Multiple Myeloma and Kidney Impairment at Diagnosis: A Nephrological Perspective from an Eastern European Country
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
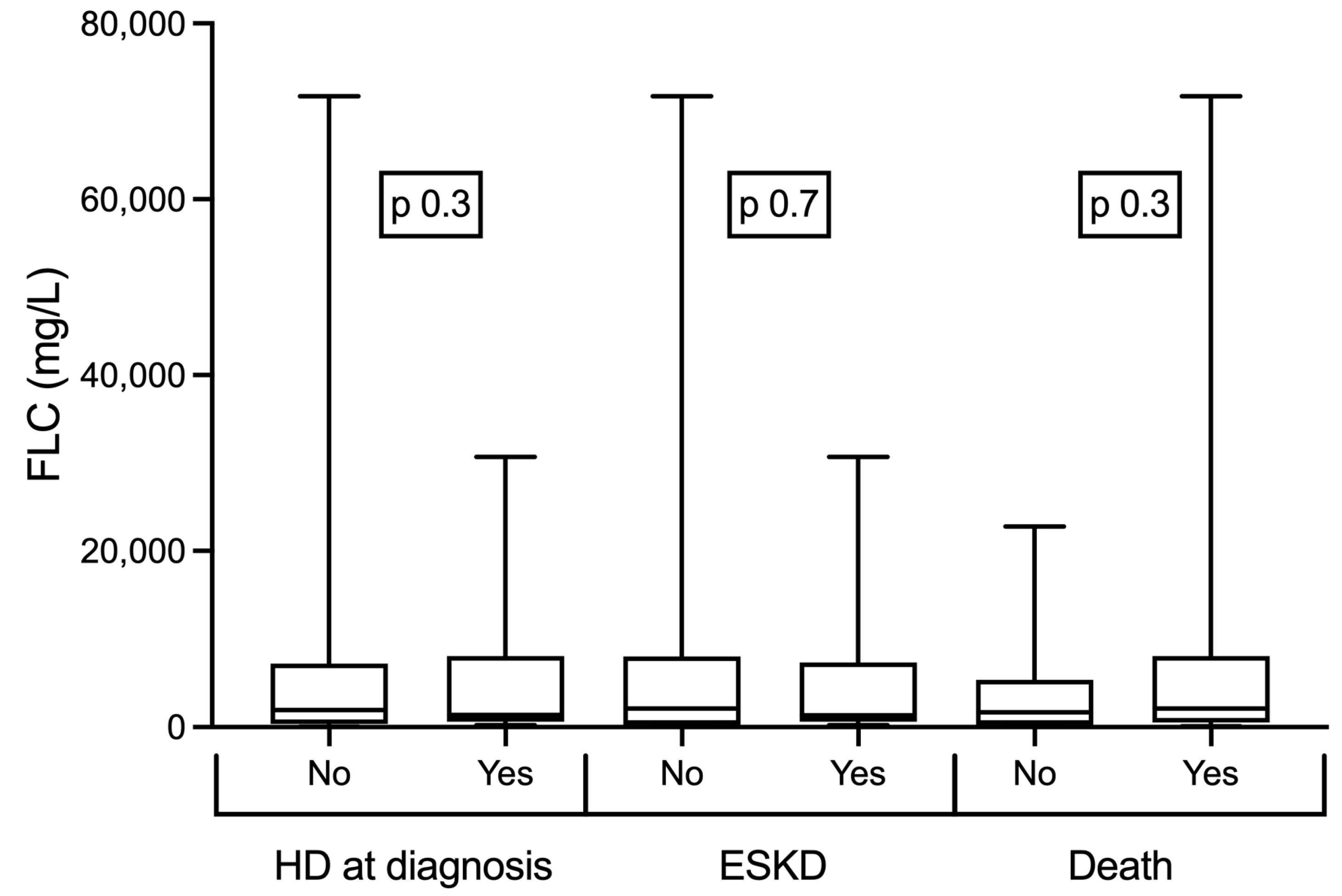
3. Results
3.1. Patient Characteristics and Clinical Features at Presentation
3.2. Multiple Myeloma Characteristics
3.3. Renal Characteristics
3.4. Treatment and Outcomes
3.5. Risk Factors for Mortality
4. Discussion
| Study/ Year/ Country | Blade et al. [9]/ 1998/ Spain | Knudsen et al. [10]/ 2000/ Denmark | Suyani et al. [15]/ 2011/ Turkey | Dimopoulos et al. [16]/ 2013/ Greece | Park et al. [17]/ 2014/ Korea | Gonsalves et al. [12]/ 2015/ USA | Dimopoulos et al. [18]/ 2017/ Greece | Ho et al. [19]/ 2019/ Australia& New Zealand | Royal et al. [13]/ 2020/ Multicenter: Europe and North America | Yadav et al. [14]/ 2020/ UK | Chen et al. [20]/ 2020/ China | Sharma et al. [21]/ 2022/ India | Present Study/ Romania |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of KI patients | 94/423 (22%); SCr >2 mg/dL | 225/775 (29%); SCr > 1.47 mg/dL | 8/30 (27%); SCr > 2 mg/dL | 133 eGFR < 60 mL/min | 117/ 379 (31%); eGFR <60 mL/min | 192/1135 (20%) eGFR < 40 mL/min | 52 (only HD patients included) | 383/ 1069 (36%) | 178 LCCN KB proven | 103 LCCN KB proven | 121/393 (31%) eGFR < 40 mL/min | 91/216 (42%) | 89 |
| eGFR at diagnosis (mL/min) | NA | NA | 11 | 32 | NA | CrS 2.6 mg/dL | NA | 39 | 13 | 7 | 16.3 | 15.3 | 9 |
| Type of FLC involved most frequently | Lambda (51%) | Kappa (31%) | Kappa (88%) | NA | NA | NA | NA | Kappa (63%) | Lambda (53%) | Kappa (54%) | NA | Kappa (61%) | Kappa (53%) |
| Involved FLC median (mg/L) | NA | NA | NA | NA | NA | 2100 | 9080 | NA | 5010 | 7531 | NA | NA | 1760 |
| LC-restricted MM in KI (%) | 32 | 42 | 38 | 21 | NA | 32 | NA | 25 | 48 | 48 | 30 | 26 | 55 |
| HD required at diagnosis (%) | 36 | 4 | 50 | 8 | 15 | 16 | 6 (52 of 796) | 4 | 47 | 67 | 17 | 17 | 38 |
| Factors associated with improved survival | SCr < 4 mg/dl; response to chemotherapy | Younger age; Stage I, II MM; hypocalcemia; renal recovery | Renal recovery | NA | Complete renal response | Age < 70 y; HD free; absence of high-risk cytogenetics | HD free; absence of high-risk cytogenetics | No KI | Higher eGFR; hematological response (VGPR or higher) | HD free under 12 mo from diagnosis | No KI | HD free; hematologic response | No HD at diagnosis; higher serum albumin |
| Mortality in KI (%) | 29 (first 2 months) | 12 (first 3 months) | 13 (first 3 months) | 8 (first 2 months) | NA | 16 (first 6 months) | 16 | 31 | 58 (at a median of 13 months after diagnosis) | 64 | 23 (first 2 months) | 15 (first 2 months) | 42 (during the study period) |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bridoux, F.; Leung, N.; Belmouaz, M.; Royal, V.; Ronco, P.; Nasr, S.H.; Fermand, J.P.; International, K.; International Kidney and Monoclonal Gammopathy Research Group. Management of acute kidney injury in symptomatic multiple myeloma. Kidney Int. 2021, 99, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Sonneveld, P.; Leung, N.; Merlini, G.; Ludwig, H.; Kastritis, E.; Goldschmidt, H.; Joshua, D.; Orlowski, R.Z.; Powles, R.; et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J. Clin. Oncol. 2016, 34, 1544–1557. [Google Scholar] [CrossRef]
- Coriu, D.; Dytfeld, D.; Niepel, D.; Spicka, I.; Markuljak, I.; Mihaylov, G.; Ostojic-Kolonic, S.; Fink, L.; Toka, K.S.; Bjorklof, K. Real-world multiple myeloma management practice patterns and outcomes in selected Central and Eastern European countries. Pol. Arch. Intern. Med. 2018, 128, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Winearls, C.G. Acute myeloma kidney. Kidney Int 1995, 48, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.C.; Mo, K.L.; Chan, C.K.; Lo, H.K.; Wong, K.S.; Chan, J.C. Renal impairment in patients with multiple myeloma. Hong Kong Med. J. 2003, 9, 78–82. [Google Scholar]
- Bridoux, F.; Arnulf, B.; Karlin, L.; Blin, N.; Rabot, N.; Macro, M.; Audard, V.; Belhadj, K.; Pegourie, B.; Gobert, P.; et al. Randomized Trial Comparing Double Versus Triple Bortezomib-Based Regimen in Patients With Multiple Myeloma and Acute Kidney Injury Due to Cast Nephropathy. J. Clin. Oncol. 2020, 38, 2647–2657. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Roussou, M.; Gavriatopoulou, M.; Psimenou, E.; Eleutherakis-Papaiakovou, E.; Migkou, M.; Matsouka, C.; Mparmparousi, D.; Gika, D.; Kafantari, E.; et al. Bortezomib-based triplets are associated with a high probability of dialysis independence and rapid renal recovery in newly diagnosed myeloma patients with severe renal failure or those requiring dialysis. Am. J. Hematol. 2016, 91, 499–502. [Google Scholar] [CrossRef]
- Blade, J.; Fernandez-Llama, P.; Bosch, F.; Montoliu, J.; Lens, X.M.; Montoto, S.; Cases, A.; Darnell, A.; Rozman, C.; Montserrat, E. Renal failure in multiple myeloma: Presenting features and predictors of outcome in 94 patients from a single institution. Arch. Intern. Med. 1998, 158, 1889–1893. [Google Scholar] [CrossRef]
- Knudsen, L.M.; Hjorth, M.; Hippe, E. Renal failure in multiple myeloma: Reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur. J. Haematol. 2000, 65, 175–181. [Google Scholar] [CrossRef]
- Yadav, P.; Hutchison, C.A.; Basnayake, K.; Stringer, S.; Jesky, M.; Fifer, L.; Snell, K.; Pinney, J.; Drayson, M.T.; Cook, M.; et al. Patients with multiple myeloma have excellent long-term outcomes after recovery from dialysis-dependent acute kidney injury. Eur. J. Haematol. 2016, 96, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, W.I.; Leung, N.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Dingli, D.; Kapoor, P.; Go, R.S.; et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015, 5, e296. [Google Scholar] [CrossRef] [PubMed]
- Royal, V.; Leung, N.; Troyanov, S.; Nasr, S.H.; Ecotiere, L.; LeBlanc, R.; Adam, B.A.; Angioi, A.; Alexander, M.P.; Asunis, A.M.; et al. Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: A multicenter retrospective study. Blood 2020, 135, 1833–1846. [Google Scholar] [CrossRef]
- Yadav, P.; Sathick, I.J.; Leung, N.; Brown, E.E.; Cook, M.; Sanders, P.W.; Cockwell, P. Serum free light chain level at diagnosis in myeloma cast nephropathy-a multicentre study. Blood Cancer J. 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Suyani, E.; Sucak, G.T.; Erten, Y.; Cakar, M.K.; Ulusal, G.; Yagci, M.; Haznedar, R. Evaluation of multiple myeloma patients presenting with renal failure in a university hospital in the year 2010. Ren. Fail. 2012, 34, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Roussou, M.; Gkotzamanidou, M.; Nikitas, N.; Psimenou, E.; Mparmparoussi, D.; Matsouka, C.; Spyropoulou-Vlachou, M.; Terpos, E.; Kastritis, E. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia 2013, 27, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Han, B.; Kim, K.; Kim, S.J.; Jang, J.H.; Kim, W.S.; Jung, C.W. Renal Insufficiency in newly-diagnosed multiple myeloma: Analysis according to International Myeloma Working Group consensus statement. Anticancer Res. 2014, 34, 4299–4306. [Google Scholar]
- Dimopoulos, M.A.; Roussou, M.; Gavriatopoulou, M.; Fotiou, D.; Ziogas, D.C.; Migkou, M.; Panagiotidis, I.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Psimenou, E.; et al. Outcomes of newly diagnosed myeloma patients requiring dialysis: Renal recovery, importance of rapid response and survival benefit. Blood Cancer J. 2017, 7, e571. [Google Scholar] [CrossRef]
- Ho, P.J.; Moore, E.M.; McQuilten, Z.K.; Wellard, C.; Bergin, K.; Augustson, B.; Blacklock, H.; Harrison, S.J.; Horvath, N.; King, T.; et al. Renal Impairment at Diagnosis in Myeloma: Patient Characteristics, Treatment, and Impact on Outcomes. Results From the Australia and New Zealand Myeloma and Related Diseases Registry. Clin. Lymphoma Myeloma Leuk. 2019, 19, e415–e424. [Google Scholar] [CrossRef]
- Chen, X.; Luo, X.; Zu, Y.; Issa, H.A.; Li, L.; Ye, H.; Yang, T.; Hu, J.; Wei, L. Severe renal impairment as an adverse prognostic factor for survival in newly diagnosed multiple myeloma patients. J. Clin. Lab. Anal. 2020, 34, e23416. [Google Scholar] [CrossRef]
- Sharma, R.; Jain, A.; Jandial, A.; Lad, D.; Khadwal, A.; Prakash, G.; Nada, R.; Aggarwal, R.; Ramachandran, R.; Varma, N.; et al. Lack of Renal Recovery Predicts Poor Survival in Patients of Multiple Myeloma With Renal Impairment. Clin. Lymphoma Myeloma Leuk. 2022, 22, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Yoo, C.; Lee, D.H.; Kim, S.W.; Lee, J.S.; Suh, C. Serum albumin level is a significant prognostic factor reflecting disease severity in symptomatic multiple myeloma. Ann. Hematol 2010, 89, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Klein, B.; Bataille, R. Interleukin-6 is a potent myeloma-cell growth factor in patients with aggressive multiple myeloma. Blood 1989, 74, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.; Tu, Y.; Fady, C.; Vescio, R.; Berenson, J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol. 1995, 162, 248–255. [Google Scholar] [CrossRef]
- Rosean, T.R.; Tompkins, V.S.; Tricot, G.; Holman, C.J.; Olivier, A.K.; Zhan, F.; Janz, S. Preclinical validation of interleukin 6 as a therapeutic target in multiple myeloma. Immunol. Res. 2014, 59, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.O.; Wisloff, F.; Gulbrandsen, N. Reduced nutritional status among multiple myeloma patients during treatment with high-dose chemotherapy and autologous stem cell support. Clin. Nutr. 2010, 29, 488–491. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Wang, W.; Wang, Y.; Liang, Y. Inflammatory and Nutritional Scoring System for Predicting Prognosis in Patients with Newly Diagnosed Multiple Myeloma. J. Inflamm. Res. 2023, 16, 7–17. [Google Scholar] [CrossRef]
- Ludwig, H.; Nachbaur, D.M.; Fritz, E.; Krainer, M.; Huber, H. Interleukin-6 is a prognostic factor in multiple myeloma. Blood 1991, 77, 2794–2795. [Google Scholar] [CrossRef]
- Greipp, P.R.; San Miguel, J.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Blade, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Jacobson, J.L.; Hussein, M.A.; Barlogie, B.; Durie, B.G.; Crowley, J.J.; Southwest Oncology, G. A new staging system for multiple myeloma patients based on the Southwest Oncology Group (SWOG) experience. Br. J. Haematol. 2003, 122, 441–450. [Google Scholar] [CrossRef]

| Total N = 89 | Survivors n = 31 | Non-Survivors n = 58 | p | |
|---|---|---|---|---|
| Age (years) | 66 (60–74) | 62 (52–66) | 70 (63–78) | <0.001 |
| Male sex (%) | 38 | 45 | 35 | 0.3 |
| Comorbidities (%) | ||||
| Arterial hypertension | 71 | 71 | 71 | 0.9 |
| Systemic atherosclerosis | 58 | 32 | 72 | <0.001 |
| Ischemic heart disease | 44 | 26 | 53 | 0.01 |
| Diabetes mellitus | 12 | 7 | 16 | 0.2 |
| Clinical presentation (%) | ||||
| Bone pain | 51 | 55 | 48 | 0.5 |
| Asthenia | 75 | 58 | 85 | <0.001 |
| Dyspnea | 6 | 3 | 7 | 0.4 |
| Edema | 10 | 10 | 10 | 0.9 |
| Orthostatic hypotension | 14 | 10 | 16 | 0.4 |
| Peripheral neuropathy | 10 | 13 | 9 | 0.5 |
| Type of MM (%) | 0.2 | |||
| IgG kappa | 21 | 16 | 24 | |
| IgG lambda | 9 | 10 | 9 | |
| IgA kappa | 6 | 7 | 5 | |
| IgA lambda | 8 | 7 | 9 | |
| IgM lambda | 1 | 3 | 0 | |
| Free kappa | 26 | 39 | 35 | |
| Free lambda | 29 | 18 | 18 | |
| FLC type (%) | 0.2 | |||
| Kappa | 53 | 61 | 48 | |
| Lambda | 47 | 39 | 52 | |
| Kappa to lambda ratio | 5.6 (0.03–271.33) | 31.5 (0.06–380.95) | 0.38 (0.01–108.90) | 0.1 |
| FLC level (mg/L) | 1760 (450–7373) | 1583 (277–5134) | 1963 (514–8000) | 0.3 |
| Marrow plasmacytosis (%) | 30 (15–60) | 38 (15–69) | 30 (15–54) | 0.3 |
| Lytic bone lesions (%) | 47 | 58 | 41 | 0.1 |
| Hemoglobin (g/dL) | 9.4 (7.8–10.6) | 9.6 (7.7–11.5) | 9.3 (8.1–10.4) | 0.2 |
| M-component presence (%) | 61 | 54 | 65 | 0.3 |
| M-component level (%) | 36.0 (18.5–49.0) | 38 (26–51) | 36 (18–49) | 0.6 |
| Total serum protein (g/dL) | 7.4 (6.7–8.4) | 7.7 (7.1–9.0) | 7.3 (6.6–8.3) | 0.08 |
| Serum albumin (g/dL) | 3.8 (3.3–4.3) | 4.4 (3.7–4.6) | 3.7 (3.1–4.0) | <0.001 |
| Total serum calcium (mg/dL) | 9.9 (9.1–10.5) | 9.9 (9.5–10.6) | 9.7 (8.9–10.3) | 0.1 |
| Ionic serum calcium (mg/dL) | 4.2 (3.8–4.5) | 4.2 (3.9–4.4) | 4.1 (3.7–4.7) | 0.8 |
| LDH (UI/L) | 219 (186–315) | 201 (180–230) | 236 (200–334) | 0.01 |
| C-reactive protein (mg/L) | 9 (3–27) | 3 (1–17) | 14 (5–44) | <0.001 |
| Nephrotic syndrome (%) | 0.2 | |||
| AKI | 81 | 71 | 86 | |
| CKD | 12 | 16 | 10 | |
| Nephrotic syndrome | 2 | 3 | 2 | |
| Isolated proteinuria | 5 | 10 | 2 | |
| Serum creatinine (mg/dL) | 5.0 (2.9–8.2) | 2.5 (1.35–8.18) | 5.80 (3.85–9.60) | 0.02 |
| Proteinuria (g/g) | 3.3 (1.3–6.0) | 3.0 (1.0–6.0) | 3.6 (1.4–6.0) | 0.3 |
| Albuminuria (g/g) | 0.27 (0.11–0.66) | 0.24 (0.09–0.46) | 0.29 (0.13–0.75) | 0.3 |
| Alb to prot-uria ratio <10% (%) | 55 | 61 | 52 | 0.4 |
| Bence Jones protein (%) | 54 | 55 | 54 | 0.9 |
| Dexamethasone treatment (%) | 74 | 71 | 76 | 0.6 |
| Dexamethasone dose (mg) | 128 (96–160) | 128 (112–160) | 128 (80–160) | 0.2 |
| Hemodialysis initiation at diagnosis (%) | 38 | 19 | 48 | <0.01 |
| Kidney survival (%) | 58 | 93 | 40 | 0.01 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (years) | 1.07 (1.03, 1.12) | 0.001 | 1.07 (0.98, 1.16) | 0.09 |
| Male sex | 1.56 (0.64, 3.81) | 0.3 | - | |
| Comorbidities vs. absence | ||||
| Systemic atherosclerosis | 5.51 (2.13, 14.22) | <0.001 | 0.11 (0.01, 1.16) | 0.06 |
| Ischemic heart disease | 3.30 (1.26, 8.58) | <0.01 | 1.48 (0.18, 11.74) | 0.7 |
| Clinical presentation | ||||
| Asthenia vs. absence | 3.93 (1.43, 10.76) | 0.01 | 0.22 (0.03, 1.35) | 0.1 |
| FLC type: kappa vs. lambda | 0.58 (0.24, 1.43) | 0.2 | - | |
| M-component (%) | 0.99 (0.96, 1.02) | 0.9 | - | |
| LC restricted MM vs. whole Ig MM | 0.82 (0.34, 2.00) | 0.6 | - | |
| Serum albumin (g/dL) | 0.28 (0.13, 0.63) | 0.01 | 0.22 (0.05, 0.90) | 0.03 |
| Total serum calcium (mg/dL) | 0.94 (0.69, 1.28) | 0.9 | - | |
| C-reactive protein (mg/L) | 1.05 (1.01, 1.09) | 0.01 | 1.02 (0.98, 1.06) | 0.2 |
| LDH (UI/L) | 1.01 (1.00, 1.02) | 0.01 | 1.00 (0.99, 1.01) | 0.1 |
| Serum creatinine (mg/dL) | 1.06 (0.96, 1.16) | 0.2 | - | |
| Alb to prot-uria ratio <10% | 0.69 (0.27, 1.77) | 0.4 | - | |
| Bence Jones protein vs. absence | 0.96 (0.34, 2.72) | 0.9 | - | |
| Dexamethasone treatment vs. absence | 1.28 (0.48, 3.43) | 0.6 | - | |
| Dexamethasone dose (mg) | 0.99 (0.98, 1.00) | 0.2 | - | |
| HD initiation at diagnosis vs. absence | 2.94 (1.04, 8.26) | 0.04 | 10.64 (1.71, 66.14) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștefan, G.; Cinca, S.; Chiriac, C.; Zugravu, A.; Stancu, S. Multiple Myeloma and Kidney Impairment at Diagnosis: A Nephrological Perspective from an Eastern European Country. Medicina 2023, 59, 1326. https://doi.org/10.3390/medicina59071326
Ștefan G, Cinca S, Chiriac C, Zugravu A, Stancu S. Multiple Myeloma and Kidney Impairment at Diagnosis: A Nephrological Perspective from an Eastern European Country. Medicina. 2023; 59(7):1326. https://doi.org/10.3390/medicina59071326
Chicago/Turabian StyleȘtefan, Gabriel, Simona Cinca, Corina Chiriac, Adrian Zugravu, and Simona Stancu. 2023. "Multiple Myeloma and Kidney Impairment at Diagnosis: A Nephrological Perspective from an Eastern European Country" Medicina 59, no. 7: 1326. https://doi.org/10.3390/medicina59071326
APA StyleȘtefan, G., Cinca, S., Chiriac, C., Zugravu, A., & Stancu, S. (2023). Multiple Myeloma and Kidney Impairment at Diagnosis: A Nephrological Perspective from an Eastern European Country. Medicina, 59(7), 1326. https://doi.org/10.3390/medicina59071326





