli, E.; et al. Neonatal Healthcare-Associated Conjunctivitis: A Descriptive Study from Saudi Arabia. Medicina 2022, 58, 1448. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, A.R.; Mobley, H.L.T. Preventing Urinary Tract Infection: Progress toward an Effective Escherichia coli Vaccine. Expert Rev. Vaccines 2012, 11, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Fenta, A.; Dagnew, M.; Eshetie, S.; Belachew, T. Bacterial Profile, Antibiotic Susceptibility Pattern and Associated Risk Factors of Urinary Tract Infection among Clinically Suspected Children Attending at Felege-Hiwot Comprehensive and Specialized Hospital, Northwest Ethiopia. A Prospective Study. BMC Infect. Dis. 2020, 20, 673. [Google Scholar] [CrossRef]
- Rosello, A.; Pouwels, K.B.; Domenech de Cellès, M.; Van Kleef, E.; Hayward, A.C.; Hopkins, S.; Robotham, J.V.; Smieszek, T.; Opatowski, L.; Deeny, S.R. Seasonality of Urinary Tract Infections in the United Kingdom in Different Age Groups: Longitudinal Analysis of The Health Improvement Network (THIN). Epidemiol. Infect. 2018, 146, 37–45. [Google Scholar] [CrossRef]
- Rajabnia, M.; Forghani, M.S.; Hasani, S.; Bahadoram, M.; Mohammadi, M.; Barahman, M. Prevalence and Antibiotic Resistance Pattern of Extended Spectrum Beta Lactamase Producing Escherichia coli Isolated from Urinary Tract Infection. J. Renal Inj. Prev. 2018, 8, 78–81. [Google Scholar] [CrossRef]
- Shields, R.K.; Zhou, Y.; Kanakamedala, H.; Cai, B. Burden of Illness in US Hospitals Due to Carbapenem-Resistant Gram-Negative Urinary Tract Infections in Patients with or without Bacteraemia. BMC Infect. Dis. 2021, 21, 572. [Google Scholar] [CrossRef]
- Martin, D.; Fougnot, S.; Grobost, F.; Thibaut-Jovelin, S.; Ballereau, F.; Gueudet, T.; de Mouy, D.; Robert, J.; Alexandre, F.; Andorin, P.; et al. Prevalence of Extended-Spectrum Beta-Lactamase Producing Escherichia coli in Community-Onset Urinary Tract Infections in France in 2013. J. Infect. 2016, 72, 201–206. [Google Scholar] [CrossRef]
- Alsohaim, S.I.A.; Bawadikji, A.A.; Elkalmi, R.; Mahmud, M.I.A.-D.M.; Hassali, M.A. Relationship between Antimicrobial Prescribing and Antimicrobial Resistance among UTI Patients at Buraidah Central Hospital, Saudi Arabia. J. Pharm. Bioallied Sci. 2019, 11, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Al Hamdan, A.S.; Alghamdi, A.A.; Alyousif, G.F.; Hamza, F.A.; Shafey, M.M.; AlAmri, A.M.; Sunki, A.A. Evaluating the Prevalence and the Risk Factors of Gram-Negative Multi-Drug Resistant Bacteria in Eastern Saudi Arabia. Infect. Drug Resist. 2022, 15, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Aloush, V.; Navon-Venezia, S.; Seigman-Igra, Y.; Cabili, S.; Carmeli, Y. Multidrug-Resistant Pseudomonas aeruginosa : Risk Factors and Clinical Impact. Antimicrob. Agents Chemother. 2006, 50, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Baig, K. Incidence of Hospital Acquired Multidrug Resistant Organisms in a Tertiary Care Facility. J. Infect. Dis. Epidemiol. 2015, 1, 4. [Google Scholar] [CrossRef]
- Al-Zahrani, I.A.; Alsiri, B.A. The emergence of carbapenem-resistant Klebsiella pneumoniae isolates producing OXA-48 and NDM in the Southern (Asir) province, Saudi Arabia. Saudi Med. J. 2018, 39, 23–30. [Google Scholar] [CrossRef]
- Barrios-Villa, E.; Mendez-Pfeiffer, P.; Valencia, D.; Caporal-Hernandez, L.; Ballesteros-Monrreal, M.G. Intracellular bacterial communities in patient with recurrent urinary tract infection caused by Staphylococcus spp and Streptococcus agalactiae: A case report and literature review. Afr. J. Urol. 2022, 28, 46. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Arenas-Hernández, M.M.P.; Barrios-Villa, E.; Juarez, J.; Álvarez-Ainza, M.L.; Taboada, P.; De la Rosa-López, R.; Bolado-Martínez, E.; Valencia, D. Bacterial Morphotypes as Important Trait for Uropathogenic E. coli Diagnostic; a Virulence-Phenotype-Phylogeny Study. Microorganisms 2021, 9, 2381. [Google Scholar] [CrossRef]
| Variable | n | % | |
|---|---|---|---|
| Gender | Male | 404 | 37.34 |
| Female | 678 | 62.66 | |
| Location | Clinic | 306 | 28.28 |
| ER | 443 | 40.94 | |
| ICU | 107 | 9.89 | |
| Ward | 226 | 20.89 | |
| Nationality | Saudi | 903 | 83.46 |
| Non-Saudi | 179 | 16.54 | |
| Origin | ND | 471 | 43.53 |
| CAI | 461 | 42.61 | |
| HAI | 150 | 13.86 | |
| Alert | No alert | 711 | 65.71 |
| CRE | 21 | 1.94 | |
| ESBL | 326 | 30.13 | |
| MDRO | 14 | 1.29 | |
| MRSA | 8 | 0.74 | |
| VRE | 2 | 0.18 | |
| Gram stain | Gram-negative | 1015 | 93.81 |
| Gram-positive | 67 | 6.19 | |
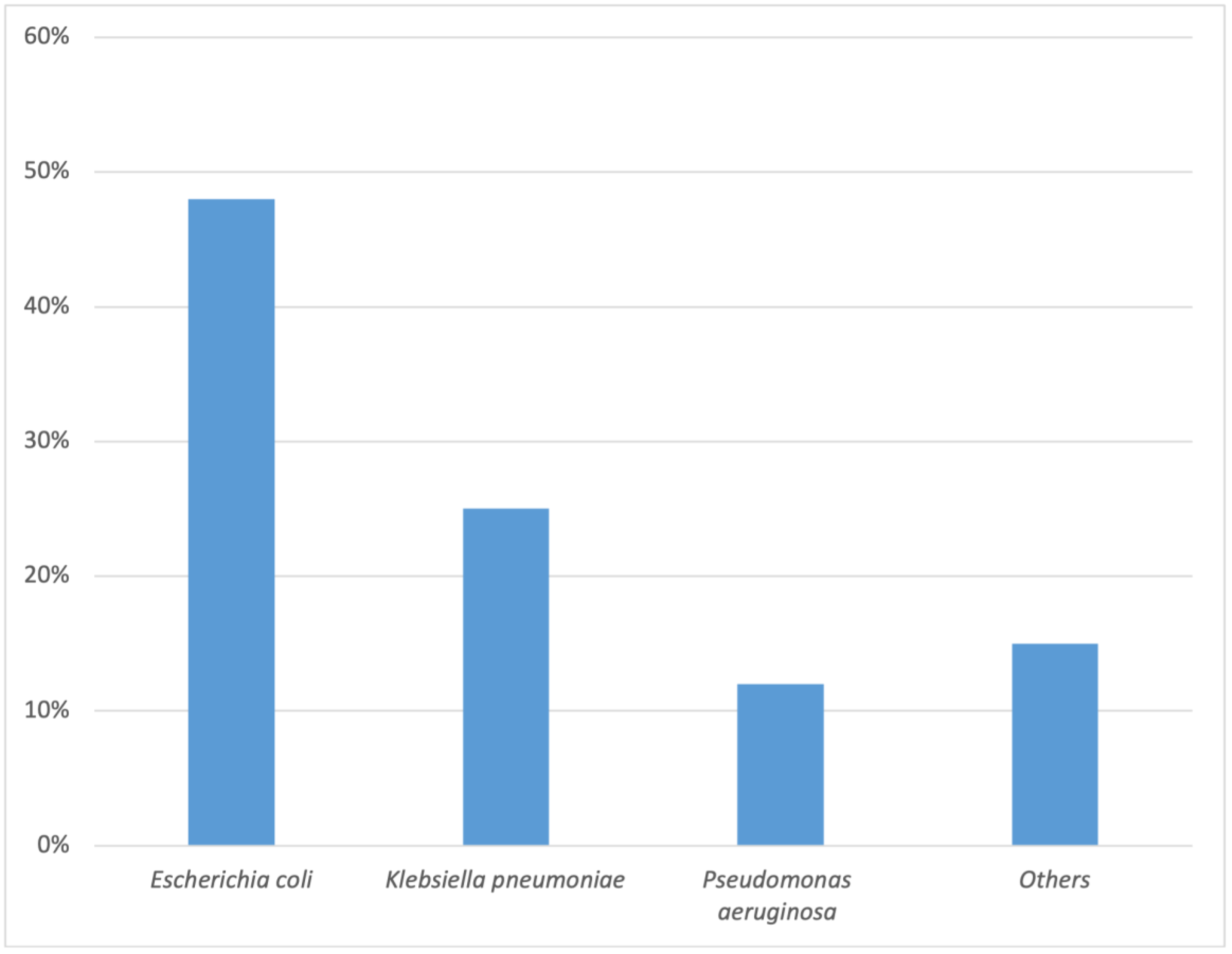
| Organism | Escherichia coli | 519 | 47.97 |
| Klebsiella pneumoniae | 266 | 24.58 | |
| Pseudomonas aeruginosa | 125 | 11.55 | |
| Enterobacter cloacae | 28 | 2.59 | |
| Enterococcus faecalis | 16 | 1.48 | |
| Staphylococcus aureus | 15 | 1.39 | |
| Proteus mirabilis | 14 | 1.29 | |
| Streptococcus agalactiae | 20 | 1.85 | |
| Acinetobacter baumannii | 12 | 1.11 | |
| Others | 67 | 6.19 | |
| Variable | Gram-Negative Bacteria (n = 1015, 94%) | Gram Positive Bacteria (n = 67, 6%) | p Value | |||
|---|---|---|---|---|---|---|
| Gender | Male | 374 | 36.85% | 30 | 44.78% | 0.195 |
| Female | 641 | 63.15% | 37 | 55.22% | ||
| Location | Clinic | 289 | 28.47% | 17 | 25.37% | 0.302 |
| ER | 414 | 40.79% | 29 | 43.28% | ||
| ICU | 104 | 10.25% | 3 | 4.48% | ||
| WARD | 208 | 20.49% | 18 | 26.87% | ||
| Nationality | Saudi | 844 | 83.15% | 59 | 88.06% | 0.395 |
| Non-Saudi | 171 | 16.85% | 8 | 11.94% | ||
| Origin | ND | 443 | 43.65% | 28 | 41.79% | 0.578 |
| CAI | 429 | 42.27% | 32 | 47.76% | ||
| HAI | 143 | 14.09% | 7 | 10.45% | ||
| Variable | Gram-Negative without Reported MDRO (n = 654, 64%) | Gram-Negative with Reported MDRO (n = 361, 36%) | p-Value | |||
|---|---|---|---|---|---|---|
| Gender | Male | 236 | 23.25% | 128 | 12.61% | 0.498 |
| Female | 418 | 41.18% | 223 | 21.97% | ||
| Location | Clinic | 197 | 19.41% | 92 | 9.06% | 0.167 |
| ER | 270 | 26.60% | 144 | 14.19% | ||
| ICU | 65 | 6.40% | 39 | 3.84% | ||
| WARD | 122 | 12.02% | 86 | 8.47% | ||
| Nationality | Saudi | 534 | 52.61% | 310 | 30.54% | 0.096 |
| Non-Saudi | 120 | 11.82% | 51 | 5.02% | ||
| Origin | ND | 347 | 34.19% | 96 | 9.46% | 0.0001 * |
| CAI | 246 | 24.24% | 183 | 18.03% | ||
| HAI | 62 | 6.11% | 82 | 8.08% | ||
| Organism | Escherichia coli | 287 | 88.04% | 232 | 34.42% | 0.0001 * |
| Klebsiella pneumoniae | 172 | 52.76% | 94 | 13.95% | ||
| Pseudomonas aeruginosa | 112 | 34.36% | 11 | 1.63% | ||
| Others | 16 | 4.91% | 12 | 1.78% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhazmi, A.H.; Alameer, K.M.; Abuageelah, B.M.; Alharbi, R.H.; Mobarki, M.; Musawi, S.; Haddad, M.; Matabi, A.; Dhayhi, N. Epidemiology and Antimicrobial Resistance Patterns of Urinary Tract Infections: A Cross-Sectional Study from Southwestern Saudi Arabia. Medicina 2023, 59, 1411. https://doi.org/10.3390/medicina59081411
Alhazmi AH, Alameer KM, Abuageelah BM, Alharbi RH, Mobarki M, Musawi S, Haddad M, Matabi A, Dhayhi N. Epidemiology and Antimicrobial Resistance Patterns of Urinary Tract Infections: A Cross-Sectional Study from Southwestern Saudi Arabia. Medicina. 2023; 59(8):1411. https://doi.org/10.3390/medicina59081411
Chicago/Turabian StyleAlhazmi, Abdulaziz H., Khalid M. Alameer, Bandar M. Abuageelah, Rena H. Alharbi, Mousa Mobarki, Shaqraa Musawi, Moayad Haddad, Abdullatif Matabi, and Nabil Dhayhi. 2023. "Epidemiology and Antimicrobial Resistance Patterns of Urinary Tract Infections: A Cross-Sectional Study from Southwestern Saudi Arabia" Medicina 59, no. 8: 1411. https://doi.org/10.3390/medicina59081411
APA StyleAlhazmi, A. H., Alameer, K. M., Abuageelah, B. M., Alharbi, R. H., Mobarki, M., Musawi, S., Haddad, M., Matabi, A., & Dhayhi, N. (2023). Epidemiology and Antimicrobial Resistance Patterns of Urinary Tract Infections: A Cross-Sectional Study from Southwestern Saudi Arabia. Medicina, 59(8), 1411. https://doi.org/10.3390/medicina59081411





