Carryover Effects of Pain Neuroscience Education on Patients with Chronic Lower Back Pain: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Search Strategy
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Strategy for Data Synthesis
3. Results
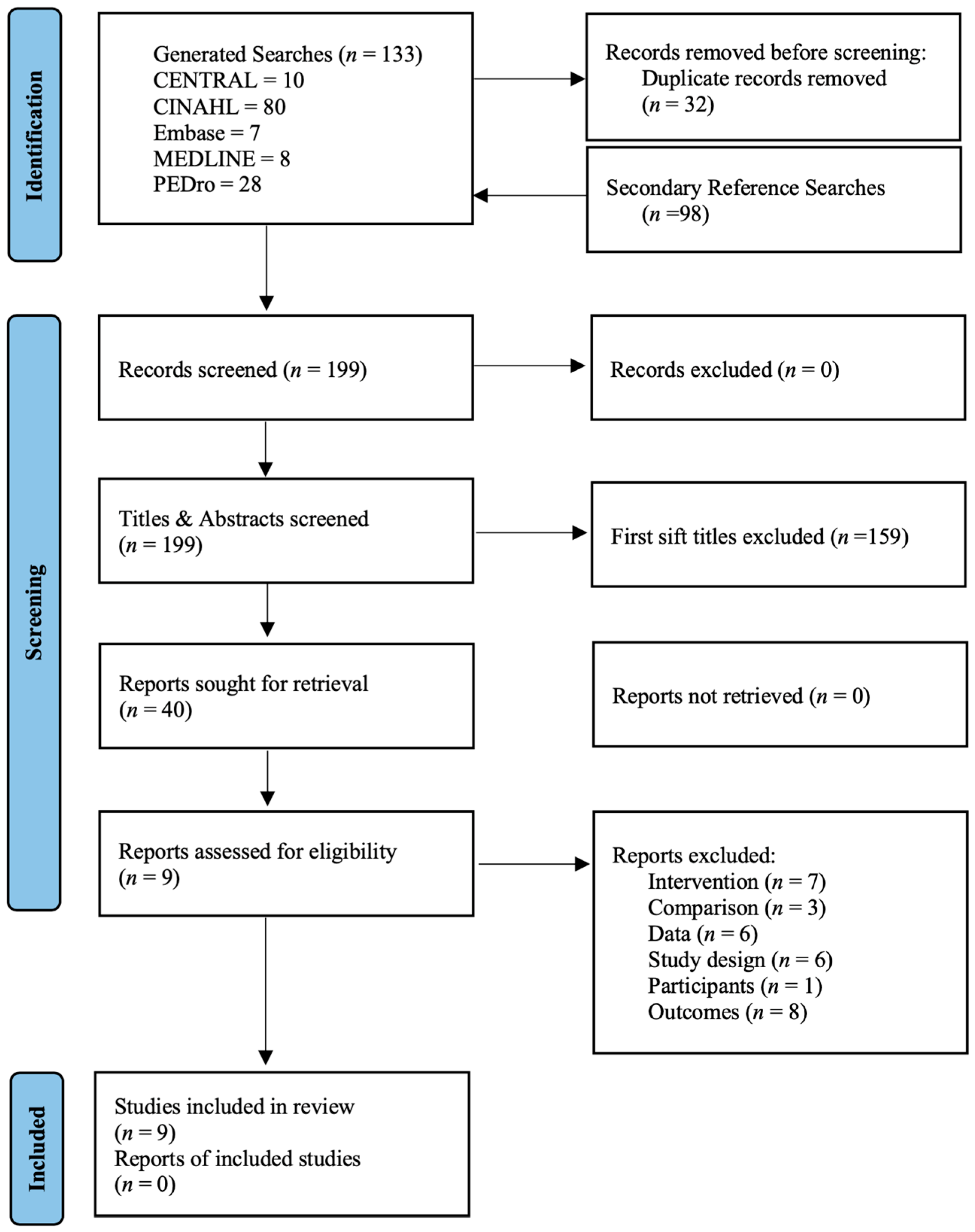
3.1. Literature Search and Characteristics of the Included Trials
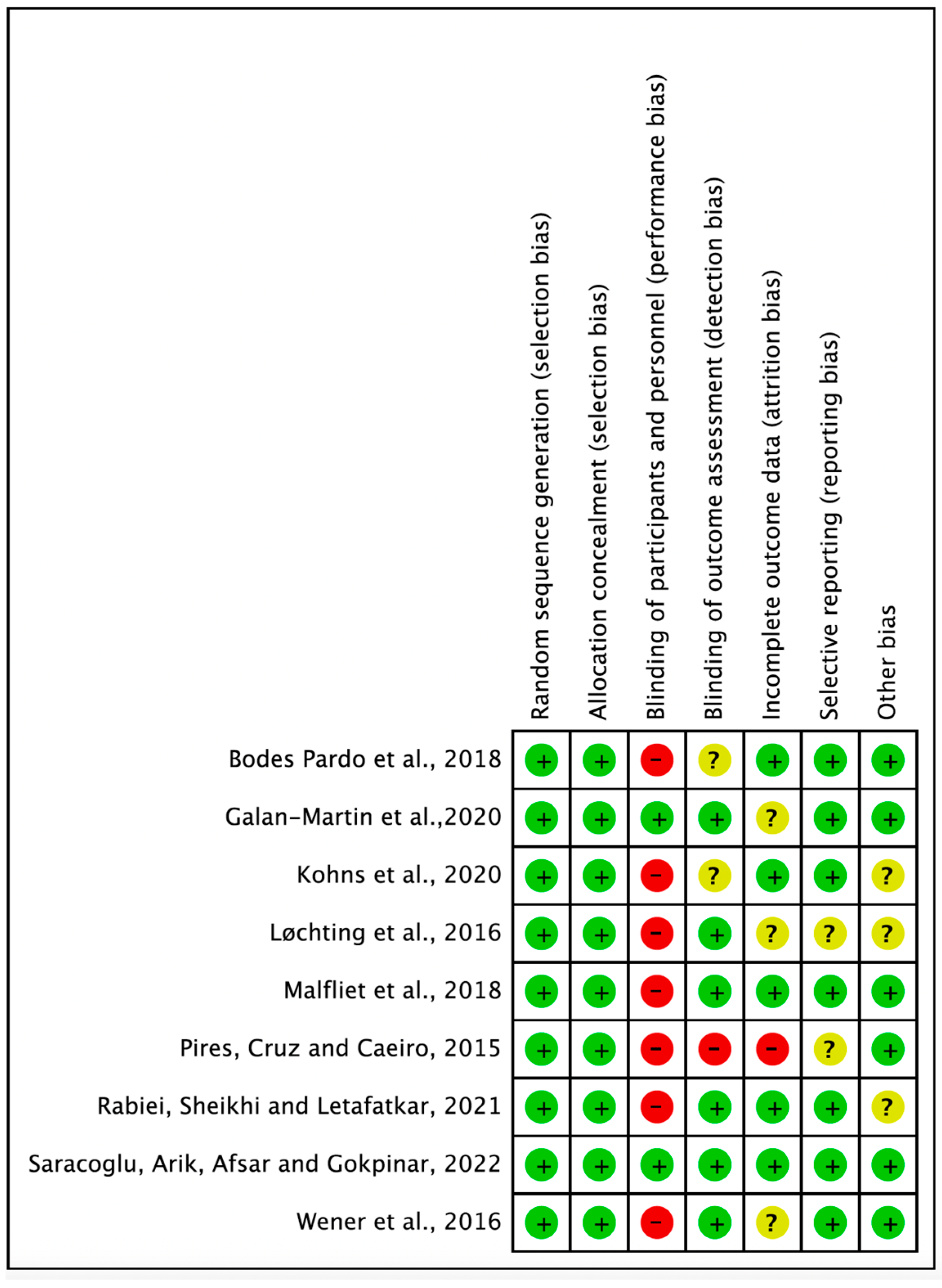
3.2. Methodological Risk of Bias Assessment
3.3. Carryover Effect of Pain Neuroscience Education for Patients with Chronic Low Back Pain
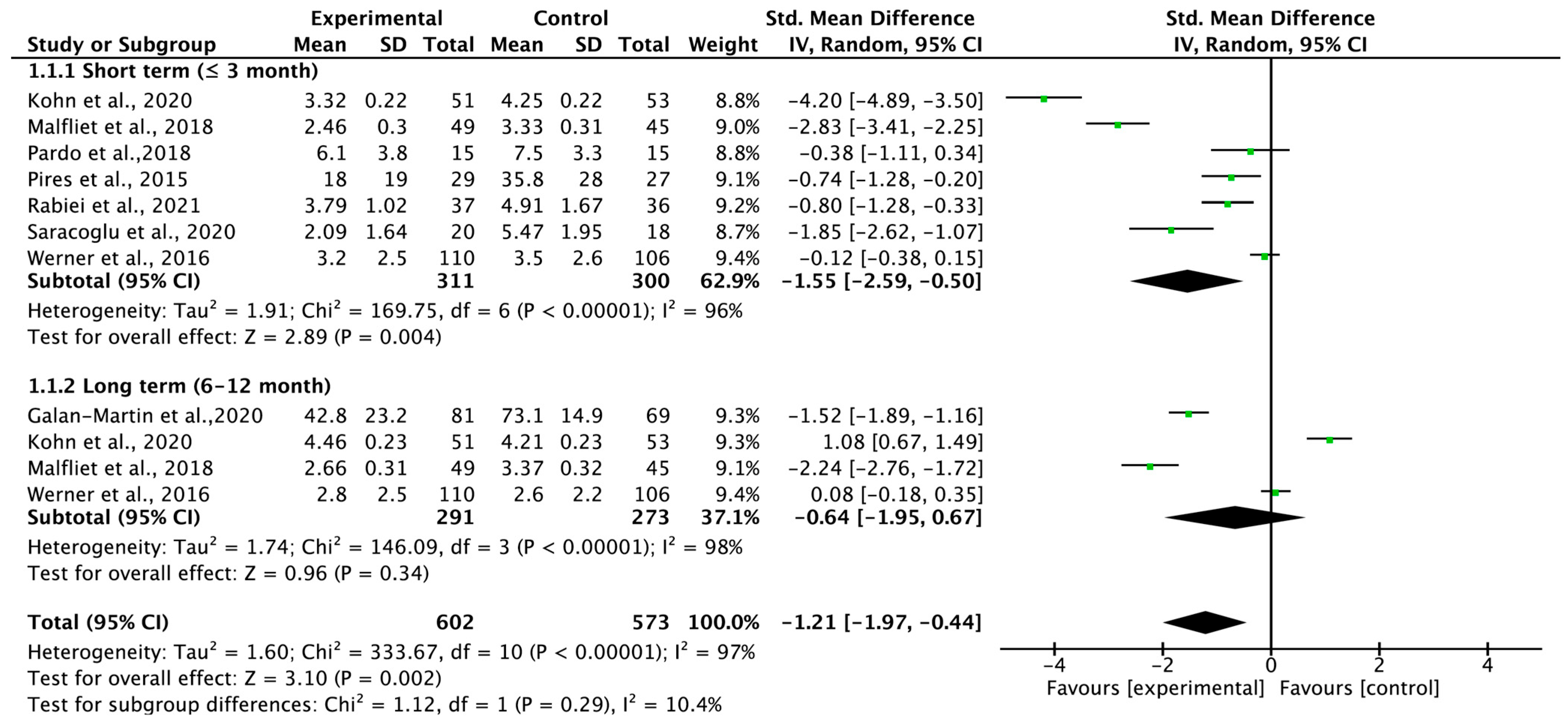
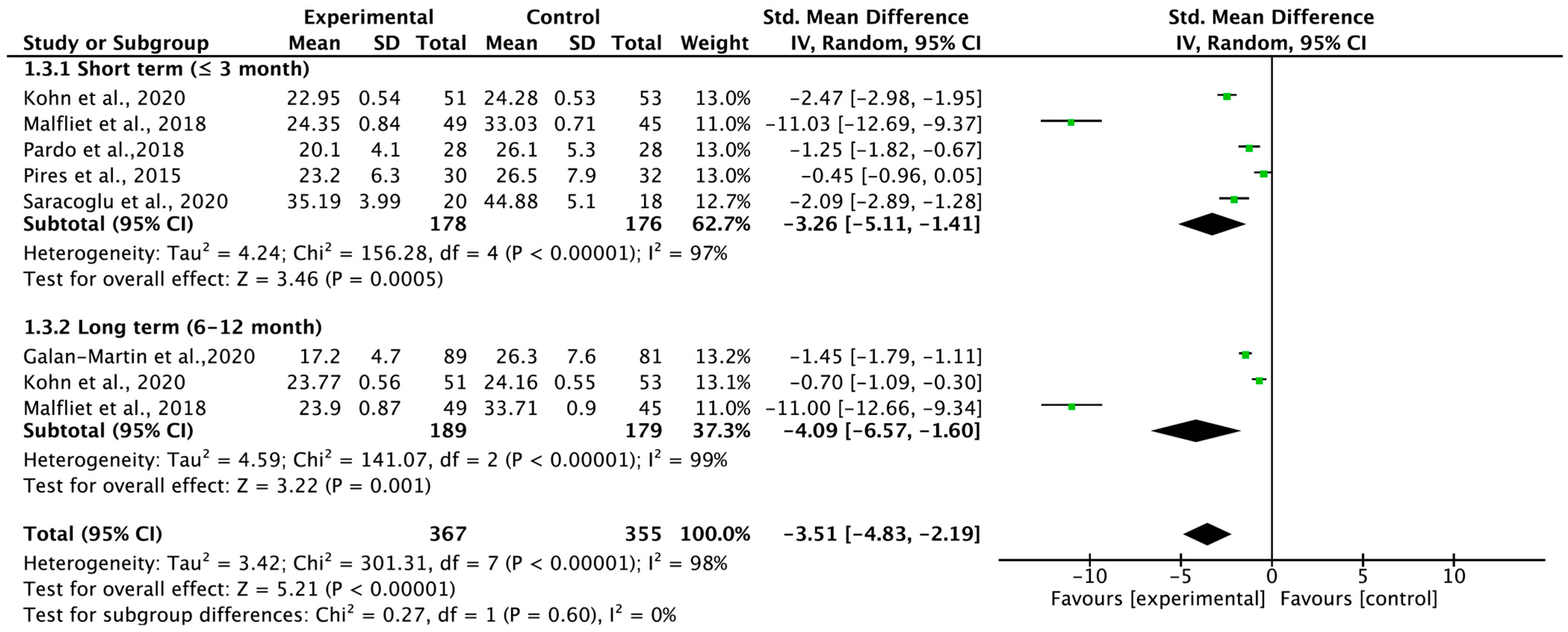
3.4. Total Carryover Effect of Pain Neuroscience Education on Pain Intensity
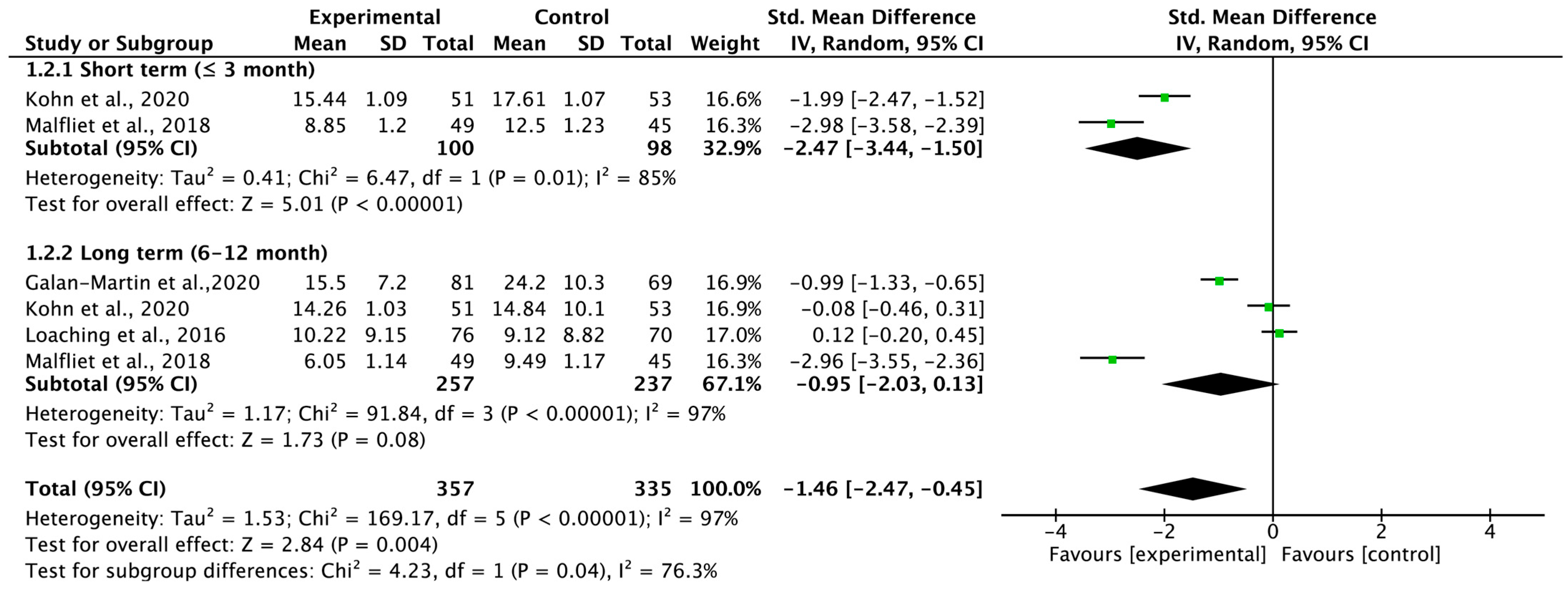
3.5. Total Carryover Effect of Pain Neuroscience Education on Pain Catastrophizing
3.6. Total Carryover Effect of Pain Neuroscience Education on Kinesiophobia
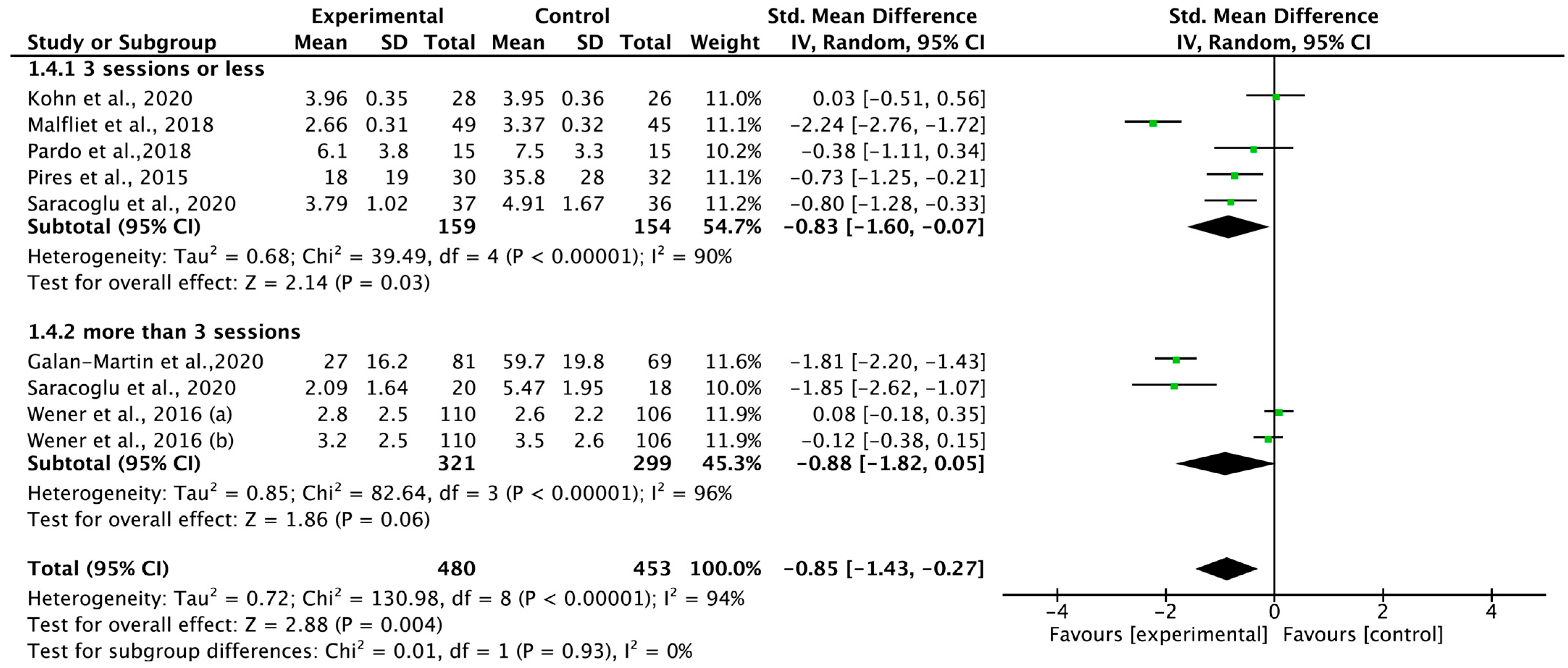
3.7. Total Effect of Pain Neuroscience Education on Pain Intensity According to Therapeutic Intensity
3.8. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koes, B.W.; van Tulder, M.; Lin, C.W.; Macedo, L.G.; McAuley, J.; Maher, C. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur. Spine J. 2010, 19, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, W.; Cai, S.; Hu, Y.; Dong, J. The efficacy of e-health in the self-management of chronic low back pain: A meta analysis. Int. J. Nurs. Stud. 2020, 106, 103507. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.D.C.M.; Maher, C.G.; Hancock, M.J.; McAuley, J.H.; Herbert, R.D.; Costa, L.O. The prognosis of acute and persistent low-back pain: A meta-analysis. CMAJ 2012, 184, E613–E624. [Google Scholar] [CrossRef]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15 (Suppl. 2), S192–S300. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, R.; Blatti, C.; Lucenti, L.; Boscarino, U.; Sapienza, M.; Pavone, V.; Testa, G. Pain Education in the Management of Patients with Chronic Low Back Pain: A Systematic Review. J. Funct. Morphol. Kinesiol. 2022, 7, 74. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Cacey, V. Pain education in the management of patients with chronic low back pain. J. Orthop. Trauma. Surg. Relat. Res. 2022, 17, 74. [Google Scholar]
- Picavet, H.S.; Vlaeyen, J.W.; Schouten, J.S. Pain catastrophizing and kinesiophobia: Predictors of chronic low back pain. Am. J. Epidemiol. 2002, 156, 1028–1034. [Google Scholar] [CrossRef]
- Wong, J.J.; Côté, P.; Sutton, D.A.; Randhawa, K.; Yu, H.; Varatharajan, S.; Goldgrub, R.; Nordin, M.; Gross, D.P.; Shearer, H.M.; et al. Clinical practice guidelines for the noninvasive management of low back pain: A systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Eur. J. Pain 2017, 21, 201–216. [Google Scholar] [CrossRef]
- Brox, J.I.; Storheim, K.; Grotle, M.; Tveito, T.H.; Indahl, A.; Eriksen, H.R. Systematic review of back schools, brief education, and fear-avoidance training for chronic low back pain. Spine J. 2008, 8, 948–958. [Google Scholar] [CrossRef]
- Galan-Martin, M.A.; Montero-Cuadrado, F.; Lluch-Girbes, E.; Coca-López, M.C.; Mayo-Iscar, A.; Cuesta-Vargas, A. Pain Neuroscience Education and Physical Therapeutic Exercise for Patients with Chronic Spinal Pain in Spanish Physiotherapy Primary Care: A Pragmatic Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L. A pain neuromatrix approach to patients with chronic pain. Man. Ther. 2003, 8, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Louw, A.; Zimney, K.; Puentedura, E.J.; Diener, I. The efficacy of pain neuroscience education on musculoskeletal pain: A systematic review of the literature. Physiother. Theory Pract. 2016, 32, 332–355. [Google Scholar] [CrossRef]
- Malfliet, A.; Kregel, J.; Coppieters, I.; De Pauw, R.; Meeus, M.; Roussel, N.; Cagnie, B.; Danneels, L.; Nijs, J. Effect of Pain Neuroscience Education Combined With Cognition-Targeted Motor Control Training on Chronic Spinal Pain: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterwijck, J.; Meeus, M.; Paul, L.; De Schryver, M.; Pascal, A.; Lambrecht, L.; Nijs, J. Pain physiology education improves health status and endogenous pain inhibition in fibromyalgia: A double-blind randomized controlled trial. Clin. J. Pain 2013, 29, 873–882. [Google Scholar] [CrossRef]
- Watson, J.A.; Ryan, C.G.; Cooper, L.; Ellington, D.; Whittle, R.; Lavender, M.; Dixon, J.; Atkinson, G.; Cooper, K.; Martin, D.J. Pain Neuroscience Education for Adults With Chronic Musculoskeletal Pain: A Mixed-Methods Systematic Review and Meta-Analysis. J. Pain 2019, 20, 1140.e1–1140.e22. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S. Effects of pain neuroscience education on kinesiophobia in patients with chronic pain: A systematic review and meta-analysis. Phys. Ther. Rehabil. Sci. 2020, 9, 309–317. [Google Scholar] [CrossRef]
- Lluch, E.; Dueñas, L.; Falla, D.; Baert, I.; Meeus, M.; Sánchez-Frutos, J.; Nijs, J. Preoperative Pain Neuroscience Education Combined With Knee Joint Mobilization for Knee Osteoarthritis: A Randomized Controlled Trial. Clin. J. Pain 2018, 34, 44–52. [Google Scholar] [CrossRef]
- Vicente-Mampel, J.; Gargallo, P.; Bautista, I.J.; Blanco-Gímenez, P.; de Bernardo Tejedor, N.; Alonso-Martín, M.; Martínez-Soler, M.; Baraja-Vegas, L. Impact of Pain Neuroscience Education Program in Community Physiotherapy Context on Pain Perception and Psychosocial Variables Associated with It in Elderly Persons: A Ranzomized Controlled Trial. Int. J. Environ. Res. Public. Health 2022, 19, 11855. [Google Scholar] [CrossRef]
- Kohns, D.J.; Urbanik, C.P.; Geisser, M.E.; Schubiner, H.; Lumley, M.A. The Effects of a Pain Psychology and Neuroscience Self-Evaluation Internet Intervention: A Randomized Controlled Trial. Clin. J. Pain 2020, 36, 683–692. [Google Scholar] [CrossRef]
- Werner, E.L.; Storheim, K.; Løchting, I.; Wisløff, T.; Grotle, M. Cognitive Patient Education for Low Back Pain in Primary Care: A Cluster Randomized Controlled Trial and Cost-Effectiveness Analysis. Spine 2016, 41, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Traeger, A.C.; Lee, H.; Hübscher, M.; Skinner, I.W.; Moseley, G.L.; Nicholas, M.K.; Henschke, N.; Refshauge, K.M.; Blyth, F.M.; Main, C.J.; et al. Effect of Intensive Patient Education vs Placebo Patient Education on Outcomes in Patients With Acute Low Back Pain: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.L.; Ryan, C.G.; Martin, D.J. Pain neurophysiology education for the management of individuals with chronic low back pain: Systematic review and meta-analysis. Man. Ther. 2011, 16, 544–549. [Google Scholar] [CrossRef]
- Moseley, G. Explaining pain to patients e recent developments. In Proceedings of the New Zealand Pain Society Annual Scientific Meeting, Rotorua, NZ, USA, 23–26 July 2019. [Google Scholar]
- Riley, R.D.; Higgins, J.P.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 241–284. [Google Scholar]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bodes Pardo, G.; Lluch Girbés, E.; Roussel, N.A.; Gallego Izquierdo, T.; Jiménez Penick, V.; Pecos Martín, D. Pain Neurophysiology Education and Therapeutic Exercise for Patients With Chronic Low Back Pain: A Single-Blind Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Cruz, E.B.; Caeiro, C. Aquatic exercise and pain neurophysiology education versus aquatic exercise alone for patients with chronic low back pain: A randomized controlled trial. Clin. Rehabil. 2015, 29, 538–547. [Google Scholar] [CrossRef]
- Saracoglu, I.; Arik, M.I.; Afsar, E.; Gokpinar, H.H. The effectiveness of pain neuroscience education combined with manual therapy and home exercise for chronic low back pain: A single-blind randomized controlled trial. Physiother. Theory Pract. 2022, 38, 868–878. [Google Scholar] [CrossRef]
- Løchting, I.; Storheim, K.; Werner, E.L.; Småstuen Cvancarova, M.; Grotle, M. Evaluation of individualized quality of life and illness perceptions in low back pain. A patient education cluster randomized controlled trial. Patient Educ. Couns. 2016, 99, 1992–1998. [Google Scholar] [CrossRef]
- Rabiei, P.; Sheikhi, B.; Letafatkar, A. Comparing Pain Neuroscience Education Followed by Motor Control Exercises With Group-Based Exercises for Chronic Low Back Pain: A Randomized Controlled Trial. Pain Pract. 2021, 21, 333–342. [Google Scholar] [CrossRef]
- Page, M.J.; Higgins, J.P.; Sterne, J.A. Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 349–374. [Google Scholar]
- Moseley, G.L. Widespread brain activity during an abdominal task markedly reduced after pain physiology education: fMRI evaluation of a single patient with chronic low back pain. Aust. J. Physiother. 2005, 51, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Julien, N.; Goffaux, P.; Arsenault, P.; Marchand, S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005, 114, 295–302. [Google Scholar] [CrossRef]
- de Souza, J.B.; Potvin, S.; Goffaux, P.; Charest, J.; Marchand, S. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin. J. Pain 2009, 25, 123–127. [Google Scholar] [CrossRef]
- Wood, L.; Hendrick, P.A. A systematic review and meta-analysis of pain neuroscience education for chronic low back pain: Short-and long-term outcomes of pain and disability. Eur. J. Pain 2019, 23, 234–249. [Google Scholar] [CrossRef]
- Moseley, G.L.; Butler, D.S. Fifteen Years of Explaining Pain: The Past, Present, and Future. J. Pain 2015, 16, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Tegner, H.; Frederiksen, P.; Esbensen, B.A.; Juhl, C. Neurophysiological Pain Education for Patients With Chronic Low Back Pain: A Systematic Review and Meta-Analysis. Clin. J. Pain 2018, 34, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Paul van Wilgen, C.; Van Oosterwijck, J.; van Ittersum, M.; Meeus, M. How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: Practice guidelines. Man. Ther. 2011, 16, 413–418. [Google Scholar] [CrossRef]
- Louw, A.; Puentedura, E.J.; Zimney, K.; Schmidt, S. Know Pain, Know Gain? A Perspective on Pain Neuroscience Education in Physical Therapy. J. Orthop. Sports Phys. Ther. 2016, 46, 131–134. [Google Scholar] [CrossRef] [PubMed]
| Study | Participants (Sample Size) | Therapeutic Intensity | Outcome Measure | Author’s Conclusions |
|---|---|---|---|---|
| Galan-Martin et al., 2020 [11] | Non-specific chronic spinal pain (166) | Total intervention period = 6 weeks EG = 6 sessions of PNE (10 h), 18 sessions exercise (18 h, three sessions per week) CG = 15 sessions (15 h) usual physiotherapy treatment | Pain intensity = VAS Pain catastrophizing = PCS Kinesiophobia = TSK | PNE and PE-based playful, dual-tasking, and socialization-promoting components are most effective in improving quality of life improvement, reduction of pain, catastrophism, kinesiophobia, CS, and disability. |
| Kohns et al., 2020 [20] | Low back pain (104) | Total intervention period = A single PPN session EG = A single PPN session lasting 20–25 min, used a 3 min instructional video CG = Single session of self-assessment of health-related behaviors, lasting 20–25 min, used an educational video. | Pain intensity = BPI | PPN helps patients learn about centralized pain and evaluate their risk factors for such pain. Moreover, this intervention resulted in some reduction in pain intensity in the short term, but not in the long term. |
| Løchting et al., 2016 [32] | Low back pain (203) | EG = 30 min, one-on-one PNE once a week for 4 consecutive weeks CG = 30 min, one-on-one sessions of usual care once a week for 4 consecutive weeks | Pain catastrophizing = PCS | The Cognitive patient education programs did not lead to improvements in individuals’ quality of life and pain catastrophizing. Through cognitive interventions need to be researched further in order to strengthen the understanding of these constructs in LBP. |
| Malfliet et al., 2018 [14] | Chronic spinal pain (120) | Total intervention period = 12 weeks EG = 3 sessions of PNE, 15 exercise sessions CG = 3 sessions of Traditional education, 15 exercise sessions | Pain intensity = NPRS Kinesiophobia = TSK Pain catastrophizing = PCS | Combining PNE with CTMCT can reduce pain and disability and improve mental and physical functioning and pain cognitions in people with nCSP. |
| Bodes Pardo et al., 2018 [29] | Chronic low back pain (56) | Total intervention period = 3 months EG = 2 sessions of PNPE (30 to 50 min), TE (daily) CG = TE (daily) | Pain intensity = NPRS Kinesiophobia = TSK | Combining PNE with TE is more effective in reducing pain, disability, and pain catastrophizing for participants with CLBP, with a large effect size, compared with TE alone. |
| Pires, Cruz and Caeiro, 2015 [30] | Chronic low back pain (62) | Total intervention period = 6 weeks EG = 2 Group sessions, 90 min each Aquatic program: 6 weeks, 2 session/week CG = 6 weeks program consisting of 12 session of aquatic exercise (30–50 min) | Pain intensity = VAS Kinesiophobia = TSK | PNE is a clinically effective addition to aquatic exercise. Further studies are necessary to better understand how pain neurophysiology education influences pain intensity and disability and to evaluate the long terms effects of this intervention on pain and disability. |
| Rabiei, Sheikhi and Letafatkar, 2021 [33] | Chronic low back pain (73) | Total intervention period = twice weekly for 8 weeks EG = 3 educational sessions PNE, each lasting 30–60 min; MCE, 2 sessions a week for 8 weeks. CG = Group-based exercise (GE) program. Proposed sessions 2 times a week for 8 weeks, each session lasting 60 min. | Pain intensity = VAS Kinesiophobia = FABQ | Individual treatment involving PNE plus MCE seem to be better at reducing pain intensity and disability compared to GE, while no significant differences were observed for fear-avoidance beliefs and self-efficacy between the 2 groups in patients with CLBP. |
| Saracoglu, Arik, Afsar and Gokpinar, 2022 [31] | Chronic low back pain (38) | Total intervention period = 4 weeks EG = MT (2 day/week, 30 min), PNE (each week, 40–45 min), HEP (3 day/week, 10 repititions) CG = MT (2 day/week, 30 min), HEP (3 day/week, 10 repetitions) | Pain intensity = NPRS Kinesiophobia = TSK | When compared to MT and HEP or HEP alone, the combination of PNE, MT, and HEP is associated with greater improvement in terms of pain intensity and kinesiophobia in the short (4 weeks) and midterm (12 weeks). |
| Werner et al., 2016 [21] | Non-specific low back pain (216) | EG = 30 min. of one-to-one PNE sessions once a week for four consecutive weeks CG = 30 min. of one-to-one sessions of usual care once a week for four consecutive weeks | Pain intensity = NPRS | The equal improvement observed in both groups in our study suggests that patient education may be useful, but no clinical or health economic benefits as a result of adding a cognitive education program to usual treatment for patients with subacute and chronic LBP. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Kim, H. Carryover Effects of Pain Neuroscience Education on Patients with Chronic Lower Back Pain: A Systematic Review and Meta-Analysis. Medicina 2023, 59, 1268. https://doi.org/10.3390/medicina59071268
Shin S, Kim H. Carryover Effects of Pain Neuroscience Education on Patients with Chronic Lower Back Pain: A Systematic Review and Meta-Analysis. Medicina. 2023; 59(7):1268. https://doi.org/10.3390/medicina59071268
Chicago/Turabian StyleShin, Seungwoo, and Hyunjoong Kim. 2023. "Carryover Effects of Pain Neuroscience Education on Patients with Chronic Lower Back Pain: A Systematic Review and Meta-Analysis" Medicina 59, no. 7: 1268. https://doi.org/10.3390/medicina59071268
APA StyleShin, S., & Kim, H. (2023). Carryover Effects of Pain Neuroscience Education on Patients with Chronic Lower Back Pain: A Systematic Review and Meta-Analysis. Medicina, 59(7), 1268. https://doi.org/10.3390/medicina59071268







