Essential Thrombocythemia and Ischemic Stroke: A Case Series of Five JAK2-Positive Patients
Abstract
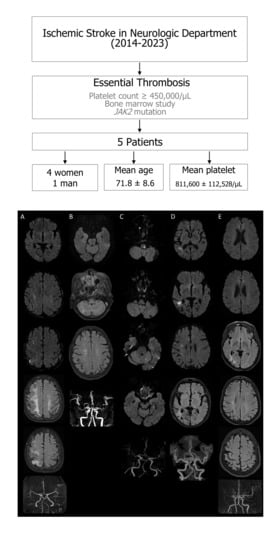
1. Introduction
2. Methods
3. Results
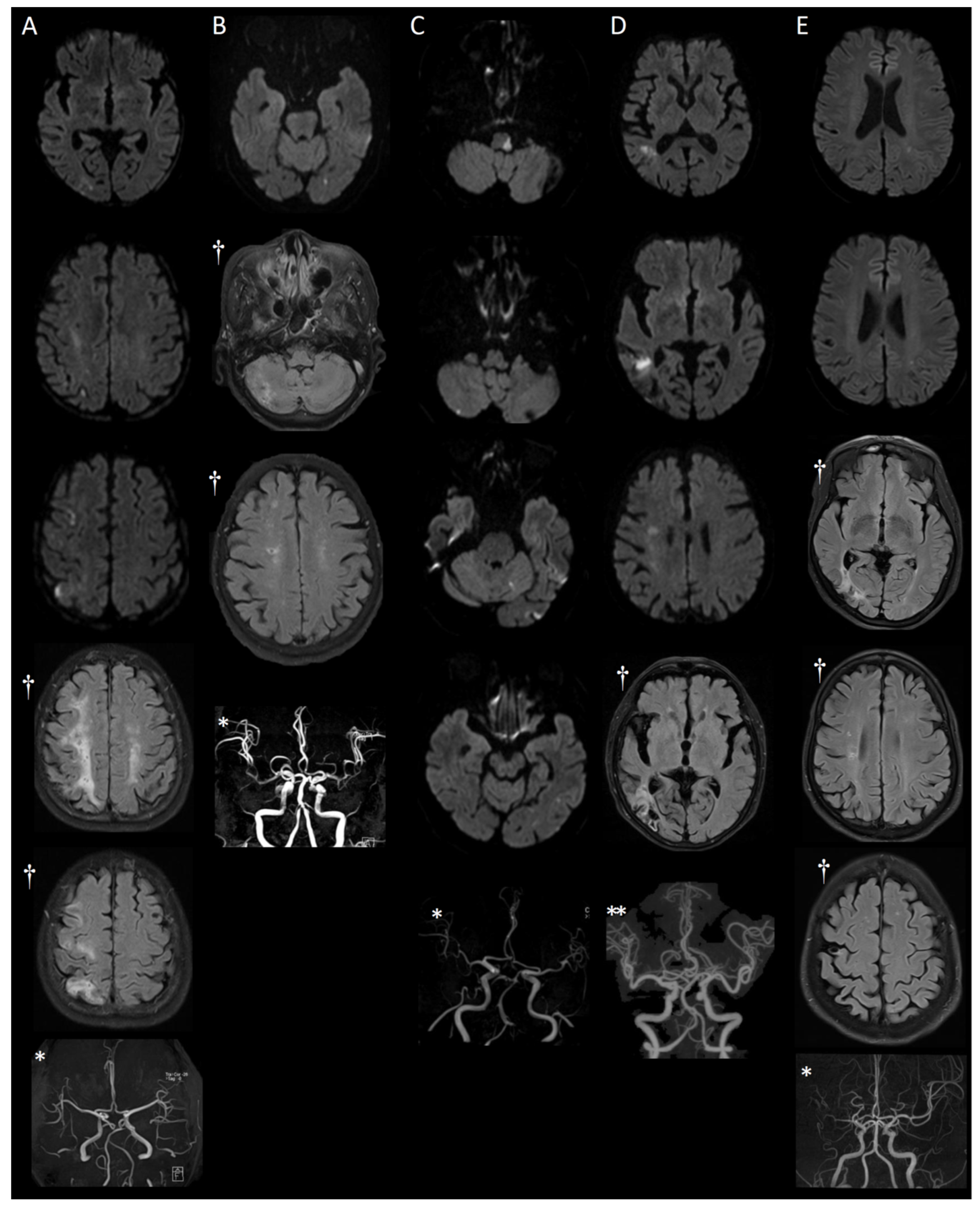
3.1. MRI Findings
3.2. Detailed Case Presentations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tefferi, A.; Thiele, J.; Vannucchi, A.M.; Barbui, T. An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia 2014, 28, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P. Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocythemia. Semin. Thromb. Hemost. 2006, 32, 171–173. [Google Scholar] [CrossRef]
- Szuber, N.; Mudireddy, M.; Nicolosi, M.; Penna, D.; Vallapureddy, R.R.; Lasho, T.L.; Tefferi, A. Mayo clinic patients with myeloproliferative neoplasms: Risk-stratified comparison of survival and outcomes data among disease subgroups. Mayo Clin. Proc. 2019, 94, 599–610. [Google Scholar] [CrossRef]
- Barbui, T.; Thiele, J.; Gisslinger, H.; Finazzi, G.; Vannucchi, A.M.; Tefferi, A. The 2016 revision of WHO classification of myeloproliferative neoplasms: Clinical and molecular advances. Blood Rev. 2016, 30, 453–459. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.J.; Scott, L.M.; Campbell, P.J.; East, C.; Fourouclas, N.; Swanton, S.; Green, A.R. Faculty Opinions recommendation of Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2016, 365, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Vainchenker, W.; Leroy, E.; Gilles, L.; Marty, C.; Plo, I.; Constantinescu, S.N. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Research 2018, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; Carobbio, A.; Thiele, J.; Passamonti, F.; Rumi, E.; Ruggeri, M.; Barbui, T. Incidence and risk factors for bleeding in 1104 patients with essential thrombocythemia or prefibrotic myelofibrosis diagnosed according to the 2008 WHO criteria. Leukemia 2012, 26, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Gall, S.L.; Tran, P.L.; Martin, K.; Blizzard, L.; Srikanth, V. Sex differences in long-term outcomes after stroke: Functional outcomes, handicap, and quality of life. Stroke 2012, 43, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Cortelazzo, S.; Finazzi, G.; Ruggeri, M.; Vestri, O.; Galli, M.; Rodeghiero, F.; Barbui, T. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N. Engl. J. Med. 1995, 332, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hayashi, T.; Sehara, Y.; Deguchi, I.; Fukuoka, T.; Maruyama, H.; Horiuchi, Y.; Nagamine, Y.; Sano, H.; Tanahashi, N. Ischemic Stroke with Essential Thrombocythemia: A Case Series. J. Stroke Cerebrovasc. Dis. 2015, 24, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Pósfai, É.; Marton, I.; Szőke, A.; Borbényi, Z.; Vécsei, L.; Csomor, A.; Sas, K. Stroke in essential thrombocythemia. J. Neurol. Sci. 2014, 336, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.; Dupriez, B.; Passamonti, F.; Vannucchi, A.M.; Morra, E.; Reilly, J.T.; Demory, J.-L.; Rumi, E.; Guglielmelli, P.; Roncoroni, E.; et al. Improving Survival Trends in Primary Myelofibrosis: An International Study. J. Clin. Oncol. 2012, 30, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Gangat, N.; Lasho, T.; Abou Hussein, A.K.; Elala, Y.C.; Hanson, C.; Tefferi, A. Validation of the revised international prognostic score of thrombosis for essential thrombocythemia (IPSET-thrombosis) in 585 Mayo clinic patients. Am. J. Hematol. 2016, 91, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Vannucchi, A.M.; Barbui, T. Essential thrombocythemia treatment algorithm 2018. Blood Cancer J. 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Rungjirajittranon, T.; Owattanapanich, W.; Ungprasert, P.; Siritanaratkul, N.; Ruchutrakool, T. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed]
| Case | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Gender | Female | Female | Male | Female | Female |
| Age | 64 | 85 | 67 | 80 | 63 |
| Presenting symptom | Left hemiparesis | Dizziness | Dizziness and impaired balance | Dizziness and gait disturbance | Right hemiparesis |
| Risk factors | |||||
| HTN | - | + | + | + | + |
| DM | - | - | - | - | |
| hyperlipidemia | + * (LDL 86) | + | + * (LDL 111) | + * (LDL 98) | + |
| Smoking | - | - | - | - | - |
| B-MRA | Unremarkable | Mild luminal irregularities in both MCA bifurcations, Focal stenosis in both VAs V4 segment | Occlusion of left VA V4 segment | Unremarkable | Steno-occlusive lesion of right MCA |
| Proximal vessel | Carotid USG (isoechoic plaque in both carotid bulb and right ICA, Calcified plaque in right carotid bulb and right ICA) | None | CTA (steno-occlusion of left VA V3–V4 segment) | CTA (mild focal stenosis with calcified plaque at both carotid bulb) Carotid USG (Hyperechoic plaque in Rt carotid bulb to ICA) | CTA (No remarkable finding in extracranial both carotid artery system) |
| TTE | Normal LV function, diastolic dysfunction grade I, No intracardiac thrombus | Concentric LVH with normal LV systolic function, Diastolic dysfunction grade I, No intracardiac thrombus | Mild concentric LVH and normal LV systolic function, diastolic dysfunction grade I, No intracardiac thrombus | Moderate AS and mild AR, Normal LV systolic function, Enlarged LA, Diastolic dysfunction grade I, No intracardiac thrombus | Concentric LVH with normal LV systolic function, Enlarged LA, Diastolic dysfunction grade I, No intracardiac thrombus |
| Holter | none | Frequent APCs Frequent VPCs | Rare APCs | Frequent APCs Rare VPCs | none |
| Antiplatelet | clopidogrel → cilostazol | clopidogrel | clopidogrel | clopidogrel | aspirin + clopidogrel |
| Cytoreductive therapy | ruxolitinib | hydroxyurea | hydroxyurea | hydroxyurea | hydroxyurea |
| NIHSS, initial | 4 | 1 | 4 | 1 | 2 |
| mRS initial | 3 | 2 | 4 | 2 | 1 |
| Admission duration (days) | 60 | 7 | 68 | 6 | 9 |
| NIHSS, at discharge | 1 | 0 | 1 | 0 | 0 |
| mRS at discharge | 1 | 1 | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-K.; Eah, K.Y.; Park, J.-M. Essential Thrombocythemia and Ischemic Stroke: A Case Series of Five JAK2-Positive Patients. Medicina 2023, 59, 1300. https://doi.org/10.3390/medicina59071300
Kim B-K, Eah KY, Park J-M. Essential Thrombocythemia and Ischemic Stroke: A Case Series of Five JAK2-Positive Patients. Medicina. 2023; 59(7):1300. https://doi.org/10.3390/medicina59071300
Chicago/Turabian StyleKim, Byong-Kyu, Kyung Yoon Eah, and Jin-Mo Park. 2023. "Essential Thrombocythemia and Ischemic Stroke: A Case Series of Five JAK2-Positive Patients" Medicina 59, no. 7: 1300. https://doi.org/10.3390/medicina59071300
APA StyleKim, B.-K., Eah, K. Y., & Park, J.-M. (2023). Essential Thrombocythemia and Ischemic Stroke: A Case Series of Five JAK2-Positive Patients. Medicina, 59(7), 1300. https://doi.org/10.3390/medicina59071300