Immersion Ultrasound Therapy in Combination with Manual Therapy in the Treatment of Ischemic Digital Ulcers in Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
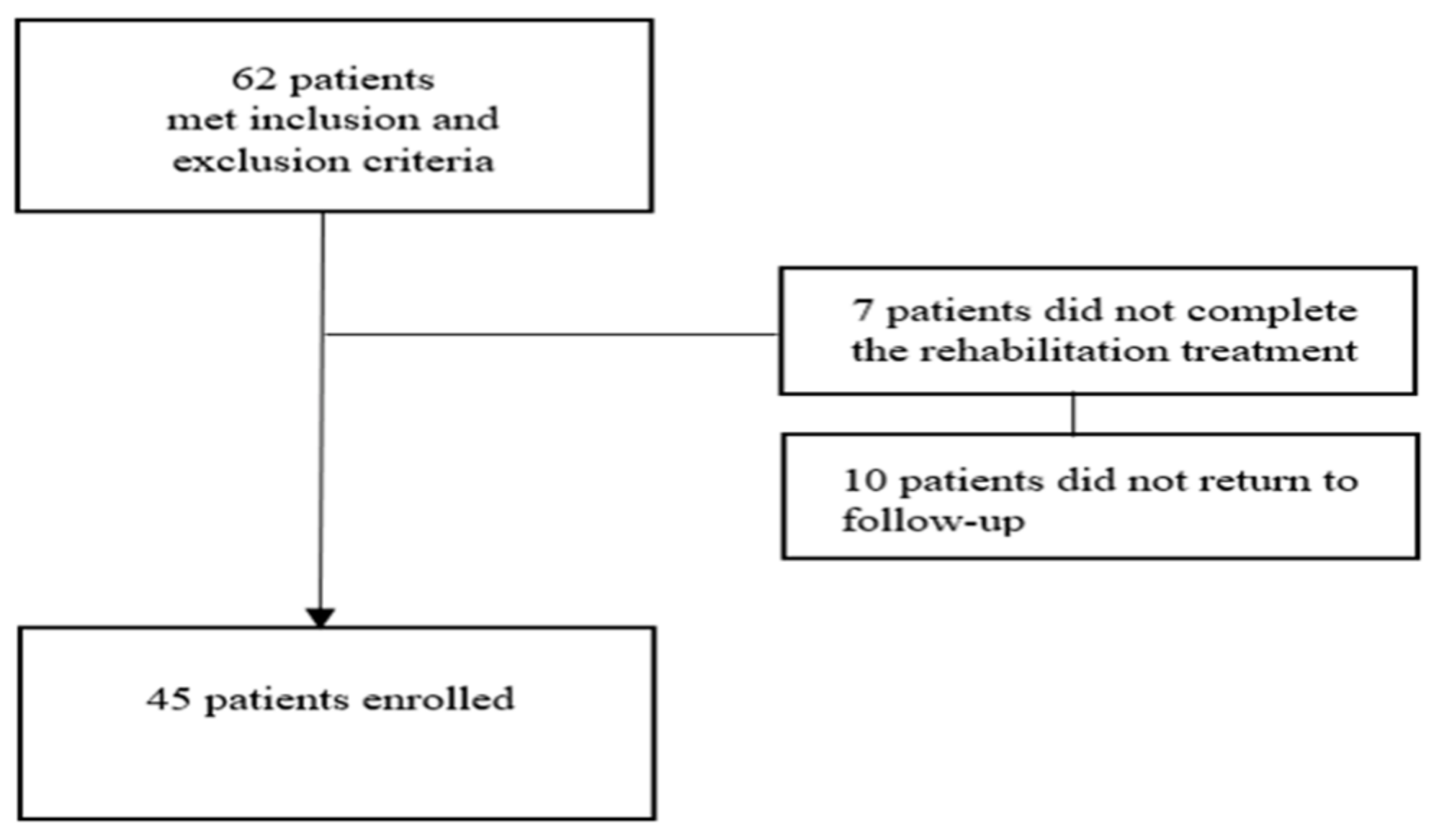
2.1. Patients
2.2. Intervention and Control
2.3. Description of Manual Therapy Techniques
2.4. Description of Therapeutic US Technique
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinchcliff, M.; Varga, J. Systemic sclerosis/scleroderma: A treatable multisystem disease. Am. Fam. Physician 2008, 78, 961–968. [Google Scholar] [PubMed]
- Di Benedetto, P.; Ruscitti, P.; Liakouli, V.; Cipriani, P.; Giacomelli, R. The Vessels Contribute to Fibrosis in Systemic Sclerosis. Isr. Med. Assoc. J. 2019, 21, 471–474. [Google Scholar] [PubMed]
- Ho, Y.Y.; Lagares, D.; Tager, A.M.; Kapoor, M. Fibrosis—A lethal component of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Vold, A.M.; Midtvedt, Ø.; Molberg, Ø.; Garen, T.; Gran, J.T. Prevalence of systemic sclerosis in south-east Norway. Rheumatology 2012, 51, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Kaipiainen-Seppänen, O.; Aho, K. Incidence of rare systemic rheumatic and connective tissue diseases in Finland. J. Intern. Med. 1996, 240, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Bernatsky, S.; Joseph, L.; Pineau, C.A.; Belisle, P.; Hudson, M.; Clarke, A.E. Scleroderma prevalence: Demographic variations in a population-based sample. Arthritis Rheum. 2009, 61, 400–404. [Google Scholar] [CrossRef]
- Nikpour, M.; Stevens, W.M.; Herrick, A.L.; Proudman, S.M. Epidemiology of systemic sclerosis. Best. Pract. Res. Clin. Rheumatol. 2010, 24, 857–869. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.; Cutolo, M.; Czirjak, L.; et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Hughes, M.; Bruni, C.; Ruaro, B.; Confalonieri, M.; Matucci-Cerinic, M.; Bellando-Randone, S. Digital Ulcers in Systemic Sclerosis. Presse Med. 2021, 50, 104064. [Google Scholar] [CrossRef]
- Abraham, S.; Steen, V. Optimal management of digital ulcers in systemic sclerosis. Ther. Clin. Risk Manag. 2015, 15, 939–947. [Google Scholar]
- Starnoni, M.; Pappalardo, M.; Spinella, A.; Testoni, S.; Lattanzi, M.; Feminò, R.; De Santis, G.; Salvarani, C.; Giuggioli, D. Systemic sclerosis cutaneous expression: Management of skin fibrosis and digital ulcers. Ann. Med. Surg. 2021, 71, 102984. [Google Scholar] [CrossRef]
- Poole, J.L.; Macintyre, N.J.; Deboer, H.N. Evidence-based management of hand and mouth disability in a woman living with diffuse systemic sclerosis (scleroderma). Physiother Can. 2013, 65, 317–320. [Google Scholar] [CrossRef]
- Badea, I.; Taylor, M.; Rosenberg, A.; Foldvari, M. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology 2009, 48, 213–221. [Google Scholar] [CrossRef]
- Kreuter, A.; Hyun, J.; Stücker, M.; Sommer, A.; Altmeyer, P.; Gambichler, T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J. Am. Acad. Dermatol. 2006, 54, 440–447. [Google Scholar] [CrossRef]
- Elst, E.F.; Van Suijlekom-Smit, L.W.; Oranje, A.P. Treatment of linear scleroderma with oral 1,25-dihydroxyvitamin D3 (calcitriol) in seven children. Pediatr. Dermatol. 1999, 16, 53–58. [Google Scholar] [CrossRef]
- Mizutani, H.; Yoshida, T.; Nouchi, N.; Hamanaka, H.; Shimizu, M. Topical tocoretinate improved hypertrophic scar, skin sclerosis in systemic sclerosis and morphea. J. Dermatol. 1999, 26, 11–17. [Google Scholar] [CrossRef]
- Gheisari, M.; Ahmadzadeh, A.; Nobari, N.; Iranmanesh, B.; Mozafari, N. Autologous Fat Grafting in the Treatment of Facial Scleroderma. Dermatol. Res. Pract. 2018, 2018, 6568016. [Google Scholar] [CrossRef]
- Griffin, M.F.; Almadori, A.; Butler, P.E. Use of Lipotransfer in Scleroderma. Aesthet. Surg. J. 2017, 37, S33–S37. [Google Scholar] [CrossRef]
- Scuderi, N.; Ceccarelli, S.; Onesti, M.G.; Fioramonti, P.; Guidi, C.; Romano, F.; Frati, L.; Angeloni, A.; Marchese, C. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013, 22, 779–795. [Google Scholar] [CrossRef]
- Pirrello, R.; Verro, B.; Grasso, G.; Ruscitti, P.; Cordova, A.; Giacomelli, R.; Ciccia, F.; Guggino, G. Hyaluronic acid and platelet-rich plasma, a new therapeutic alternative for scleroderma patients: A prospective open-label study. Arthritis Res. Ther. 2019, 21, 286. [Google Scholar] [CrossRef] [PubMed]
- Pirrello, R.; Schinocca, C.; Scaturro, D.; Rizzo, C.; Terrana, P.; Tumminelli, L.; Ruscitti, P.; Cordova, A.; Giacomelli, R.; Ciccia, F.; et al. Additional Treatment for Digital Ulcers in Patients with Systemic Sclerosis A Prospective Open-Label Multi-Arm Study for the use of Platelet Rich Plasma Lipofilling and Ultrasound-Based Treatments. J. Biotechnol. Biomed. 2021, 4, 84–107. [Google Scholar] [CrossRef]
- Samuels, J.A.; Weingarten, M.S.; Margolis, D.J.; Zubkov, L.; Sunny, Y.; Bawiec, C.R.; Conover, D.; Lewin, P.A. Low-frequency (<100 kHz), low-intensity (<100 mW/cm2) ultrasound to treat venous ulcers: A human study and in vitro experiments. J. Acoust. Soc. Am. 2013, 134, 1541–1547. [Google Scholar] [PubMed]
- Lai, J.; Pittelkow, M.R. Physiological effects of ultrasound mist on fibroblasts. Int. J. Dermatol. 2007, 46, 587–593. [Google Scholar] [CrossRef]
- Scaturro, D.; Guggino, G.; Terrana, P.; Vitagliani, F.; Falco, V.; Cuntrera, D.; Benedetti, M.G.; Moretti, A.; Iolascon, G.; Mauro, G.L. Rehabilitative interventions for ischaemic digital ulcers, pain, and hand functioning in systemic sclerosis: A prospective before-after study. BMC Musculoskelet. Disord. 2022, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Maddali Bongi, S.; Del Rosso, A.; Galluccio, F.; Sigismondi, F.; Miniati, I.; Conforti, M.L.; Nacci, F.; Matucci Cerinic, M. Efficacy of connective tissue massage and McMennell joint manipulation in the rehabilitative treatment of the hands in systemic sclerosis. Clin. Rheumatol. 2009, 28, 1167–1173. [Google Scholar] [CrossRef]
- Greenman, P.E. Mobilization with and without impulse technique. In Principles of Manual Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003; pp. 107–112. [Google Scholar]
- Alpiner, N.; Oh, T.H.; Hinderer, S.R.; Brander, V.A. Rehabilitation in joint and connective tissue diseases. 1. Systemic diseases. Arch. Phys. Med. Rehabil. 1995, 75, S32–S40. [Google Scholar] [CrossRef]
- Hylands-White, N.; Duarte, R.V.; Raphael, J.H. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef]
- Brower, L.M.; Poole, J.L. Reliability and validity of the Duruoz Hand Index in persons with systemic sclerosis (scleroderma). Arthritis Rheum. 2004, 51, 805–809, Correction in Arthritis Rheum. 2005, 53, 303. [Google Scholar] [CrossRef]
- Sugihara, F.; Inoue, N.; Venkateswarathirukumara, S. Ingestion of bioactive collagen hydrolysates enhanced pressure ulcer healing in a randomized double-blind placebo-controlled clinical study. Sci. Rep. 2018, 8, 11403. [Google Scholar] [CrossRef]
- Li, L.; Cui, Y.; Chen, S.; Zhao, Q.; Fu, T.; Ji, J.; Li, L.; Gu, Z. The impact of systemic sclerosis on health-related quality of life assessed by SF-36: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2018, 21, 1884–1893. [Google Scholar] [CrossRef]
- Korn, J.H.; Mayes, M.; Cerinic, M.M.; Rainisio, M.; Pope, J.; Hachulla, E.; Rich, E.; Carpentier, P.; Molitor, J.; Seibold, J.R.; et al. Digital ulcers in systemic sclerosis: Prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004, 50, 3985–3993. [Google Scholar] [CrossRef]
- Schiopu, E.; Impens, A.J.; Phillips, K. Digital ischemia in scleroderma spectrum of diseases. Int. J. Rheumatol. 2010, 2010, 923743. [Google Scholar] [CrossRef]
- Mouthon, L.; Carpentier, P.H.; Lok, C.; Clerson, P.; Gressin, V.; Hachulla, E.; Bérezné, A.; Diot, E.; Van Kien, A.K.; Jego, P.; et al. Ischemic digital ulcers affect hand disability and pain in systemic sclerosis. J. Rheumatol. 2014, 41, 1317–1323. [Google Scholar] [CrossRef]
- Mugii, N.; Hamaguchi, Y.; Maddali-Bongi, S. Clinical significance and usefulness of rehabilitation for systemic sclerosis. J. Scleroderma Relat. Disord. 2018, 3, 71–80. [Google Scholar] [CrossRef]
- Moran, M.E. Scleroderma and evidence based non-pharmaceutical treatment modalities for digital ulcers: A systematic review. J. Wound Care 2014, 23, 510–516. [Google Scholar] [CrossRef]
- Abousenna, M.M.H.S.; Shalaby, A.S.M.; Chahal, A.; Shaphe, A. A comparison of low dose ultrasound and far-infrared therapies in patients with mechanical neck pain. J. Pak. Med. Assoc. 2021, 71, 397–401. [Google Scholar] [CrossRef]
- Morishita, K.; Karasuno, H.; Yokoi, Y.; Morozumi, K.; Ogihara, H.; Ito, T.; Hanaoka, M.; Fujiwara, T.; Fujimoto, T.; Abe, K. Effects of therapeutic ultrasound on range of motion and stretch pain. J. Phys. Ther. Sci. 2014, 26, 711–715. [Google Scholar] [CrossRef]
- Liguori, P.A.; Peters, K.L.; Bowers, J.M. Combination of negative pressure wound therapy and acoustic pressure wound therapy for treatment of infected surgical wounds: A case series. Ostomy Wound Manag. 2008, 54, 50–53. [Google Scholar]
| Characteristics | Total (n = 45) | Treatment Group (n = 24) | Standard Care Group (n = 21) | p-Value |
|---|---|---|---|---|
| Age, mean ± SD | 61.12 ± 8.83 | 61.05 ± 9.3 | 61.2 ± 8.34 | 0.96 |
| Sex, n° (%) | ||||
| Male | 12 (26.7) | 7 (29.1) | 5 (23.8) | 0.88 |
| Female | 33 (73.3) | 17 (70.9) | 16 (76.2) | |
| BMI (kg/m2), mean ± SD | 24.6 ± 2.11 | 24.3 ± 1.88 | 25.1 ± 1.55 | 0.56 |
| DHI, mean±SD | 28.5 ± 10.4 | 28.15 ± 11.0 | 28.85 ± 9.72 | 0.82 |
| NRS, mean ± SD | 5.52 ± 1.22 | 5.55 ± 1.2 | 5.5 ± 1.24 | 0.89 |
| PSST, mean ± SD | 24.32 ± 4.14 | 24.4 ± 4.0 | 24.25 ± 4.27 | 0.90 |
| SF-36, mean ± SD | 57.2 ± 7.98 | 57.05 ± 9.1 | 57.35 ± 6.66 | 0.65 |
| Characteristics | T0 | T1 | p-Value |
|---|---|---|---|
| DHI, mean ± SD | 28.15 ± 11.0 | 19.05 ± 8.83 | <0.05 |
| NRS, mean ± SD | 5.55 ± 1.2 | 2.9 ± 1.09 | <0.05 |
| PSST, mean ± SD | 24.4 ± 4.0 | 16.2 ± 2.36 | <0.05 |
| SF-36, mean ± SD | 57.05 ± 9.1 | 52.0 ± 8.75 | 0.08 |
| Characteristics | T0 | T1 | p-Value |
|---|---|---|---|
| DHI, mean ± SD | 28.85 ± 9.72 | 23.7 ± 7.68 | <0.05 |
| NRS, mean ± SD | 5.5 ± 1.24 | 4.5 ± 1.07 | 0.08 |
| PSST, mean ± SD | 24.25 ± 4.27 | 20.4 ± 4.02 | 0.16 |
| SF-36, mean ± SD | 57.35 ± 6.66 | 54.5 ± 6.54 | 0.18 |
| Characteristics | Treatment Group | Standard Care Group | p-Value |
|---|---|---|---|
| DHI, mean ± SD | 19.05 ± 8.83 | 23.7 ± 7.68 | 0.07 |
| NRS, mean ± SD | 2.9 ± 1.09 | 4.5 ± 1.07 | <0.05 |
| PSST, mean ± SD | 16.2 ± 2.36 | 20.4 ± 4.02 | <0.05 |
| SF-36, mean ± SD | 52.0 ± 8.75 | 54.5 ± 6.54 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaturro, D.; Moretti, A.; Vitagliani, F.; Guggino, G.; Tomasello, S.; Lo Nardo, D.; Lauricella, L.; Iolascon, G.; Letizia Mauro, G. Immersion Ultrasound Therapy in Combination with Manual Therapy in the Treatment of Ischemic Digital Ulcers in Systemic Sclerosis. Medicina 2023, 59, 1335. https://doi.org/10.3390/medicina59071335
Scaturro D, Moretti A, Vitagliani F, Guggino G, Tomasello S, Lo Nardo D, Lauricella L, Iolascon G, Letizia Mauro G. Immersion Ultrasound Therapy in Combination with Manual Therapy in the Treatment of Ischemic Digital Ulcers in Systemic Sclerosis. Medicina. 2023; 59(7):1335. https://doi.org/10.3390/medicina59071335
Chicago/Turabian StyleScaturro, Dalila, Antimo Moretti, Fabio Vitagliani, Giuliana Guggino, Sofia Tomasello, Davide Lo Nardo, Lorenza Lauricella, Giovanni Iolascon, and Giulia Letizia Mauro. 2023. "Immersion Ultrasound Therapy in Combination with Manual Therapy in the Treatment of Ischemic Digital Ulcers in Systemic Sclerosis" Medicina 59, no. 7: 1335. https://doi.org/10.3390/medicina59071335
APA StyleScaturro, D., Moretti, A., Vitagliani, F., Guggino, G., Tomasello, S., Lo Nardo, D., Lauricella, L., Iolascon, G., & Letizia Mauro, G. (2023). Immersion Ultrasound Therapy in Combination with Manual Therapy in the Treatment of Ischemic Digital Ulcers in Systemic Sclerosis. Medicina, 59(7), 1335. https://doi.org/10.3390/medicina59071335








