Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases
Abstract
1. Introduction
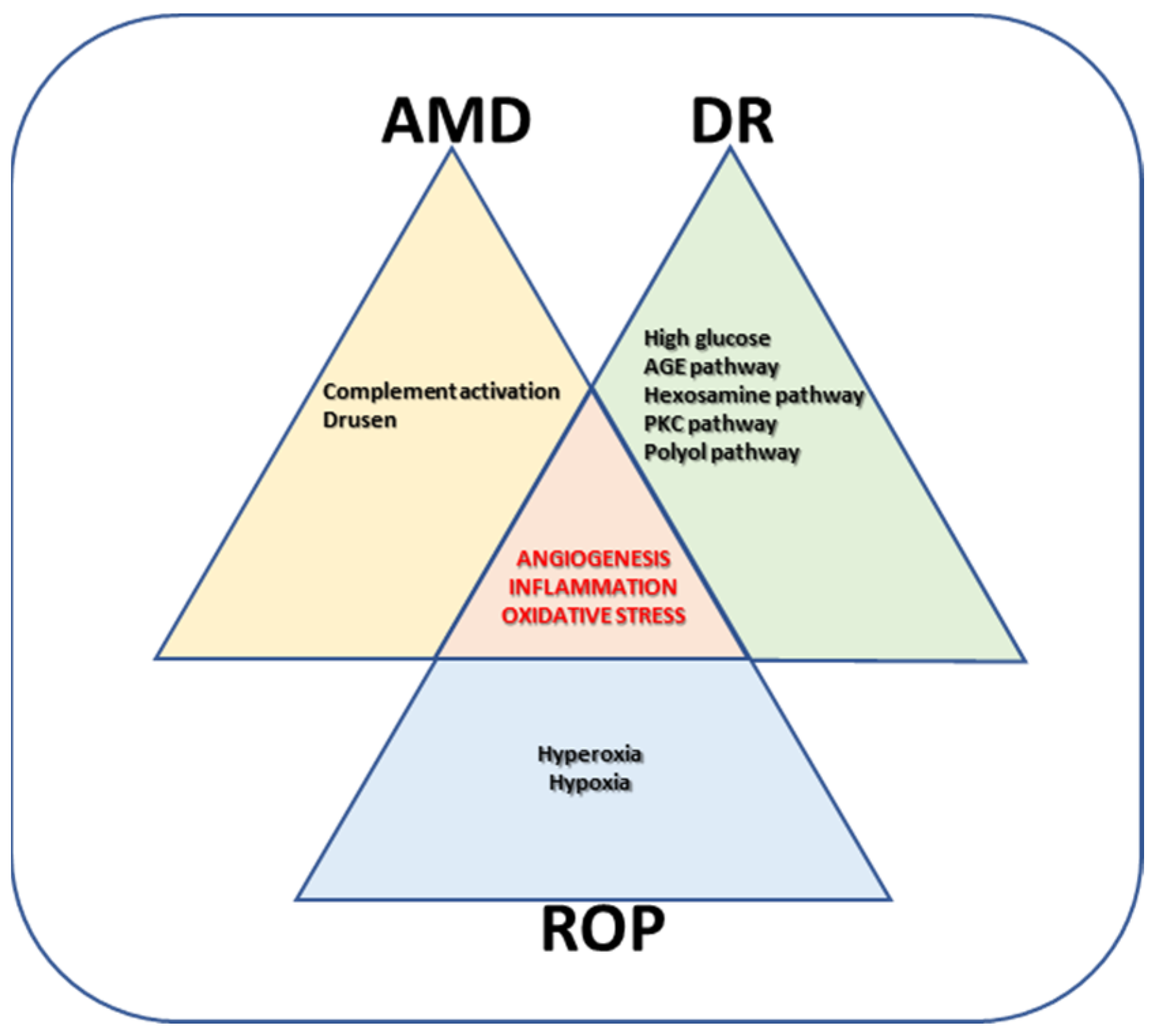
2. Proliferative Retinopathies
3. Animal Models
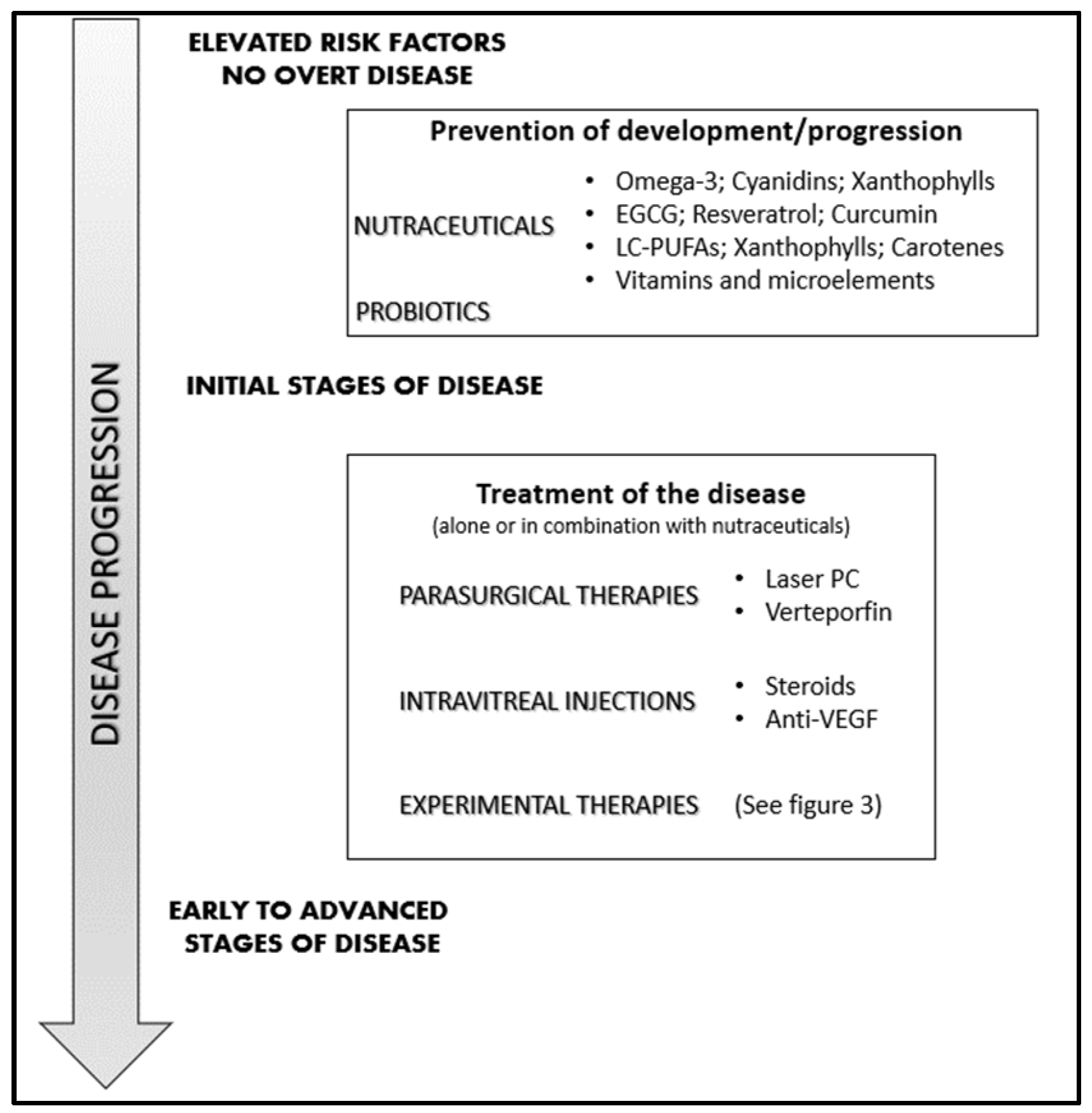
4. Management of Neovascular Retinal Diseases
4.1. Laser Photocoagulation and Vitrectomy
4.2. Photodynamic Therapy
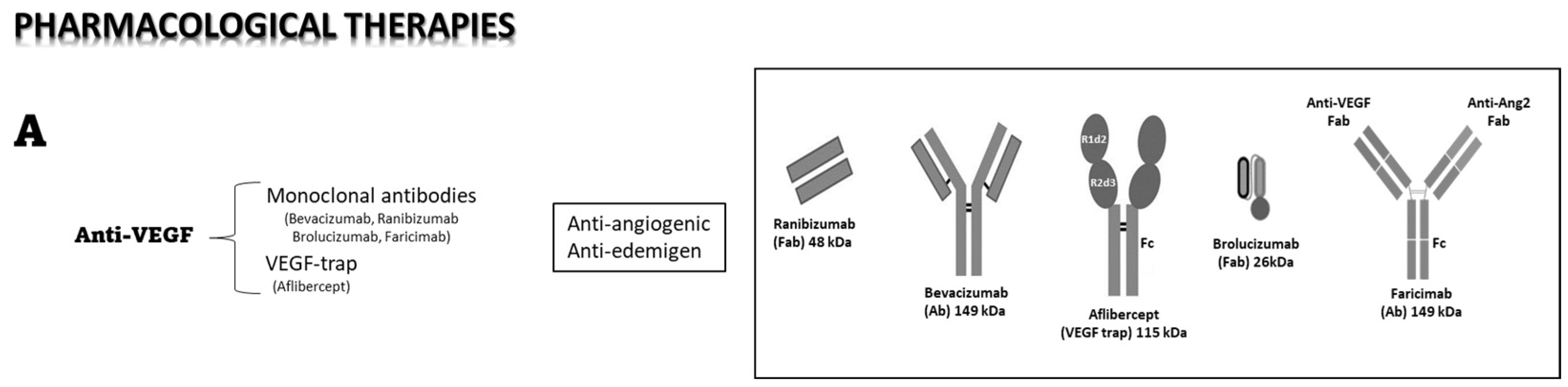
4.3. Pharmacological Therapies
4.4. Intraocular Anti-VEGF Therapy
4.5. Steroid Intravitreal Implants
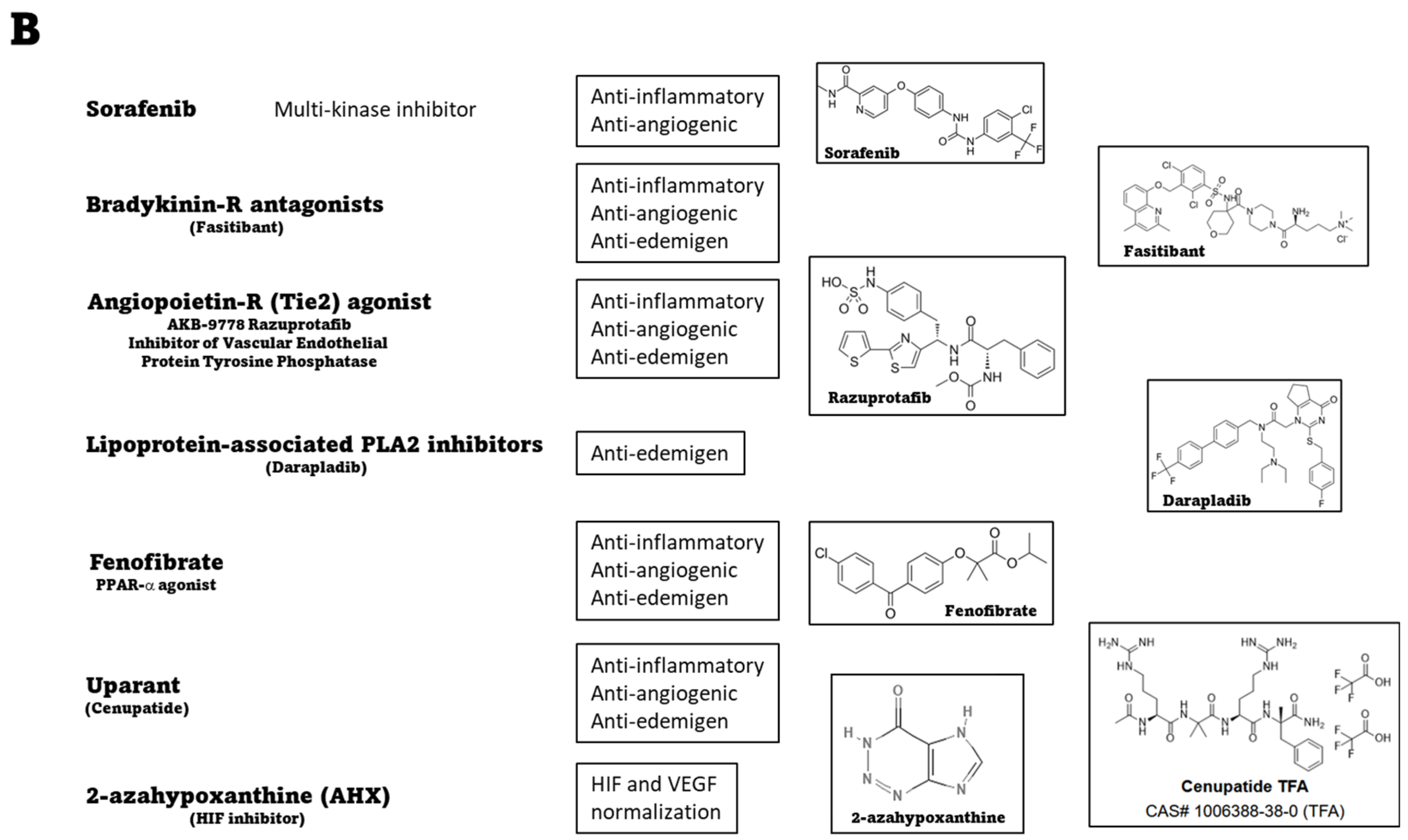
4.6. Systemic Therapies: Preclinical Evidence of Novel Treatments
5. Nutraceuticals: Which Place in the Management of Neovascular Eye Diseases?
5.1. Association #1
5.2. Association #2
5.3. Association #3
5.4. Further Elements in the Associations
5.5. Role of the Gut’s Microbiota
5.6. Final Considerations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Loscalzo, J.; Kohane, I.; Barabasi, A.L. Human disease classification in the postgenomic era: A complex systems approach to human pathobiology. Mol. Syst. Biol. 2007, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Santini, A.; Novellino, E. To Nutraceuticals and Back: Rethinking a Concept. Foods 2017, 6, 74. [Google Scholar] [CrossRef]
- Horowitz, A.; Brennan, M.; Reinhardt, J.P. Prevalence and risk factors for self-reported visual impairment among middle-aged and older adults. Res. Aging 2005, 27, 307–326. [Google Scholar] [CrossRef]
- Johnson, G.J.; Minassian, D.C.; Weale, R.A.; West, S.K. (Eds.) Epidemiology of Eye Disease, 3rd ed.; World Scientific: Singapore, 2012. [Google Scholar]
- Khoo, H.E.; Ng, H.S.; Yap, W.S.; Goh, H.J.H.; Yim, H.S. Nutrients for Prevention of Macular Degeneration and Eye-Related Diseases. Antioxidants 2019, 8, 85. [Google Scholar] [CrossRef]
- Choo, P.P.; Woi, P.J.; Bastion, M.C.; Omar, R.; Mustapha, M.; Din, N. Review of Evidence for the Usage of Antioxidants for Eye Aging. Biomed. Res. Int. 2022, 2022, 5810373. [Google Scholar] [CrossRef]
- Kim, D.; Choi, S.W.; Cho, J.; Been, J.H.; Choi, K.; Jiang, W.; Han, J.; Oh, J.; Park, C.; Choi, S.; et al. Discovery of Novel Small-Molecule Antiangiogenesis Agents to Treat Diabetic Retinopathy. J. Med. Chem. 2021, 64, 5535–5550. [Google Scholar] [CrossRef]
- Kumar Dubey, S.; Pradhan, R.; Hejmady, S.; Singhvi, G.; Choudhury, H.; Gorain, B.; Kesharwani, P. Emerging innovations in nano-enabled therapy against age-related macular degeneration: A paradigm shift. Int. J. Pharm. 2021, 600, 120499. [Google Scholar] [CrossRef]
- EUROSTAT. 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Preventive_health_care_expenditure_statistics#Among_EU_Member_States.2C_spending_on_preventive_healthcare_ranged_between_1.0_.25_and_5.6_.25_of_current_healthcare_expenditure_in_2020. (accessed on 10 March 2023).
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef]
- Eichler, W.; Yafai, Y.; Wiedemann, P.; Fengler, D. Antineovascular agents in the treatment of eye diseases. Curr. Pharm. Des. 2006, 12, 2645–2660. [Google Scholar] [CrossRef]
- Morbidelli, L.; Terzuoli, E.; Donnini, S. Use of Nutraceuticals in Angiogenesis-Dependent Disorders. Molecules 2018, 23, 2676. [Google Scholar] [CrossRef]
- Caldwell, R.B.; Bartoli, M.; Behzadian, M.A.; El-Remessy, A.E.; Al-Shabrawey, M.; Platt, D.H.; Caldwell, R.W. Vascular endothelial growth factor and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Diabetes Metab. Res. Rev. 2003, 19, 442–455. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Hietala, K.; Harjutsalo, V.; Forsblom, C.; Summanen, P.; Groop, P.H.; Finndlane Study Group. Age at onset and the risk of proliferative retinopathy in type 1 diabetes. Diabetes Care 2010, 33, 1315–1319. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, L.; Jia, P.; Xin, Z.; Yang, J.K. Early Onset Age Increased the Risk of Diabetic Retinopathy in Type 2 Diabetes Patients with Duration of 10-20 Years and HbA1C ≥7%: A Hospital-Based Case-Control Study. Int. J. Endocrinol. 2021, 2021, 5539654. [Google Scholar] [CrossRef]
- Gange, W.S.; Lopez, J.; Xu, B.Y.; Lung, K.; Seabury, S.A.; Toy, B.C. Incidence of Proliferative Diabetic Retinopathy and Other Neovascular Sequelae at 5 Years Following Diagnosis of Type 2 Diabetes. Diabetes Care 2021, 44, 2518–2526. [Google Scholar] [CrossRef]
- Im, J.H.B.; Jin, Y.P.; Chow, R.; Yan, P. Prevalence of diabetic macular edema based on optical coherence tomography in people with diabetes: A systematic review and meta-analysis. Surv. Ophthalmol. 2022, 67, 1244–1251. [Google Scholar] [CrossRef]
- Kokotas, H.; Grigoriadou, M.; Petersen, M.B. Age-related macular degeneration: Genetic and clinical findings. Clin. Chem. Lab. Med. 2011, 49, 601–616. [Google Scholar] [CrossRef]
- Silvestri, G.; Williams, M.A.; McAuley, C.; Oakes, K.; Sillery, E.; Henderson, D.C.; Ferguson, S.; Silvestri, V.; Muldrew, K.A. Drusen prevalence and pigmentary changes in Caucasians aged 18-54 years. Eye 2012, 26, 1357–1362. [Google Scholar] [CrossRef]
- de Jong, S.; Tang, J.; Clark, S.J. Age-related macular degeneration: A disease of extracellular complement amplification. Immunol. Rev. 2022, 313, 279–297. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Hubbell, M.; Jairam, P.; Ambati, B. Neovascular Macular Degeneration: A Review of Etiology, Risk Factors, and Recent Advances in Research and Therapy. Int. J. Mol. Sci. 2021, 22, 1170. [Google Scholar] [CrossRef] [PubMed]
- Gorin, M.B.; daSilva, M.J. Predictive genetics for AMD: Hype and hopes for genetics-based strategies for treatment and prevention. Exp. Eye Res. 2020, 191, 107894. [Google Scholar] [CrossRef] [PubMed]
- Csader, S.; Korhonen, S.; Kaarniranta, K.; Schwab, U. The Effect of Dietary Supplementations on Delaying the Progression of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4273. [Google Scholar] [CrossRef] [PubMed]
- Vinekar, A.; Gangwe, A.; Agarwal, S.; Kulkarni, S.; Azad, R. Improving Retinopathy of Prematurity Care: A Medico-Legal Perspective. Asia Pac. J. Ophthalmol. 2021, 10, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.C.; Holm, M.; Austeng, D.; Morken, T.S.; Zhou, T.E.; Beaudry-Richard, A.; Sierra, E.M.; Dammann, O.; Chemtob, S. Retinopathy of prematurity: Inflammation, choroidal degeneration, and novel promising therapeutic strategies. J. Neuroinflamm. 2017, 14, 165. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age-related macular degeneration. Mol. Aspects Med. 2012, 33, 487–509. [Google Scholar] [CrossRef]
- Robinson, R.; Barathi, V.A.; Chaurasia, S.S.; Wong, T.Y.; Kern, T.S. Update on animal models of diabetic retinopathy: From molecular approaches to mice and higher mammals. Dis. Model. Mech. 2012, 5, 444–456. [Google Scholar] [CrossRef]
- Scott, A.; Fruttiger, M. Oxygen-induced retinopathy: A model for vascular pathology in the retina. Eye 2010, 24, 416–421. [Google Scholar] [CrossRef]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.L.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Smith, A.G.; Kaiser, P.K. Emerging treatments for wet age-related macular degeneration. Expert. Opin. Emerg. Drugs. 2014, 19, 157–164. [Google Scholar] [CrossRef]
- Palmer, E.A.; Hardy, R.J.; Dobson, V.; Phelps, D.L.; Quinn, G.E.; Summers, C.G.; Krom, C.P.; Tung, B.; Cryotherapy for Retinopathy of Prematurity Cooperative Group. 15-year outcomes following threshold retinopathy of prematurity: Final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch. Ophthalmol. 2005, 123, 311–318. [Google Scholar] [CrossRef]
- Lenis, T.L.; Gunzenhauser, R.C.; Fung, S.S.M.; Dhindsa, Y.K.; Sarraf, D.; Pineles, S.L.; Tsui, I. Myopia and anterior segment optical coherence tomography findings in laser-treated retinopathy of prematurity eyes. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, 86.e1–86.e7. [Google Scholar] [CrossRef]
- Tsang, J.K.W.; Liu, J.; Lo, A.C.Y. Vascular and Neuronal Protection in the Developing Retina: Potential Therapeutic Targets for Retinopathy of Prematurity. Int. J. Mol. Sci. 2019, 20, 4321. [Google Scholar] [CrossRef]
- Kong, H.B.; Zheng, G.Y.; He, B.M.; Zhang, Y.; Zhou, Q. Clinical Efficacy and Safety of Propranolol in the Prevention and Treatment of Retinopathy of Prematurity: A Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 2021, 9, 631673. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Hasan, T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 195–214. [Google Scholar] [CrossRef]
- Mammo, Z.; Forooghian, F. Incidence of acute exudative maculopathy after reduced-fluence photodynamic therapy. Retin. Cases Brief Rep. 2017, 11, 217–220. [Google Scholar] [CrossRef]
- Wallsh, J.O.; Gallemore, R.P. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells 2021, 10, 1049. [Google Scholar] [CrossRef]
- Jermak, C.M.; Dellacroce, J.T.; Heffez, J.; Peyman, G.A. Triamcinolone acetonide in ocular therapeutics. Surv. Ophthalmol. 2007, 52, 503–522. [Google Scholar] [CrossRef]
- Cáceres-del-Carpio, J.; Costa, R.D.; Haider, A.; Narayanan, R.; Kuppermann, B.D. Corticosteroids: Triamcinolone, Dexamethasone and Fluocinolone. Dev. Ophthalmol. 2016, 55, 221–231. [Google Scholar] [CrossRef]
- Irigoyen, C.; Alonso, A.A.; Sanchez-Molina, J.; Rodríguez-Hidalgo, M.; Lara-López, A.; Ruiz-Ederra, J. Subretinal Injection Techniques for Retinal Disease: A Review. J. Clin. Med. 2022, 11, 4717. [Google Scholar] [CrossRef]
- Ghoraba, H.H.; Akhavanrezayat, A.; Karaca, I.; Yavari, N.; Lajevardi, S.; Hwang, J.; Regenold, J.; Matsumiya, W.; Pham, B.; Zaidi, M.; et al. Ocular Gene Therapy: A Literature Review with Special Focus on Immune and Inflammatory Responses. Clin. Ophthalmol. 2022, 16, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Peral, A.; Mateo, J.; Domínguez-Godínez, C.O.; Carracedo, G.; Gómez, J.A.; Crooke, A.; Pintor, J. Therapeutic potential of topical administration of siRNAs against HIF-1α for corneal neovascularization. Exp. Eye Res. 2022, 219, 109036. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tsirukis, D.I.; Sun, Y. Targeting Neuroinflammation in Neovascular Retinal Diseases. Front. Pharmacol. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.; Henriques, J.; Carneiro, Â.; Marques-Neves, C.; Flores, R.; Castro-Sousa, J.P.; Meireles, A.; Gomes, N.; Nascimento, J.; Amaro, M.; et al. Guidelines for the Management of Center-Involving Diabetic Macular Edema: Treatment Options and Patient Monitorization. Clin. Ophthalmol. 2021, 15, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Mettu, P.S.; Allingham, M.J.; Cousins, S.W. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021, 82, 100906. [Google Scholar] [CrossRef]
- Cai, S.; Yang, Q.; Li, X.; Zhang, Y. The efficacy and safety of aflibercept and conbercept in diabetic macular edema. Drug Des. Dev. Ther. 2018, 12, 3471–3483. [Google Scholar] [CrossRef]
- Halim, S.; Nugawela, M.; Chakravarthy, U.; Peto, T.; Madhusudhan, S.; Lenfestey, P.; Hamill, B.; Zheng, Y.; Parry, D.; Nicholson, L.; et al. Topographical Response of Retinal Neovascularization to Aflibercept or Panretinal Photocoagulation in Proliferative Diabetic Retinopathy: Post Hoc Analysis of the CLARITY Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 501–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stewart, M.W.; Rosenfeld, P.J.; Penha, F.M.; Wang, F.; Yehoshua, Z.; Bueno-Lopez, E.; Lopez, P.F. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina 2012, 32, 434–457. [Google Scholar] [CrossRef]
- Wang, J.K.; Huang, T.L.; Chang, P.Y.; Chen, Y.T.; Chang, C.W.; Chen, F.T.; Hsu, Y.R.; Chen, Y.J. Intravitreal aflibercept versus bevacizumab for treatment of myopic choroidal neovascularization. Sci. Rep. 2018, 8, 14389. [Google Scholar] [CrossRef]
- Ross, E.L.; Hutton, D.W.; Stein, J.D.; Bressler, N.M.; Jampol, L.M.; Glassman, A.R.; Diabetic Retinopathy Clinical Research Network. Cost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema Treatment: Analysis from the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol. 2016, 134, 888–896. [Google Scholar] [CrossRef]
- Binder, S. Loss of reactivity in intravitreal anti-VEGF therapy: Tachyphylaxis or tolerance? Br. J. Ophthalmol. 2012, 96, 1–2. [Google Scholar] [CrossRef]
- Cheema, M.R.; DaCosta, J.; Talks, J. Ten-Year Real-World Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Neovascular Age-Related Macular Degeneration. Clin. Ophthalmol. 2021, 15, 279–287. [Google Scholar] [CrossRef]
- Bae, K.W.; Kim, D.I.; Hwang, D.D. The effect of intravitreal brolucizumab on choroidal thickness in patients with neovascular age-related macular degeneration. Sci. Rep. 2022, 12, 19855. [Google Scholar] [CrossRef]
- Nair, A.A.; Finn, A.P.; Sternberg, P., Jr. Spotlight on Faricimab in the Treatment of Wet Age-Related Macular Degeneration: Design, Development and Place in Therapy. Drug Des. Dev. Ther. 2022, 16, 3395–3400. [Google Scholar] [CrossRef]
- Lin, F.L.; Wang, P.Y.; Chuang, Y.F.; Wang, J.H.; Wong, V.H.Y.; Bui, B.V.; Liu, G.S. Gene Therapy Intervention in Neovascular Eye Disease: A Recent Update. Mol. Ther. 2020, 28, 2120–2138. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, A.; Zhang, H.; Wang, M.; Tang, Q.; Huang, Y.; Wang, L. Inhibition of retinal neovascularization by VEGF siRNA delivered via bioreducible lipid-like nanoparticles. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2407–2418. [Google Scholar] [CrossRef]
- Garba, A.O.; Mousa, S.A. Bevasiranib for the treatment of wet, age-related macular degeneration. Ophthalmol. Eye Dis. 2010, 2, 75–83. [Google Scholar] [CrossRef]
- Froger, N.; Matonti, F.; Roubeix, C.; Forster, V.; Ivkovic, I.; Brunel, N.; Baudouin, C.; Sahel, J.A.; Picaud, S. VEGF is an autocrine/paracrine neuroprotective factor for injured retinal ganglion neurons. Sci. Rep. 2020, 10, 12409. [Google Scholar] [CrossRef]
- Wingard, J.B.; Delzell, D.A.; Houlihan, N.V.; Lin, J.; Gieser, J.P. Incidence of Glaucoma or Ocular Hypertension After Repeated Anti-Vascular Endothelial Growth Factor Injections for Macular Degeneration. Clin. Ophthalmol. 2019, 13, 2563–2572. [Google Scholar] [CrossRef]
- Lind, J.T.; Gill, Z.; Seibold, L.K. Anti-VEGF Injection IOP Elevations. Eye-Wiki, the American Academy of Ophthalmology. 2022. Available online: https://eyewiki.aao.org/Anti-VEGF_Injection_IOP_Elevations (accessed on 10 March 2023).
- Fico, E.; Rosso, P.; Triaca, V.; Segatto, M.; Lambiase, A.; Tirassa, P. NGF Prevents Loss of TrkA/VEGFR2 Cells, and VEGF Isoform Dysregulation in the Retina of Adult Diabetic Rats. Cells 2022, 11, 3246. [Google Scholar] [CrossRef]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z.; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Yeh, P.T.; Chen, S.N.; Yang, C.M.; Lai, C.C.; Kuo, H.K. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in Taiwan. Ophthalmology 2011, 118, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.A.; Schwartz, S.; García-Aguirre, G.; Quiroz-Mercado, H. Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br. J. Ophthalmol. 2013, 97, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castellanos, M.A.; Schwartz, S.; Hernández-Rojas, M.L.; Kon-Jara, V.A.; García-Aguirre, G.; Guerrero-Naranjo, J.L.; Chan, R.V.; Quiroz-Mercado, H. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina 2013, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Urbančič, M.; Gardašević Topčić, I. Dexamethasone implant in the management of diabetic macular edema from clinician’s perspective. Clin. Ophthalmol. 2019, 13, 829–840. [Google Scholar] [CrossRef]
- Choi, M.Y.; Jee, D.; Kwon, J.W. Characteristics of diabetic macular edema patients refractory to anti-VEGF treatments and a dexamethasone implant. PLoS ONE 2019, 14, e0222364. [Google Scholar] [CrossRef]
- Levin, A.M.; Chaya, C.J.; Kahook, M.Y.; Wirostko, B.M. Intraocular Pressure Elevation Following Intravitreal Anti-VEGF Injections: Short- and Long-term Considerations. J. Glaucoma. 2021, 30, 1019–1026. [Google Scholar] [CrossRef]
- Catanzaro, O.; Labal, E.; Andornino, A.; Capponi, J.A.; Di Martino, I.; Sirois, P. Blockade of early and late retinal biochemical alterations associated with diabetes development by the selective bradykinin B1 receptor antagonist R-954. Peptides 2012, 34, 349–352. [Google Scholar] [CrossRef]
- Othman, R.; Cagnone, G.; Joyal, J.S.; Vaucher, E.; Couture, R. Kinins and Their Receptors as Potential Therapeutic Targets in Retinal Pathologies. Cells 2021, 10, 1913. [Google Scholar] [CrossRef]
- Terzuoli, E.; Morbidelli, L.; Nannelli, G.; Giachetti, A.; Donnini, S.; Ziche, M. Involvement of Bradykinin B2 Receptor in Pathological Vascularization in Oxygen-Induced Retinopathy in Mice and Rabbit Cornea. Int. J. Mol. Sci. 2018, 19, 330. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Peters, K.G. Targeting Tie2 for Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diab. Rep. 2016, 16, 126. [Google Scholar] [CrossRef]
- Khan, M.; Aziz, A.A.; Shafi, N.A.; Abbas, T.; Khanani, A.M. Targeting Angiopoietin in Retinal Vascular Diseases: A Literature Review and Summary of Clinical Trials Involving Faricimab. Cells 2020, 9, 1869. [Google Scholar] [CrossRef]
- Khanani, A.M.; Russell, M.W.; Aziz, A.A.; Danzig, C.J.; Weng, C.Y.; Eichenbaum, D.A.; Singh, R.P. Angiopoietins as Potential Targets in Management of Retinal Disease. Clin. Ophthalmol. 2021, 15, 3747–3755. [Google Scholar] [CrossRef]
- Canning, P.; Kenny, B.A.; Prise, V.; Glenn, J.; Sarker, M.H.; Hudson, N.; Brandt, M.; Lopez, F.J.; Gale, D.; Luthert, P.J.; et al. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc. Natl. Acad. Sci. USA 2016, 113, 7213–7218. [Google Scholar] [CrossRef]
- Stewart, S.; Lois, N. Fenofibrate for Diabetic Retinopathy. Asia Pac. J. Ophthalmol. 2018, 7, 422–426. [Google Scholar] [CrossRef]
- Duran, C.L.; Howell, D.W.; Dave, J.M.; Smith, R.L.; Torrie, M.E.; Essner, J.J.; Bayless, K.J. Molecular Regulation of Sprouting Angiogenesis. Compr. Physiol. 2017, 8, 153–235. [Google Scholar] [CrossRef]
- Cammalleri, M.; Dal Monte, M.; Pavone, V.; De Rosa, M.; Rusciano, D.; Bagnoli, P. The uPAR System as a Potential Therapeutic Target in the Diseased Eye. Cells 2019, 8, 925. [Google Scholar] [CrossRef]
- Santonocito, M.; Zappulla, C.; Viola, S.; La Rosa, L.R.; Solfato, E.; Abbate, I.; Tarallo, V.; Apicella, I.; Platania, C.B.M.; Maugeri, G.; et al. Assessment of a New Nanostructured Microemulsion System for Ocular Delivery of Sorafenib to Posterior Segment of the Eye. Int. J. Mol. Sci. 2021, 22, 4404. [Google Scholar] [CrossRef]
- Vinores, S.A.; Xiao, W.H.; Aslam, S.; Shen, J.; Oshima, Y.; Nambu, H.; Liu, H.; Carmeliet, P.; Campochiaro, P.A. Implication of the hypoxia response element of the Vegf promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J. Cell Physiol. 2006, 206, 749–758. [Google Scholar] [CrossRef]
- Martinez-Alejo, J.M.; Baiza-Duran, L.M.; Quintana-Hau, J.D. Novel therapies for proliferative retinopathies. Ther. Adv. Chronic Dis. 2022, 13, 20406223221140395. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, S.; Li, S.; Yu, C.; Huang, D.; Chen, H.; Yin, X. CircPDE4B inhibits retinal pathological angiogenesis via promoting degradation of HIF-1α though targeting miR-181c. IUBMB Life 2020, 72, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Medori, M.C.; Naureen, Z.; Dhuli, K.; Placidi, G.; Falsini, B.; Bertelli, M. Dietary supplements in retinal diseases, glaucoma, and other ocular conditions. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E189–E199. [Google Scholar] [CrossRef]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef]
- Milluzzo, A.; Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; Mazzone, M.G.; Sciacca, L.; Agodi, A. Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? A Systematic Review. Nutrients 2022, 14, 4430. [Google Scholar] [CrossRef]
- Castro-Castaneda, C.R.; Altamirano-Lamarque, F.; Ortega-Macías, A.G.; Santa Cruz-Pavlovich, F.J.; Gonzalez-De la Rosa, A.; Armendariz-Borunda, J.; Santos, A.; Navarro-Partida, J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients 2022, 14, 5014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruamviboonsuk, V.; Grzybowski, A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. J. Clin. Med. 2022, 11, 6490. [Google Scholar] [CrossRef]
- Rusciano, D.; Pezzino, S.; Olivieri, M.; Cristaldi, M.; Spampinato, G. Food Supplements in the Treatment of Ophthalmic Diseases: Preclinical and Clinical Studies. J. Pharmacol. Pharm. Res. 2020, 3, 1–34. [Google Scholar] [CrossRef]
- Reinders, M.E.; Sho, M.; Izawa, A.; Wang, P.; Mukhopadhyay, D.; Koss, K.E.; Geehan, C.S.; Luster, A.D.; Sayegh, M.H.; Briscoe, D.M. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Investig. 2003, 112, 1655–1665. [Google Scholar] [CrossRef]
- Kim, Y.W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Díaz-López, A.; Babio, N.; Martínez-González, M.A.; Corella, D.; Amor, A.J.; Fitó, M.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Mediterranean Diet, Retinopathy, Nephropathy, and Microvascular Diabetes Complications: A Post Hoc Analysis of a Randomized Trial. Diabetes Care 2015, 38, 2134–2141, Erratum in: Diabetes Care 2018, 41, 2260–2261. [Google Scholar] [CrossRef]
- Pall, M.L.; Levine, S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef]
- Sapieha, P.; Chen, J.; Stahl, A.; Seaward, M.R.; Favazza, T.L.; Juan, A.M.; Hatton, C.J.; Joyal, J.-S.; Krah, N.M.; Dennison, R.J.; et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutr. Diabetes. 2012, 2, e36. [Google Scholar] [CrossRef]
- Eynard, A.R.; Repossi, G. Role of ω3 polyunsaturated fatty acids in diabetic retinopathy: A morphological and metabolically cross talk among blood retina barriers damage, autoimmunity and chronic inflammation. Lipids Health Dis. 2019, 18, 114. [Google Scholar] [CrossRef]
- Chew, E.Y. Dietary Intake of Omega-3 Fatty Acids from Fish and Risk of Diabetic Retinopathy. JAMA 2017, 317, 2226–2227. [Google Scholar] [CrossRef]
- Rosenberg, K. Omega-3 Fatty Acid Intake Lowers Risk of Diabetic Retinopathy. Am. J. Nurs. 2017, 117, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.; Ortín, L.; Argente, M.; Guindo, J.L.; López-Bernal, M.D.; López-Román, F.J.; García, M.J.; Domingo, J.C.; Lajara, J. Combined intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema: Two-Year Randomized Single-Blind Controlled Trial Results. Retina 2017, 37, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.; Ortín, L.; Argente, M.; Guindo, J.L.; López-Bernal, M.D.; López-Román, F.J.; Domingo, J.C.; Lajara, J. Three-year outcomes in a randomized single-blind controlled trial of intravitreal ranibizumab and oral supplementation with docosahexaenoic acid and antioxidants for diabetic macular edema. Retina 2019, 39, 1083–1090. [Google Scholar] [CrossRef]
- Duda, M.; Kawula, K.; Pawlak, A.; Sarna, T.; Wisniewska-Becker, A. EPR Studies on the Properties of Model Photoreceptor Membranes Made of Natural and Synthetic Lipids. Cell Biochem. Biophys. 2017, 75, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, M.; Dal Monte, M.; Locri, F.; Lardner, E.; Kvanta, A.; Rusciano, D.; André, H.; Bagnoli, P. Efficacy of a Fatty Acids Dietary Supplement in a Polyethylene Glycol-Induced Mouse Model of Retinal Degeneration. Nutrients 2017, 9, 1079. [Google Scholar] [CrossRef]
- Gong, Y.; Fu, Z.; Liegl, R.; Chen, J.; Hellström, A.; Smith, L.E. ω-3 and ω-6 long-chain PUFAs and their enzymatic metabolites in neovascular eye diseases. Am. J. Clin. Nutr. 2017, 106, 16–26. [Google Scholar] [CrossRef]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef]
- Aiello, L.P.; Pierce, E.A.; Foley, E.D.; Takagi, H.; Chen, H.; Riddle, L.; Ferrara, N.; King, G.L.; Smith, L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10457–10461. [Google Scholar] [CrossRef]
- NoA, L. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436, Erratum in: Arch. Ophthalmol. 2008, 126, 1251. [Google Scholar] [CrossRef]
- NoA, L. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015, Erratum in: JAMA 2013, 310, 208. [Google Scholar] [CrossRef]
- Liu, Y.; Bell, B.A.; Song, Y.; Zhang, K.; Anderson, B.; Axelsen, P.H.; Bohannan, W.; Agbaga, M.; Park, H.G.; James, G.; et al. Deuterated docosahexaenoic acid protects against oxidative stress and geographic atrophy-like retinal degeneration in a mouse model with iron overload. Aging Cell 2022, 21, e13579. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, X.; Fan, Y.; Wang, D.; Li, B.; Zhou, J.; Pei, C.; Ma, L. Dietary omega-3 polyunsaturated fatty acids and fish intake and risk of age-related macular degeneration. Clin. Nutr. 2021, 40, 5662–5673. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.; AREDS and AREDS2 Research Groups. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Meng, X.T.; Shi, Y.Y.; Hong-Yan, Z. Dietary omega-3 LCPUFA intake in the prevention of neovascular age-related macular degeneration: A systematic review and meta-analysis. Nutr. Hosp. 2022, 39, 910–915. (In English) [Google Scholar]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Paik, S.S.; Jeong, E.; Jung, S.W.; Ha, T.J.; Kang, S.; Sim, S.; Jeon, J.H.; Chun, M.H.; Kim, I.B. Anthocyanins from the seed coat of black soybean reduce retinal degeneration induced by N-methyl-N-nitrosourea. Exp. Eye Res. 2012, 97, 55–62. [Google Scholar] [CrossRef]
- Song, Y.; Huang, L.; Yu, J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 2016, 301, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296, Erratum in: J. Nat. Prod. 1999, 62, 802. [Google Scholar] [CrossRef]
- Canovai, A.; Amato, R.; Melecchi, A.; Dal Monte, M.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Preventive Efficacy of an Antioxidant Compound on Blood Retinal Barrier Breakdown and Visual Dysfunction in Streptozotocin-Induced Diabetic Rats. Front. Pharmacol. 2022, 12, 811818. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef]
- Silván, J.M.; Reguero, M.; de Pascual-Teresa, S. A protective effect of anthocyanins and xanthophylls on UVB-induced damage in retinal pigment epithelial cells. Food Funct. 2016, 7, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Nakamura, Y.; Tachibanaki, S.; Kawamura, S.; Hirayama, M. Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. J. Agric. Food Chem. 2003, 51, 3560–3563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Exp. Eye Res. 2017, 160, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Wu, H.; Li, D.J.; Song, J.F.; Xiao, Y.D.; Liu, C.Q.; Zhou, J.Z.; Sui, Z.Q. Protective Effects of Blueberry Anthocyanins against H2O2-Induced Oxidative Injuries in Human Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2018, 66, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, C.; Wu, W.; Liu, T.; Ji, B.; Zhou, F. Cyanidin-3-glucoside Alleviates 4-Hydroxyhexenal-Induced NLRP3 Inflammasome Activation via JNK-c-Jun/AP-1 Pathway in Human Retinal Pigment Epithelial Cells. J. Immunol. Res. 2018, 2018, 5604610. [Google Scholar] [CrossRef]
- Amato, R.; Canovai, A.; Melecchi, A.; Pezzino, S.; Corsaro, R.; Monte, M.D.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Dietary Supplementation of Antioxidant Compounds Prevents Light-Induced Retinal Damage in a Rat Model. Biomedicines 2021, 9, 1177. [Google Scholar] [CrossRef]
- Huang, S.; Yang, N.; Liu, Y.; Hu, L.; Zhao, J.; Gao, J.; Li, Y.; Li, C.; Zhang, X.; Huang, T. Grape seed proanthocyanidins inhibit angiogenesis via the downregulation of both vascular endothelial growth factor and angiopoietin signaling. Nutr. Res. 2012, 32, 530–536. [Google Scholar] [CrossRef]
- Mohn, E.S.; Erdman, J.W., Jr.; Kuchan, M.J.; Neuringer, M.; Johnson, E.J. Lutein accumulates in subcellular membranes of brain regions in adult rhesus macaques: Relationship to DHA oxidation products. PLoS ONE 2017, 12, e0186767. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary identification of the human macular pigment. Vis. Vision. Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Menon, B.; Gierhart, D.L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1645–1651. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010, 53, 971–979. [Google Scholar] [CrossRef]
- Brazionis, L.; Rowley, K.; Itsiopoulos, C.; O’Dea, K. Plasma carotenoids and diabetic retinopathy. Br. J. Nutr. 2009, 101, 270–277. [Google Scholar] [CrossRef]
- Zhang, P.C.; Wu, C.R.; Wang, Z.L.; Wang, L.Y.; Han, Y.; Sun, S.L.; Li, Q.S.; Ma, L. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac. J. Clin. Nutr. 2017, 26, 406–411. [Google Scholar] [CrossRef]
- Hu, B.J.; Hu, Y.N.; Lin, S.; Ma, W.J.; Li, X.R. Application of Lutein and Zeaxanthin in nonproliferative diabetic retinopathy. Int. J. Ophthalmol. 2011, 4, 303–306. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Pinazo-Duran, M.D.; Garcia-Medina, M.; Zanon-Moreno, V.; Pons-Vazquez, S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 637–643. [Google Scholar] [CrossRef]
- Lawlor, S.M.; O’Brien, N.M. Astaxanthin: Antioxidant effects in chicken embryo fibroblasts. Nutr. Res. 1995, 15, 1695–1704. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef]
- Yeh, P.T.; Huang, H.W.; Yang, C.M.; Yang, W.S.; Yang, C.H. Astaxanthin Inhibits Expression of Retinal Oxidative Stress and Inflammatory Mediators in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, F.; Hu, X.; Chen, J.; Wen, X.; Sun, Y.; Liu, Y.; Tang, R.; Zheng, K.; Song, Y. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mice. Physiol. Behav. 2015, 151, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.P.; Sun, L.; Yu, H.S.; Liang, L.P.; Li, W.; Ding, H.; Song, X.B.; Zhang, L.J. The Pharmacological Effects of Lutein and Zeaxanthin on Visual Disorders and Cognition Diseases. Molecules 2017, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, A.; Subczynski, W.K. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic. Biol. Med. 2006, 41, 1257–1265. [Google Scholar] [CrossRef]
- Wisniewska-Becker, A.; Nawrocki, G.; Duda, M.; Subczynski, W.K. Structural aspects of the antioxidant activity of lutein in a model of photoreceptor membranes. Acta Biochim. Pol. 2012, 59, 119–124. [Google Scholar] [CrossRef]
- Subczynski, W.; Wisniewska-Becker, A.; Widomska, J. Xanthophyll–membrane interactions. In Carotenoids and Retinal Disease; Landrum, J.T., Nolan, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 203–222. [Google Scholar]
- Biswal, M.R.; Justis, B.D.; Han, P.; Li, H.; Gierhart, D.; Dorey, C.K.; Lewin, A.S. Daily zeaxanthin supplementation prevents atrophy of the retinal pigment epithelium (RPE) in a mouse model of mitochondrial oxidative stress. PLoS ONE 2018, 13, e0203816. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fletcher, L.M.; Roos, F.; Wittwer, J.; Schalch, W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8583–8589. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Thurnham, D.I. Macular zeaxanthins and lutein—A review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr. Res. Rev. 2007, 20, 163–179. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Simple isocratic method for simultaneous determination of caffeine and catechins in tea products by HPLC. Springerplus 2016, 5, 970. [Google Scholar] [CrossRef]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: A patent review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin Gallate Is the Most Effective Catechin Against Antioxidant Stress via Hydrogen Peroxide and Radical Scavenging Activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Chu, K.O.; Chan, K.P.; Yang, Y.P.; Qin, Y.J.; Li, W.Y.; Chan, S.O.; Wang, C.C.; Pang, C.P. Effects of EGCG content in green tea extract on pharmacokinetics, oxidative status and expression of inflammatory and apoptotic genes in the rat ocular tissues. J. Nutr. Biochem. 2015, 26, 1357–1367. [Google Scholar] [CrossRef]
- Zhang, B.; Osborne, N.N. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 2006, 1124, 176–187. [Google Scholar] [CrossRef]
- Peng, P.H.; Ko, M.L.; Chen, C.F. Epigallocatechin-3-gallate reduces retinal ischemia/reperfusion injury by attenuating neuronal nitric oxide synthase expression and activity. Exp. Eye Res. 2008, 86, 637–646. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, L.; Shen, C.; Wan, H.; Xu, L.; Wang, N.; Jonas, J.B. Neuroprotective effect of epigallocatechin-3-gallate against N-methyl-D-aspartate-induced excitotoxicity in the adult rat retina. Acta Ophthalmol. 2012, 90, e609–e615. [Google Scholar] [CrossRef]
- Silva, K.C.; Rosales, M.A.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.; Faria, J.B.; Faria, J.M. Green tea is neuroprotective in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, J.H.; Lin, H.H.; Chiang, H.S.; Chen, B.H.; Hong, J.Y.; Hung, C.F. Protective effects of (-)-epigallocatechin gallate on UVA-induced damage in ARPE19 cells. Mol. Vis. 2008, 14, 2528–2534. [Google Scholar]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother. 2017, 87, 73–81. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, R. Angiogenesis inhibited by drinking tea. Nature 1999, 398, 381. [Google Scholar] [CrossRef]
- Garbisa, S.; Sartor, L.; Biggin, S.; Salvato, B.; Benelli, R.; Albini, A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer 2001, 91, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aica, I.; Donà, M.; Sartor, L.; Pezzato, E.; Garbisa, S. (-)Epigallocatechin-3-gallate directly inhibits MT1-MMP activity, leading to accumulation of nonactivated MMP-2 at the cell surface. Lab. Investig. 2002, 82, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.D.; Ellis, L.M. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int. J. Exp. Pathol. 2001, 82, 309–316. [Google Scholar] [CrossRef]
- Lee, H.S.; Jun, J.H.; Jung, E.H.; Koo, B.A.; Kim, Y.S. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 2014, 19, 12150–12172. [Google Scholar] [CrossRef]
- Shankar, S.; Chen, Q.; Srivastava, R.K. Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to enhance antiangiogenic effects of EGCG through activation of FOXO transcription factor. J. Mol. Signal. 2008, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.K.; Liang, S. Epigallocatechin-3-gallate protects retinal vascular endothelial cells from high glucose stress in vitro via the MAPK/ERK-VEGF pathway. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Xu, J.; Tu, Y.; Wang, Y.; Xu, X.; Sun, X.; Xie, L.; Zhao, Q.; Guo, Y.; Gu, Y.; Du, J.; et al. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed. Pharmacother. 2020, 121, 109606. [Google Scholar] [CrossRef] [PubMed]
- Bola, C.; Bartlett, H.; Eperjesi, F. Resveratrol and the eye: Activity and molecular mechanisms. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 699–713. [Google Scholar] [CrossRef]
- Bryl, A.; Falkowski, M.; Zorena, K.; Mrugacz, M. The Role of Resveratrol in Eye Diseases-A Review of the Literature. Nutrients 2022, 14, 2974. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Ying, J.; Shi, T.; Wang, P. Resveratrol Prevents ROS-Induced Apoptosis in High Glucose-Treated Retinal Capillary Endothelial Cells via the Activation of AMPK/Sirt1/PGC-1α Pathway. Oxid. Med. Cell Longev. 2017, 2017, 7584691. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, C.W.; Hsieh, M.C.; Wu, H.J.; Wu, W.S.; Wu, W.C.; Kao, Y.H. High mobility group B1 up-regulates angiogenic and fibrogenic factors in human retinal pigment epithelial ARPE-19 cells. Cell Signal. 2017, 40, 248–257. [Google Scholar] [CrossRef]
- Losso, J.N.; Truax, R.E.; Richard, G. Trans-resveratrol inhibits hyperglycemia-induced inflammation and connexin downregulation in retinal pigment epithelial cells. J. Agric. Food Chem. 2010, 58, 8246–8252. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, J.; Li, H.; Wei, H.; Bi, F.; Liu, S.; Tang, K.; Guo, H.; Liu, W. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp. Eye Res. 2019, 181, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, C.H.; Li, H.J.; Hsiao, C.Y.; Su, C.C.; Lee, P.L.; Hung, C.F. Protective effects of resveratrol against UVA-induced damage in ARPE19 cells. Int. J. Mol. Sci. 2015, 16, 5789–5802. [Google Scholar] [CrossRef]
- Nagai, N.; Kubota, S.; Tsubota, K.; Ozawa, Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. J. Nutr. Biochem. 2014, 25, 1218–1225. [Google Scholar] [CrossRef]
- Subramani, M.; Ponnalagu, M.; Krishna, L.; Jeyabalan, N.; Chevour, P.; Sharma, A.; Jayadev, C.; Shetty, R.; Begum, N.; Archunan, G.; et al. Resveratrol reverses the adverse effects of bevacizumab on cultured ARPE-19 cells. Sci. Rep. 2017, 7, 12242. [Google Scholar] [CrossRef] [PubMed]
- Bråkenhielm, E.; Cao, R.; Cao, Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001, 15, 1798–1800. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fu, Z.D.; Wang, F.; Liu, H.Y.; Han, R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J. Asian Nat. Prod. Res. 2005, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-Oxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Resveratrol in Ocular Diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Guerin, K.I.; Chen, J.; Michán, S.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Juan, A.M.; Hatton, C.J.; et al. Resveratrol inhibits pathologic retinal neovascularization in Vldlr(−/−) mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2809–2816. [Google Scholar] [CrossRef]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. Role of Curcumin in Retinal Diseases-A review. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1457–1473. [Google Scholar] [CrossRef]
- Allegrini, D.; Raimondi, R.; Borgia, A.; Sorrentino, T.; Montesano, G.; Tsoutsanis, P.; Cancian, G.; Verma, Y.; De Rosa, F.P.; Romano, M.R. Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside. Int. J. Mol. Sci. 2022, 23, 3557. [Google Scholar] [CrossRef] [PubMed]
- Franzone, F.; Nebbioso, M.; Pergolizzi, T.; Attanasio, G.; Musacchio, A.; Greco, A.; Limoli, P.G.; Artico, M.; Spandidos, D.A.; Taurone, S.; et al. Anti-inflammatory role of curcumin in retinal disorders (Review). Exp. Ther. Med. 2021, 22, 790. [Google Scholar] [CrossRef]
- Nebbioso, M.; Franzone, F.; Greco, A.; Gharbiya, M.; Bonfiglio, V.; Polimeni, A. Recent Advances and Disputes About Curcumin in Retinal Diseases. Clin. Ophthalmol. 2021, 15, 2553–2571. [Google Scholar] [CrossRef]
- NoA, L. Joint Expert Committee on Food Additives Evaluation of Certain Food Additives and Contaminants: Eightieth Report of the Joint FAO/WHO Expert Committee on Food Additives: Rome, 16–25 June 2015; WHO Technical Report Series; WHO: Geneva, Switzerland, 2016; ISBN 978-92-4-120995-3.
- Cheng, A.L.; Hsu, C.-H.; Lin, J.K.; Hsu, M.M.; Ho, Y.-F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.-R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal Protection and Distribution of Curcumin in Vitro and in Vivo. Front. Pharmacol. 2018, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Premanand, C.; Rema, M.; Sameer, M.Z.; Sujatha, M.; Balasubramanyam, M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2179–2184. [Google Scholar] [CrossRef]
- Woo, J.M.; Shin, D.Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012, 18, 901–908. [Google Scholar]
- Li, Y.; Zou, X.; Cao, K.; Xu, J.; Yue, T.; Dai, F.; Zhou, B.; Lu, W.; Feng, Z.; Liu, J. Curcumin analog 1, 5-bis (2-trifluoromethylphenyl)-1, 4-pentadien-3-one exhibits enhanced ability on Nrf2 activation and protection against acrolein-induced ARPE-19 cell toxicity. Toxicol. Appl. Pharmacol. 2013, 272, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.F.; Zhang, Q.; Liu, X.Z. Protective effects of curcumin on retinal Müller cell in early diabetic rats. Int. J. Ophthalmol. 2013, 6, 422–424. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kumar, B.; Nag, T.C.; Agrawal, S.S.; Agrawal, R.; Agrawal, P.; Saxena, R.; Srivastava, S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 2011, 27, 123–130. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab. 2007, 4, 8. [Google Scholar] [CrossRef]
- Yadav, V.R.; Aggarwal, B.B. Curcumin: A component of the golden spice, targets multiple angiogenic pathways. Cancer Biol. Ther. 2011, 11, 236–241. [Google Scholar] [CrossRef]
- Arbiser, J.L.; Klauber, N.; Rohan, R.; van Leeuwen, R.; Huang, M.T.; Fisher, C.; Flynn, E.; Byers, H.R. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998, 4, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Gururaj, A.E.; Belakavadi, M.; Venkatesh, D.A.; Marmé, D.; Salimath, B.P. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem. Biophys. Res. Commun. 2002, 297, 934–942. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, J.H.; Cho, H.Y.; Yum, Y.N.; Kim, S.H.; Park, H.J.; Shim, B.S.; Choi, S.H.; Kwon, H.J. Irreversible inhibition of CD13/aminopeptidase N by the antiangiogenic agent curcumin. Chem. Biol. 2003, 10, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A. Curcumin and Wnt/β catenin signaling in exudative age related macular degeneration (Review). Int. J. Mol. Med. 2022, 49, 79. [Google Scholar] [CrossRef]
- Allegrini, D.; Raimondi, R.; Angi, M.; Ricciardelli, G.; Montericcio, A.; Borgia, A.; Romano, M.R. Curcuma-Based Nutritional Supplement in Patients with Neovascular Age-Related Macular Degeneration. J. Med. Food 2021, 24, 1191–1196. [Google Scholar] [CrossRef]
- Cota, F.; Costa, S.; Giannantonio, C.; Purcaro, V.; Catenazzi, P.; Vento, G. Lutein supplementation and retinopathy of prematurity: A meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Meng, S.S.; Burnim, S.B.; Smith, L.E.; Lo, A.C. Lutein facilitates physiological revascularization in a mouse model of retinopathy of prematurity. Clin. Exp. Ophthalmol. 2017, 45, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, B.; Ramaprasad, T.R.; Baskaran, V. Dietary fatty acid determines the intestinal absorption of lutein in lutein deficient mice. Food Res. Int. 2014, 64, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, B.; Mamatha, B.S.; Baskaran, V. Olive oil improves the intestinal absorption and bioavailability of lutein in lutein-deficient mice. Eur. J. Nutr. 2014, 53, 117–126. [Google Scholar] [CrossRef]
- Baack, M.L.; Puumala, S.E.; Messier, S.E.; Pritchett, D.K.; Harris, W.S. What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels? Prostaglandins Leukot. Essent. Fat. Acids 2015, 100, 5–11. [Google Scholar] [CrossRef]
- Khalesi, N.; Bordbar, A.; Khosravi, N.; Kabirian, M.; Karimi, A. The Efficacy of Omega-3 Supplement on Prevention of Retinopathy of Prematurity in Premature Infants: A Randomized Double-blinded Controlled trial. Curr. Pharm. Des. 2018, 24, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Pivodic, A.; Gränse, L.; Lundgren, P.; Sjöbom, U.; Nilsson, A.K.; Söderling, H.; Hård, A.L.; Smith, L.E.H.; Löfqvist, C.A. Association of Docosahexaenoic Acid and Arachidonic Acid Serum Levels with Retinopathy of Prematurity in Preterm Infants. JAMA Netw. Open 2021, 4, e2128771. [Google Scholar] [CrossRef]
- Hellström, A.; Nilsson, A.K.; Wackernagel, D.; Pivodic, A.; Vanpee, M.; Sjöbom, U.; Hellgren, G.; Hallberg, B.; Domellöf, M.; Klevebro, S.; et al. Effect of Enteral Lipid Supplement on Severe Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2021, 175, 359–367. [Google Scholar] [CrossRef]
- Tu, C.F.; Lee, C.H.; Chen, H.N.; Tsao, L.Y.; Chen, J.Y.; Hsiao, C.C. Effects of fish oil-containing lipid emulsions on retinopathy of prematurity in very low birth weight infants. Pediatr. Neonatol. 2020, 61, 224–230. [Google Scholar] [CrossRef]
- Sun, H.; Cheng, R.; Wang, Z. Early vitamin A supplementation improves the outcome of retinopathy of prematurity in extremely preterm infants. Retina 2020, 40, 1176–1184. [Google Scholar] [CrossRef]
- Garofoli, F.; Barillà, D.; Angelini, M.; Mazzucchelli, I.; De Silvestri, A.; Guagliano, R.; Decembrino, L.; Tzialla, C. Oral vitamin A supplementation for ROP prevention in VLBW preterm infants. Ital. J. Pediatr. 2020, 46, 77. [Google Scholar] [CrossRef] [PubMed]
- Okai, Y.; Higashi-Okai, K.; FSato, E.; Konaka, R.; Inoue, M. Potent radical-scavenging activities of thiamin and thiamin diphosphate. J. Clin. Biochem. Nutr. 2007, 40, 42–48. [Google Scholar] [CrossRef]
- Berrone, E.; Beltramo, E.; Solimine, C.; Ape, A.U.; Porta, M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J. Biol. Chem. 2006, 281, 9307–9313. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, P.; Airen, S.; Brown, C.; Liu, Z.; Townsend, J.H.; Wang, J.; Jiang, H. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis. 2020, 7, 33. [Google Scholar] [CrossRef]
- NoA, L. Vitamin C-Health Professional Fact Sheet. National Institutes of Health. 2020. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 10 March 2023).
- Shang, F.; Lu, M.; Dudek, E.; Reddan, J.; Taylor, A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic Biol. Med. 2003, 34, 521–530. [Google Scholar] [CrossRef]
- Guan, Y.; Dai, P.; Wang, H. Effects of vitamin C supplementation on essential hypertension: A systematic review and meta-analysis. Medicine 2020, 99, e19274. [Google Scholar] [CrossRef]
- Thosar, S.S.; Bielko, S.L.; Wiggins, C.C.; Klaunig, J.E.; Mather, K.J.; Wallace, J.P. Antioxidant vitamin C prevents decline in endothelial function during sitting. Med. Sci. Monit. 2015, 21, 1015–1021. [Google Scholar] [CrossRef]
- Park, S.W.; Ghim, W.; Oh, S.; Kim, Y.; Park, U.C.; Kang, J.; Yu, H.G. Association of vitreous vitamin C depletion with diabetic macular ischemia in proliferative diabetic retinopathy. PLoS ONE 2019, 14, e0218433. [Google Scholar] [CrossRef]
- Gurreri, A.; Pazzaglia, A.; Schiavi, C. Role of Statins and Ascorbic Acid in the Natural History of Diabetic Retinopathy: A New, Affordable Therapy? Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, S23–S27. [Google Scholar] [CrossRef]
- Ulker, E.; Parker, W.H.; Raj, A.; Qu, Z.C.; May, J.M. Ascorbic acid prevents VEGF-induced increases in endothelial barrier permeability. Mol. Cell Biochem. 2016, 412, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Wang, C.; Liu, D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr. Diabetes. 2017, 7, e281. [Google Scholar] [CrossRef] [PubMed]
- Bursell, S.E.; Clermont, A.C.; Aiello, L.P.; Aiello, L.M.; Schlossman, D.K.; Feener, E.P.; Laffel, L.; King, G.L. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999, 22, 1245–1251. [Google Scholar] [CrossRef]
- Chatziralli, I.P.; Theodossiadis, G.; Dimitriadis, P.; Charalambidis, M.; Agorastos, A.; Migkos, Z.; Platogiannis, N.; Moschos, M.M.; Theodossiadis, P.; Keryttopoulos, P. The Effect of Vitamin E on Oxidative Stress Indicated by Serum Malondialdehyde in Insulin-dependent Type 2 Diabetes Mellitus Patients with Retinopathy. Open Ophthalmol. J. 2017, 11, 51–58. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Goldman, R.; Darrow, R.M.; Organisciak, D.T.; Kagan, V.E. Endogenous ascorbate regenerates vitamin E in the retina directly and in combination with exogenous dihydrolipoic acid. Curr. Eye Res. 1995, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Schaffer, D.; Quinn, G.; Goldstein, D.; Mathis, M.J.; Otis, C.; Boggs, T.R., Jr. Vitamin E supplementation and the retinopathy of prematurity. Ann. N. Y. Acad. Sci. 1982, 393, 473–495. [Google Scholar] [CrossRef]
- Hittner, H.M.; Rudolph, A.J.; Kretzer, F.L. Suppression of severe retinopathy of prematurity with vitamin E supplementation. Ultrastructural mechanism of clinical efficacy. Ophthalmology 1984, 91, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, M.; Polat, O. Clinical Efficacy of Topical CoQ10 and Vitamin-E Eye-drop in Retinopathy of Prematurity. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 291–297. [Google Scholar]
- Robison, W.G.; Kuwabara, T.; Bieri, J.G. The roles of vitamin E and unsaturated fatty acids in the visual process. Retina 1982, 2, 263–281. [Google Scholar] [CrossRef]
- Tanito, M.; Yoshida, Y.; Kaidzu, S.; Chen, Z.H.; Cynshi, O.; Jishage, K.; Niki, E.; Ohira, A. Acceleration of age-related changes in the retina in alpha-tocopherol transfer protein null mice fed a Vitamin E-deficient diet. Investig. Ophthalmol. Vis. Sci. 2007, 48, 396–404. [Google Scholar] [CrossRef]
- Katz, M.L.; Eldred, G.E. Failure of vitamin E to protect the retina against damage resulting from bright cyclic light exposure. Investig. Ophthalmol. Vis. Sci. 1989, 30, 29–36. [Google Scholar]
- Belda, J.I.; Romá, J.; Vilela, C.; Puertas, F.J.; Díaz-Llopis, M.; Bosch-Morell, F.; Romero, F.J. Serum vitamin E levels negatively correlate with severity of age-related macular degeneration. Mech. Ageing Dev. 1999, 107, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, W.; El-Sherbiny, S. Evidence-based nutritional advice for patients affected by age-related macular degeneration. Ophthalmologica 2014, 231, 185–190. [Google Scholar] [CrossRef] [PubMed]
- de Koning-Backus, A.P.M.; Buitendijk, G.H.S.; Kiefte-de Jong, J.C.; Colijn, J.M.; Hofman, A.; Vingerling, J.R.; Haverkort, E.B.; Franco, O.H.; Klaver, C.C.W. Intake of Vegetables, Fruit, and Fish is Beneficial for Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019, 198, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group; Sangiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Rd, F.F.; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Sperduto, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Błasiak, J. Can vitamin D protect against age-related macular degeneration or slow its progression? Acta Biochim. Pol. 2019, 66, 147–158. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857, Erratum in: JAMA 2008, 299, 765–766. [Google Scholar] [CrossRef]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef]
- Miao, X.; Sun, W.; Miao, L.; Fu, Y.; Wang, Y.; Su, G.; Liu, Q. Zinc and diabetic retinopathy. J. Diabetes Res. 2013, 2013, 425854. [Google Scholar] [CrossRef]
- Dascalu, A.M.; Anghelache, A.; Stana, D.; Costea, A.C.; Nicolae, V.A.; Tanasescu, D.; Costea, D.O.; Tribus, L.C.; Zgura, A.; Serban, D.; et al. Serum levels of copper and zinc in diabetic retinopathy: Potential new therapeutic targets (Review). Exp. Ther. Med. 2022, 23, 324. [Google Scholar] [CrossRef] [PubMed]
- NoA, L.; EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Draft Scientific Opinion on Dietary Reference Values for Zinc. EFSA Journal 2014. Available online: https://www.efsa.europa.eu/sites/default/files/consultation/140514%2C0.pdf (accessed on 10 March 2023).
- Terrin, G.; Berni Canani, R.; Passariello, A.; Messina, F.; Conti, M.G.; Caoci, S.; Smaldore, A.; Bertino, E.; De Curtis, M. Zinc supplementation reduces morbidity and mortality in very-low-birth-weight preterm neonates: A hospital-based randomized, placebo-controlled trial in an industrialized country. Am. J. Clin. Nutr. 2013, 98, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Staub, E.; Evers, K.; Askie, L.M. Enteral zinc supplementation for prevention of morbidity and mortality in preterm neonates. Cochrane Database Syst. Rev. 2021, 3, CD012797. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium, glucose intolerance and diabetes. J. Am. Coll. Nutr. 1998, 17, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W. Interaction of chromium with insulin: A progress report. Nutr. Rev. 1998, 56, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Cheng, N.; Bryden, N.A.; Polansky, M.M.; Cheng, N.; Chi, J.; Feng, J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes 1997, 46, 1786–1791. [Google Scholar] [CrossRef]
- Erie, J.C.; Good, J.A.; Butz, J.A.; Pulido, J.S. Reduced zinc and copper in the retinal pigment epithelium and choroid in age-related macular degeneration. Am. J. Ophthalmol. 2009, 147, 276–282.e1. [Google Scholar] [CrossRef]
- Arteel, G.E.; Sies, H. The biochemistry of selenium and the glutathione system. Environ. Environ. Toxicol. Pharmacol. 2001, 10, 153–158. [Google Scholar] [CrossRef]
- Farnsworth, C.C.; Stone, W.L.; Dratz, E.A. Effects of vitamin E and selenium deficiency on the fatty acid composition of rat retinal tissues. Biochim. Biophys. Acta 1979, 552, 281–293. [Google Scholar] [CrossRef]
- Rinninella, E.; Mele, M.C.; Merendino, N.; Cintoni, M.; Anselmi, G.; Caporossi, A.; Gasbarrini, A.; Minnella, A.M. The Role of Diet, Micronutrients and the Gut Microbiota in Age-Related Macular Degeneration: New Perspectives from the Gut–Retina Axis. Nutrients 2018, 10, 1677. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.L.; Grant, M.B. The Gut-Eye Axis: Lessons Learned from Murine Models. Ophthalmol. Ther. 2020, 9, 499–513. [Google Scholar] [CrossRef]
- Bu, Y.; Chan, Y.K.; Wong, H.L.; Poon, S.H.; Lo, A.C.; Shih, K.C.; Tong, L. A Review of the Impact of Alterations in Gut Microbiome on the Immunopathogenesis of Ocular Diseases. J. Clin. Med. 2021, 10, 4694. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Miwa, Y.; Jounai, K.; Fujiwara, D.; Kurihara, T.; Kanauchi, O. Lactobacillus paracasei KW3110 Prevents Blue Light-Induced Inflammation and Degeneration in the Retina. Nutrients 2018, 10, 1991. [Google Scholar] [CrossRef]
- Morita, Y.; Jounai, K.; Sakamoto, A.; Tomita, Y.; Sugihara, Y.; Suzuki, H.; Ohshio, K.; Otake, M.; Fujiwara, D.; Kanauchi, O.; et al. Long-term intake of Lactobacillus paracasei KW3110 prevents age-related chronic inflammation and retinal cell loss in physiologically aged mice. Aging 2018, 10, 2723–2740. [Google Scholar] [CrossRef] [PubMed]
- Lima-Fontes, M.; Meira, L.; Barata, P.; Falcão, M.; Carneiro, Â. Gut microbiota and age-related macular degeneration: A growing partnership. Surv. Ophthalmol. 2022, 67, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Jabbehdari, S.; Sallam, A.B. Gut microbiome and diabetic retinopathy. Eur. J. Ophthalmol. 2022, 32, 2494–2497. [Google Scholar] [CrossRef]
- Bai, J.; Wan, Z.; Zhang, Y.; Wang, T.; Xue, Y.; Peng, Q. Composition and diversity of gut microbiota in diabetic retinopathy. Front. Microbiol. 2022, 13, 926926. [Google Scholar] [CrossRef]
- Liu, K.; Zou, J.; Fan, H.; Hu, H.; You, Z. Causal effects of gut microbiota on diabetic retinopathy: A Mendelian randomization study. Front. Immunol. 2022, 13, 930318. [Google Scholar] [CrossRef]
- Feuerbach, C.M. “Man is what he eats”: A rectification. J. Hist. Ideas 1963, 24, 397–406. [Google Scholar]
- Cizza, G.; Rother, K.I. Was Feuerbach right: Are we what we eat? J. Clin. Investig. 2011, 121, 2969–2971. [Google Scholar] [CrossRef]
- Pache, M.; Flammer, J. A sick eye in a sick body? Systemic findings in patients with primary open-angle glaucoma. Surv. Ophthalmol. 2006, 51, 179–212. [Google Scholar] [CrossRef]
- Man, A.W.C.; Li, H.; Xia, N. Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function. Oxid. Med. Cell Longev. 2020, 2020, 1496462. [Google Scholar] [CrossRef]
- Hirschberg, S.; Gisevius, B.; Duscha, A.; Haghikia, A. Implications of Diet and The Gut Microbiome in Neuroinflammatory and Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3109. [Google Scholar] [CrossRef]
- Rezende, F.A.; Lapalme, E.; Qian, C.X.; Smith, L.E.; Sangiovanni, J.P.; Sapieha, P. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 1071–1078. [Google Scholar] [CrossRef]
- Semeraro, F.; Gambicordi, E.; Cancarini, A.; Morescalchi, F.; Costagliola, C.; Russo, A. Treatment of exudative age-related macular degeneration with aflibercept combined with pranoprofen eye drops or nutraceutical support with omega-3: A randomized trial. Br. J. Clin. Pharmacol. 2019, 85, 908–913. [Google Scholar] [CrossRef]

|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusciano, D.; Bagnoli, P. Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases. Medicina 2023, 59, 1334. https://doi.org/10.3390/medicina59071334
Rusciano D, Bagnoli P. Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases. Medicina. 2023; 59(7):1334. https://doi.org/10.3390/medicina59071334
Chicago/Turabian StyleRusciano, Dario, and Paola Bagnoli. 2023. "Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases" Medicina 59, no. 7: 1334. https://doi.org/10.3390/medicina59071334
APA StyleRusciano, D., & Bagnoli, P. (2023). Pharmacotherapy and Nutritional Supplements for Neovascular Eye Diseases. Medicina, 59(7), 1334. https://doi.org/10.3390/medicina59071334









