Failed Back Surgery Syndrome: No Longer a Surgeon’s Defeat—A Narrative Review
Abstract
1. Introduction
2. Proposed New Nomenclature
3. Epidemiology
4. Molecular Mechanisms Underlying the Pathology of the PSPS-T1 (Surgery-Related)
5. Etiology—Risk Factors
5.1. Risk Factors for PSPS-T1
5.2. Risk Factors for PSPS-T2
6. Diagnosis
6.1. History
| Symptoms and Signs | Explanation |
|---|---|
| Red Flags [4,5] | |
| Recent Trauma | Onset under 20 or over 50 years |
| History of Cancer | Any trauma, minor or major, especially in people over 50 |
| Unexplained Weight Loss | Especially if new back pain in patients with known cancer history |
| Failure to Improve | Can indicate cancer or systemic disease |
| Night Pain | Pain not improving after 4–6 weeks of appropriate conservative treatment |
| Fever, Chills, Sweats | Pain that wakes you up at night |
| Pain Not Relieved by Rest or Lying Down | May indicate infection or systemic disease |
| History of Intravenous Drug Use | Often indicates a serious condition |
| History of Long-Term Steroid Use | Increased risk of infection |
| Neurologic Symptoms | Increased risk of osteoporosis and vertebral fractures |
| Bowel or Bladder Dysfunction | Such as weakness, numbness, or altered sensation in lower extremities |
| Severe or Progressive Neurological Deficit | Could indicate cauda equina syndrome, a surgical emergency |
| Recent Trauma | Indicates possible nerve involvement and requires urgent attention |
| Yellow Flags [56] | |
| Fear-Avoidance Behavior | Avoiding movement due to fear of causing more pain |
| Belief That Pain Means Harm | Incorrectly associating all pain with harm can inhibit recovery |
| Catastrophizing | An exaggerated negative view of the pain’s impact |
| Expectation of Passive Treatments Only | Belief that only treatments performed on the person (like surgery, injections) will help, rather than active participation |
| Depression, Anxiety, or Stress | Psychological factors can influence the perception of pain and recovery |
| Over-reliance on Medication | May indicate lack of active coping strategies |
| Poor Job Satisfaction or Difficulties at Work | Could influence chronicity and disability |
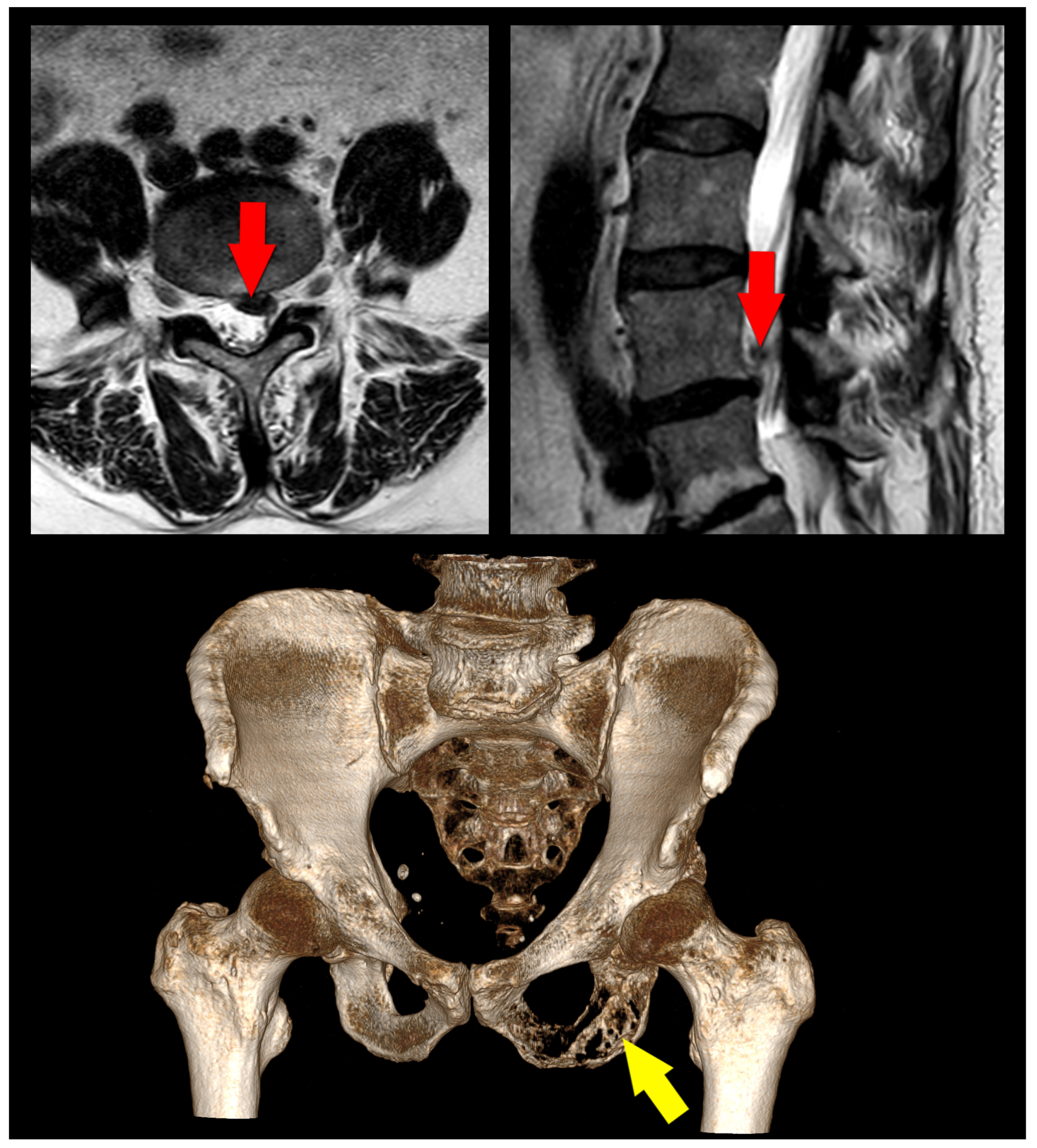
6.2. Imaging
6.3. Other
7. Management
7.1. Causative Treatment
7.2. Symptomatic Treatment
| Name of Drug | Main Indication in PSPS-T1/2 | Potential Side Effects |
|---|---|---|
| Tier 1 (Non-opioid analgesics) | ||
| Acetaminophen (Paracetamol) [101] | Mild to moderate axial pain | Liver damage, skin reactions, kidney damage |
| Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), e.g., Ibuprofen, Naproxen [101], | Axial pain, sciatica/radicular pain | Stomach ulcers/bleeds, increased risk of heart attack or stroke |
| Acetaminophen (Paracetamol) [101] | Mild to moderate axial pain | Liver damage, skin reactions, kidney damage |
| Tier 2 (Mild opioids for moderate to severe pain) | ||
| Codeine [42] (often combined with Acetaminophen) | Moderate to severe axial pain | Drowsiness, constipation, nausea |
| Tramadol [42] | Moderate to severe axial pain | Nausea, dizziness, constipation, risk of addiction |
| Tier 3 (Strong opioids for severe pain) | ||
| Morphine [42] | Severe axial pain | Drowsiness, constipation, nausea, risk of addiction |
| Fentanyl [42] | Severe axial pain | Drowsiness, constipation, nausea, risk of addiction |
| Adjuvant analgesics (medications that can enhance pain relief or combat side effects) | ||
| Anticonvulsants—Gabapentin [102], Pregabalin [103] | Neuropathic pain related to nerve damage in PSPS-T1/2 | Dizziness, fatigue, weight gain |
| Antidepressants—Amitriptyline, Duloxetine [104] | Neuropathic pain and associated depressive symptoms in PSPS-T1/2 | Drowsiness, dry mouth, constipation, weight gain |
| Anticonvulsants—Gabapentin [102], Pregabalin [103] | Neuropathic pain related to nerve damage in PSPS-T1/2 | Dizziness, fatigue, weight gain |
8. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global Low Back Pain Prevalence and Years Lived with Disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef]
- Urits, I.; Burshtein, A.; Sharma, M.; Testa, L.; Gold, P.A.; Orhurhu, V.; Viswanath, O.; Jones, M.R.; Sidransky, M.A.; Spektor, B.; et al. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr. Pain Headache Rep. 2019, 23, 23. [Google Scholar] [CrossRef]
- Hurwitz, E.L.; Randhawa, K.; Yu, H.; Côté, P.; Haldeman, S. The Global Spine Care Initiative: A Summary of the Global Burden of Low Back and Neck Pain Studies. Eur. Spine J. 2018, 27, 796–801. [Google Scholar] [CrossRef]
- Finucane, L.M.; Downie, A.; Mercer, C.; Greenhalgh, S.M.; Boissonnault, W.G.; Pool-Goudzwaard, A.L.; Beneciuk, J.M.; Leech, R.L.; Selfe, J. International Framework for Red Flags for Potential Serious Spinal Pathologies. J. Orthop. Sport. Phys. Ther. 2020, 50, 350–372. [Google Scholar] [CrossRef]
- Galliker, G.; Scherer, D.E.; Trippolini, M.A.; Rasmussen-Barr, E.; LoMartire, R.; Wertli, M.M. Low Back Pain in the Emergency Department: Prevalence of Serious Spinal Pathologies and Diagnostic Accuracy of Red Flags. Am. J. Med. 2020, 133, 60–72. [Google Scholar] [CrossRef]
- Geurts, J.W.; Willems, P.C.; Kallewaard, J.W.; van Kleef, M.; Dirksen, C. The Impact of Chronic Discogenic Low Back Pain: Costs and Patients’ Burden. Pain Res. Manag. 2018, 2018, 4696180. [Google Scholar] [CrossRef]
- Traeger, A.C.; Buchbinder, R.; Elshaug, A.G.; Croft, P.R.; Maher, C.G. Care for Low Back Pain: Can Health Systems Deliver? Bull. World Health Organ. 2019, 97, 423. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, C. Alternative Payment Models and Physician Treatment Decisions: Evidence from Lower Back Pain. J. Health Econ. 2021, 80, 102548. [Google Scholar] [CrossRef]
- Baber, Z.; Erdek, M.A. Failed Back Surgery Syndrome: Current Perspectives. J. Pain Res. 2016, 9, 979–987. [Google Scholar] [CrossRef]
- Chan, C.W.; Peng, P. Failed Back Surgery Syndrome. Pain Med. 2011, 12, 577–606. [Google Scholar] [CrossRef]
- al Kaisy, A.; Pang, D.; Desai, M.J.; Pries, P.; North, R.; Taylor, R.S.; Mc Cracken, L.; Rigoard, P. Failed Back Surgery Syndrome: Who Has Failed? Neurochirurgie 2015, 61 (Suppl. 1), S6–S14. [Google Scholar] [CrossRef]
- Rigoard, P.; Desai, M.J.; Taylor, R.S. Failed Back Surgery Syndrome: What’s in a Name? A Proposal to Replace “FBSS” by “POPS”…. Neurochirurgie 2015, 61 (Suppl. 1), S16–S21. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Christelis, N.; Simpson, B.; Russo, M.; Stanton-Hicks, M.; Barolat, G.; Thomson, S.; Schug, S.; Baron, R.; Buchser, E.; Carr, D.B.; et al. Persistent Spinal Pain Syndrome: A Proposal for Failed Back Surgery Syndrome and ICD-11. Pain Med. 2021, 22, 807–818. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Primary Pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef]
- Naiditch, N.; Billot, M.; Goudman, L.; Cornet, P.; Roulaud, M.; Ounajim, A.; Page, P.; Lorgeoux, B.; Baron, S.; Nivole, K.; et al. Professional Status of Persistent Spinal Pain Syndrome Patients after Spinal Surgery (PSPS-T2): What Really Matters? A Prospective Study Introducing the Concept of “Adapted Professional Activity” Inferred from Clinical, Psychological and Social Influence. J. Clin. Med. 2021, 10, 5055. [Google Scholar] [CrossRef]
- Naiditch, N.; Billot, M.; Moens, M.; Goudman, L.; Cornet, P.; le Breton, D.; Roulaud, M.; Ounajim, A.; Page, P.; Lorgeoux, B.; et al. Persistent Spinal Pain Syndrome Type 2 (PSPS-T2), a Social Pain? Advocacy for a Social Gradient of Health Approach to Chronic Pain. J. Clin. Med. 2021, 10, 2817. [Google Scholar] [CrossRef]
- Petersen, E.A.; Schatman, M.E.; Sayed, D.; Deer, T. Persistent Spinal Pain Syndrome: New Terminology for a New Era. J. Pain Res. 2021, 14, 1627–1630. [Google Scholar] [CrossRef]
- Ampat, G.; George, J.S.; Clynch, A.L.; Sims, J.M.G. Spinal Fusion Surgery—The Need to Follow the ‘BRAN’ Toolkit (Benefits, Risks, Alternatives, Nothing): A Case Report. J. Surg. Case Rep. 2022, 2022, rjac431. [Google Scholar] [CrossRef]
- Stanton, E.W.; Chang, K.E.; Formanek, B.; Buser, Z.; Wang, J. The Incidence of Failed Back Surgery Syndrome Varies between Clinical Setting and Procedure Type. J. Clin. Neurosci. 2022, 103, 56–61. [Google Scholar] [CrossRef]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of Inflammatory Mediators with Pain Perception. Biomed. Pharmacother. 2017, 96, 1445–1452. [Google Scholar] [CrossRef]
- Rigoard, P.; Gatzinsky, K.; Deneuville, J.P.; Duyvendak, W.; Naiditch, N.; Van Buyten, J.P.; Eldabe, S. Optimizing the Management and Outcomes of Failed Back Surgery Syndrome: A Consensus Statement on Definition and Outlines for Patient Assessment. Pain Res. Manag. 2019, 2019, 3126464. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Malfliet, A.; Kregel, J.; Meeus, M.; Danneels, L.; Cagnie, B.; Roussel, N.; Nijs, J. Patients with Chronic Spinal Pain Benefit from Pain Neuroscience Education Regardless the Self-Reported Signs of Central Sensitization: Secondary Analysis of a Randomized Controlled Multicenter Trial. PM&R 2018, 10, 1330–1343.e1. [Google Scholar] [CrossRef]
- Satyanarayanan, S.K.; Shih, Y.H.; Wen, Y.R.; Palani, M.; Lin, Y.W.; Su, H.; Gałecki, P.; Su, K.P. MiR-200a-3p Modulates Gene Expression in Comorbid Pain and Depression: Molecular Implication for Central Sensitization. Brain Behav. Immun. 2019, 82, 230–238. [Google Scholar] [CrossRef]
- Gazerani, P. Satellite Glial Cells in Pain Research: A Targeted Viewpoint of Potential and Future Directions. Front. Pain Res. 2021, 2, 4. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An Update on Reactive Astrocytes in Chronic Pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef]
- Yue, Z.; Hu, B.; Chen, Z.; Zheng, G.; Wang, Y.; Yang, C.; Cao, P.; Wu, X.; Liang, L.; Zang, F.; et al. Continuous Release of Mefloquine Featured in Electrospun Fiber Membranes Alleviates Epidural Fibrosis and Aids in Sensory Neurological Function after Lumbar Laminectomy. Mater. Today Bio 2022, 17, 100469. [Google Scholar] [CrossRef]
- Ding, Q.; Wei, Q.; Sheng, G.; Wang, S.; Jing, S.; Ma, T.; Zhang, R.; Wang, T.; Li, W.; Tang, X.; et al. The Preventive Effect of Decorin on Epidural Fibrosis and Epidural Adhesions After Laminectomy. Front. Pharmacol. 2021, 12, 3482. [Google Scholar] [CrossRef]
- Crosio, A.; Ronchi, G.; Fornasari, B.E.; Odella, S.; Raimondo, S.; Tos, P. Experimental Methods to Simulate and Evaluate Postsurgical Peripheral Nerve Scarring. J. Clin. Med. 2021, 10, 1613. [Google Scholar] [CrossRef]
- Peng, X.Q.; Sun, C.G.; Fei, Z.G.; Zhou, Q.J. Risk Factors for Surgical Site Infection After Spinal Surgery: A Systematic Review and Meta-Analysis Based on Twenty-Seven Studies. World Neurosurg. 2019, 123, e318–e329. [Google Scholar] [CrossRef] [PubMed]
- Souslian, F.G.; Patel, P.D. Review and Analysis of Modern Lumbar Spinal Fusion Techniques. Br. J. Neurosurg. 2021, 1–7. [Google Scholar] [CrossRef]
- Alhaug, O.K.; Dolatowski, F.; Austevoll, I.; Mjønes, S.; Lønne, G. Incidental Dural Tears Associated with Worse Clinical Outcomes in Patients Operated for Lumbar Spinal Stenosis. Acta Neurochir. 2023, 165, 99. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wang, Y.C.; Chang, C.W.; Wong, C.B.; Cheng, Y.H.; Fu, T.S. Surgical Outcomes for Upper Lumbar Disc Herniation: Decompression Alone versus Fusion Surgery. J. Clin. Med. 2019, 8, 1435. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, Y.; Bozkurt, I.; Yaman, M.E.; Guvenc, Y.; Tolunay, T.; Bayram, P.; Hayirli, N.; Billur, D.; Erbay, F.K.; Senturk, S.; et al. Histopathologic Analysis of Tamoxifen on Epidural Fibrosis. World Neurosurg. 2018, 111, e941–e948. [Google Scholar] [CrossRef]
- Godlewski, C.A.; Kalagara, H.; Do Campo, R.V.; Northern, T.; Kukreja, P. Post-Surgical Inflammatory Neuropathy: An Underappreciated but Critical and Treatable Cause of Postoperative Neuropathy. Cureus 2020, 12, e11927. [Google Scholar] [CrossRef]
- Charalampidis, A.; Jiang, F.; Wilson, J.R.F.; Badhiwala, J.H.; Brodke, D.S.; Fehlings, M.G. The Use of Intraoperative Neurophysiological Monitoring in Spine Surgery. Glob. Spine J. 2020, 10, 104S–114S. [Google Scholar] [CrossRef]
- Derman, P.B.; Singh, K. Surgical Strategies for the Treatment of Lumbar Pseudarthrosis in Degenerative Spine Surgery: A Literature Review and Case Study. HSS J. 2020, 16, 183–187. [Google Scholar] [CrossRef]
- Peters, M.J.M.; Bastiaenen, C.H.G.; Brans, B.T.; Weijers, R.E.; Willems, P.C. The Diagnostic Accuracy of Imaging Modalities to Detect Pseudarthrosis after Spinal Fusion—A Systematic Review and Meta-Analysis of the Literature. Skelet. Radiol. 2019, 48, 1499–1510. [Google Scholar] [CrossRef]
- Hills, J.M.; Kim, E.; Khan, I.; Devin, C.J. Evaluation and Workup in Revision Spine Surgery. Semin. Spine Surg. 2019, 31, 44–52. [Google Scholar] [CrossRef]
- Dessouky, R.; Khaleel, M.; Khalifa, D.N.; Tantawy, H.I.; Chhabra, A. Magnetic Resonance Neurography of the Lumbosacral Plexus in Failed Back Surgery Syndrome. Spine 2018, 43, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lee, C.Y.; Chen, S.J. Narcotic Addiction in Failed Back Surgery Syndrome. Cell Transplant. 2019, 28, 239–247. [Google Scholar] [CrossRef]
- Le Huec, J.C.; Seresti, S.; Bourret, S.; Cloche, T.; Monteiro, J.; Cirullo, A.; Roussouly, P. Revision after Spinal Stenosis Surgery. Eur. Spine J. 2020, 29, 22–38. [Google Scholar] [CrossRef]
- Ahn, Y.; Keum, H.J.; Shin, S.H.; Choi, J.J. Laser-Assisted Endoscopic Lumbar Foraminotomy for Failed Back Surgery Syndrome in Elderly Patients. Lasers Med. Sci. 2019, 35, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Haro, R.; de Andrés-Serrano, C.; Noriega González, D.C.; Bordes-García, C.; de Andrés, J. Adjacent Segment Syndrome after Failed Back Surgery: Biomechanics, Diagnosis, and Treatment. Minerva Anestesiol. 2021, 88, 282–292. [Google Scholar] [CrossRef]
- Rogerson, A.; Aidlen, J.; Jenis, L.G. Persistent Radiculopathy after Surgical Treatment for Lumbar Disc Herniation: Causes and Treatment Options. Int. Orthop. 2019, 43, 969–973. [Google Scholar] [CrossRef]
- Guler, S.; Akcali, O.; Sen, B.; Micili, S.C.; Sanli, N.K.; Cankaya, D. Effect of platelet-rich plasma, fat pad and dural matrix in preventing epidural fibrosis. Acta Ortop. Bras. 2020, 28, 31–35. [Google Scholar] [CrossRef]
- Canizares, M.; Gleenie, R.A.; Perruccio, A.V.; Abraham, E.; Ahn, H.; Attabib, N.; Christie, S.; Johnson, M.G.; Nataraj, A.; Nicholls, F.; et al. Patients’ Expectations of Spine Surgery for Degenerative Conditions: Results from the Canadian Spine Outcomes and Research Network (CSORN). Spine J. 2020, 20, 399–408. [Google Scholar] [CrossRef]
- Strøm, J.; Nielsen, C.V.; Jørgensen, L.B.; Andersen, N.T.; Laursen, M. A Web-Based Platform to Accommodate Symptoms of Anxiety and Depression by Featuring Social Interaction and Animated Information in Patients Undergoing Lumbar Spine Fusion: A Randomized Clinical Trial. Spine J. 2019, 19, 827–839. [Google Scholar] [CrossRef]
- Strøm, J.; Bjerrum, M.B.; Nielsen, C.V.; Thisted, C.N.; Nielsen, T.L.; Laursen, M.; Jørgensen, L.B. Anxiety and Depression in Spine Surgery—A Systematic Integrative Review. Spine J. 2018, 18, 1272–1285. [Google Scholar] [CrossRef]
- Ondeck, N.T.; Bohl, D.D.; Bovonratwet, P.; McLynn, R.P.; Cui, J.J.; Shultz, B.N.; Lukasiewicz, A.M.; Grauer, J.N. Discriminative Ability of Commonly Used Indices to Predict Adverse Outcomes after Poster Lumbar Fusion: A Comparison of Demographics, ASA, the Modified Charlson Comorbidity Index, and the Modified Frailty Index. Spine J. 2018, 18, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Shim, K.D.; Song, Y.S.; Park, Y.S. Risk Factor Analysis of Adjacent Segment Disease Requiring Surgery after Short Lumbar Fusion: The Influence of Rheumatoid Arthritis. Spine J. 2018, 18, 1578–1583. [Google Scholar] [CrossRef]
- Friedman, G.N.; Benton, J.A.; Echt, M.; De la Garza Ramos, R.; Shin, J.H.; Coumans, J.V.C.E.; Gitkind, A.I.; Yassari, R.; Leveque, J.C.; Sethi, R.K.; et al. Multidisciplinary Approaches to Complication Reduction in Complex Spine Surgery: A Systematic Review. Spine J. 2020, 20, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Impellizzeri, F.M.; Leunig, M.; Jeszenszy, D.; Becker, H.J.; Haschtmann, D.; Preiss, S.; Fekete, T.F. Eurospine 2017 Full Paper Award: Time to Remove Our Rose-Tinted Spectacles: A Candid Appraisal of the Relative Success of Surgery in over 4500 Patients with Degenerative Disorders of the Lumbar Spine, Hip or Knee. Eur. Spine J. 2018, 27, 778–788. [Google Scholar] [CrossRef]
- Abubakar, M.K.; Mohammad, S. Management of Failed Back Surgery Syndrome (FBSS). Arch. Int. Surg. 2018, 8, 47. [Google Scholar] [CrossRef]
- Glattacker, M.; Heyduck, K.; Jakob, T. Yellow Flags as Predictors of Rehabilitation Outcome in Chronic Low Back Pain. Rehabil. Psychol. 2018, 63, 408–417. [Google Scholar] [CrossRef]
- Borkar, S.A.; Sharma, R.; Mansoori, N.; Sinha, S.; Kale, S.S. Spinopelvic Parameters in Patients with Lumbar Degenerative Disc Disease, Spondylolisthesis, and Failed Back Syndrome: Comparison Vis-à-Vis Normal Asymptomatic Population and Treatment Implications. J. Craniovertebr. Junction Spine 2019, 10, 167. [Google Scholar] [CrossRef]
- Zárate-Kalfópulos, B.; Reyes-Tarrago, F.; Navarro-Aceves, L.A.; García-Ramos, C.L.; Reyes-Sánchez, A.A.; Alpízar-Aguirre, A.; Rosales-Olivarez, L.M. Characteristics of Spinopelvic Sagittal Alignment in Lumbar Degenerative Disease. World Neurosurg. 2019, 126, e417–e421. [Google Scholar] [CrossRef]
- Greenberg, J.K.; Whiting, B.B.; Martinez, O.M.; Butt, B.B.; Badhiwala, J.H.; Clifton, W.E. Age-Adjusted Alignment Goals in Adult Spinal Deformity Surgery. Semin. Spine Surg. 2023, 35, 101027. [Google Scholar] [CrossRef]
- Celestre, P.C.; Dimar, J.R.; Glassman, S.D. Spinopelvic Parameters: Lumbar Lordosis, Pelvic Incidence, Pelvic Tilt, and Sacral Slope: What Does a Spine Surgeon Need to Know to Plan a Lumbar Deformity Correction? Neurosurg. Clin. N. Am. 2018, 29, 323–329. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Z.; Shi, B.; Liu, Z.; Zhu, Z.; Chu, W.C.W.; Lam, T.P.; Cheng, J.C.Y.; Qiu, Y. Global Alignment and Proportion (GAP) Score in Asymptomatic Individuals: Is It Universal? Spine J. 2022, 22, 1566–1575. [Google Scholar] [CrossRef]
- Passias, P.G.; Pierce, K.E.; Williamson, T.K.; Krol, O.; Lafage, R.; Lafage, V.; Schoenfeld, A.J.; Protopsaltis, T.S.; Vira, S.; Line, B.; et al. Pelvic Nonresponse Following Treatment of Adult Spinal Deformity: Influence of Realignment Strategies on Occurrence. Spine 2023, 48, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Im, S.K.; Lee, J.H.; Kang, K.C.; Shin, S.J.; Lee, K.Y.; Park, J.J.; Kim, M.H. Proximal Junctional Kyphosis in Degenerative Sagittal Deformity after Under- and Overcorrection of Lumbar Lordosis: Does Overcorrection of Lumbar Lordosis Instigate PJK? Spine 2020, 45, E933–E942. [Google Scholar] [CrossRef] [PubMed]
- Witkam, R.L.; Buckens, C.F.; van Goethem, J.W.M.; Vissers, K.C.P.; Henssen, D.J.H.A. The Current Role and Future Directions of Imaging in Failed Back Surgery Syndrome Patients: An Educational Review. Insights Imaging 2022, 13, 117. [Google Scholar] [CrossRef]
- Corona-Cedillo, R.; Saavedra-Navarrete, M.T.; Espinoza-Garcia, J.J.; Mendoza-Aguilar, A.N.; Ternovoy, S.K.; Roldan-Valadez, E. Imaging Assessment of the Postoperative Spine: An Updated Pictorial Review of Selected Complications. BioMed Res. Int. 2021, 2021, 9940001. [Google Scholar] [CrossRef] [PubMed]
- Germann, C.; Nanz, D.; Sutter, R. Magnetic Resonance Imaging Around Metal at 1.5 Tesla: Techniques from Basic to Advanced and Clinical Impact. Investig. Radiol. 2021, 56, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Ibrahim, E.S.H.; Bs, N.D.; Lu, J.C.; Kalia, V.; Runge, M.; Srinivasan, A.; Stojanovska, J.; Agarwal, P.P. Improving Mr Image Quality in Patients with Metallic Implants. Radiographics 2021, 41, E126–E137. [Google Scholar] [CrossRef]
- Chhabra, A.; Kanchustambham, P.; Mogharrabi, B.; Ratakonda, R.; Gill, K.; Xi, Y. MR Neurography of Lumbosacral Plexus: Incremental Value Over XR, CT, and MRI of L Spine with Improved Outcomes in Patients with Radiculopathy and Failed Back Surgery Syndrome. J. Magn. Reson. Imaging 2023, 57, 139–150. [Google Scholar] [CrossRef]
- Choi, T.Y.; Chang, M.Y.; Lee, S.H.; Park, Y.; Ha, J.W.; Park, J.H. Differences in Time-to-Fusion Based on “Absence of Peri-Graft Radiolucency” and “Trabecular Bone Bridging” Criteria after Transforaminal Lumbar Interbody Fusion in Patients with Low and Normal Bone Density. Skelet. Radiol. 2023, 52, 733–742. [Google Scholar] [CrossRef]
- Zou, D.; Muheremu, A.; Sun, Z.; Zhong, W.; Jiang, S.; Li, W. Computed Tomography Hounsfield Unit-Based Prediction of Pedicle Screw Loosening after Surgery for Degenerative Lumbar Spine Disease. J. Neurosurg. Spine 2020, 32, 716–721. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Kim, H.S.; Singh, R.; Kashlan, O.N. Radiographic Outcomes of Endoscopic Decompression for Lumbar Spinal Stenosis. Neurosurg. Focus 2019, 46, E10. [Google Scholar] [CrossRef]
- Garcia, D.; Sousa-Pinto, B.; Akinduro, O.O.; De Biase, G.; Filho, L.M.; Qu, W.; Atchison, J.W.; Deen, H.G.; Nottmeier, E.; Chen, S.; et al. SPECT-CT as a Predictor of Pain Generators in Patients Undergoing Intra-Articular Injections for Chronic Neck and Back Pain. World Neurosurg. 2022, 164, e1243–e1250. [Google Scholar] [CrossRef]
- Weisenthal, B.W.; Glassman, S.D.; Mkorombindo, T.; Nelson, L.; Carreon, L.Y. When Does CT Myelography Add Value beyond MRI for Lumbar Degenerative Disease? Spine J. 2022, 22, 787–792. [Google Scholar] [CrossRef]
- Hanscom, D.; Grunert, P. Revision Lumber Decompressions. In The Resident’s Guide to Spine Surgery; Springer: Cham, Switzerland, 2020; pp. 259–279. [Google Scholar] [CrossRef]
- Patel, D.M.; Weinberg, B.D.; Hoch, M.J. CT Myelography: Clinical Indications and Imaging Findings. Radiographics 2020, 40, 470–484. [Google Scholar] [CrossRef]
- Al-Zaghal, A.; Ayubcha, C.; Kothekar, E.; Alavi, A. Clinical Applications of Positron Emission Tomography in the Evaluation of Spine and Joint Disorders. PET Clin. 2019, 14, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Follenfant, E.; Balamoutoff, N.; Lawson-Ayayi, S.; Dutronc, H.; Dupon, M.; Vital, J.M.; Delobel, P.; Durox, H.; de Clermont-Gallerande, H.; Fernandez, P.; et al. Added Value of [18F]Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for the Diagnosis of Post-Operative Instrumented Spine Infection. Jt. Bone Spine 2019, 86, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.H. Epidurography. Anaesth. Pain Intensive Care 2019, 13, 31–44. [Google Scholar]
- Funao, H.; Yokosuka, K.; Ukai, J.; Nakanishi, K.; Paku, M.; Tomita, T.; Hoshino, M.; Saito, T.; Ishii, K.; Sato, K. Efficacy of Minimally Invasive Trans-Sacral Canal Plasty between Patients with and without Failed Back Surgery Syndrome. Medicina 2022, 58, 251. [Google Scholar] [CrossRef]
- Perper, Y.; Ivanov, Y.; Serebnitsky, S. Algorithm for Performing Cervical Epidural Steroid Injections with Contrast Spread Technique. J. Orthop. Trauma Surg. Relat. Res. 2020, 15, 27–32. [Google Scholar]
- Wong, O.; Zhang, G.; Matthews, H.; Skalski, M.; Asadi, H.; Lalloo, S.; Kurda, D. Image-Guided Spinal Injection for Pain Management. J. Med. Imaging Radiat. Oncol. 2022, 66, 79–91. [Google Scholar] [CrossRef]
- Ah. Shin, D. Percutaneous Epidural Neuroplasty. In Endoscopic Procedures on the Spine; Springer: Singapore, 2019; pp. 353–359. [Google Scholar] [CrossRef]
- Kim, D.H.; Ji, G.Y.; Kwon, H.J.; Na, T.; Shin, J.W.; Shin, D.A.; Choi, S.S. Contrast Dispersion on Epidurography May Be Associated with Clinical Outcomes After Percutaneous Epidural Neuroplasty Using an Inflatable Balloon Catheter. Pain Med. 2020, 21, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Hur, J.W.; Lee, J.-B.; Park, J.Y. Early Clinical Outcome of Comparative Study between Revision Operation and Radiofrequency Treatment for Management of Failed Back Surgery Syndrome. Asian J. Pain 2021, 7, 3. [Google Scholar] [CrossRef]
- Gatzinsky, K.; Eldabe, S.; Deneuville, J.P.; Duyvendak, W.; Naiditch, N.; Van Buyten, J.P.; Rigoard, P.; Peng, B. Optimizing the Management and Outcomes of Failed Back Surgery Syndrome: A Proposal of a Standardized Multidisciplinary Team Care Pathway. Pain Res. Manag. 2019, 2019, 8184592. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.J.; Cupler, Z.A.; Gliedt, J.A.; Walters, S.; Schielke, A.L.; Hinkeldey, N.A.; Golley, D.J.; Hawk, C. Manipulative and Manual Therapies in the Management of Patients with Prior Lumbar Surgery: A Systematic Review. Complement. Ther. Clin. Pract. 2021, 42, 101261. [Google Scholar] [CrossRef]
- Avila, L.; Neves, M.L.; Abreu, A.R.; Fiuza, C.R.; Fukusawa, L.; Meziat-Filho, N.; Soares Santos, A.R. Cognitive Functional Therapy (CFT) Compared with Core Training Exercise (CTE) in Patients with Failed Back Surgery Syndrome (FBSS): A Study Protocol for a Randomized Controlled Trial. J. Bodyw. Mov. Ther. 2021, 26, 428–434. [Google Scholar] [CrossRef]
- Malfliet, A.; Kregel, J.; Coppieters, I.; De Pauw, R.; Meeus, M.; Roussel, N.; Cagnie, B.; Danneels, L.; Nijs, J. Effect of Pain Neuroscience Education Combined with Cognition-Targeted Motor Control Training on Chronic Spinal Pain: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 808–817. [Google Scholar] [CrossRef]
- Trager, R.J.; Daniels, C.J.; Meyer, K.W.; Stout, A.C.; Dusek, J.A. Protocol: Clinical Decision-Making for Spinal Manipulation for Persistent Spinal Pain Following Lumbar Surgery: A Protocol for a Systematic Review and Meta-Analysis of Individual Participant Data. BMJ Open 2021, 11, 54070. [Google Scholar] [CrossRef]
- Papalia, G.F.; Russo, F.; Vadalà, G.; Pascarella, G.; De Salvatore, S.; Ambrosio, L.; Di Martino, S.; Sammartini, D.; Sammartini, E.; Carassiti, M.; et al. Non-Invasive Treatments for Failed Back Surgery Syndrome: A Systematic Review. Glob. Spine J. 2023, 13, 1153–1162. [Google Scholar] [CrossRef]
- Zeng, F.; Mallozzi, S.; Moss, I.; Cote, M.; Sakalkale, D. Role of Simultaneous Bilateral Transforaminal Epidural Steroid Injections in Patients with Prior Lumbar Fusions or Laminectomies: A Retrospective Case Series. Interv. Pain Med. 2022, 1, 100066. [Google Scholar] [CrossRef]
- Solmaz, İ.; Akpancar, S.; Örsçelik, A.; Yener-Karasimav, Ö.; Gül, D. Dextrose Injections for Failed Back Surgery Syndrome: A Consecutive Case Series. Eur. Spine J. 2019, 28, 1610–1617. [Google Scholar] [CrossRef]
- Gewandter, J.S.; Frazer, M.E.; Cai, X.; Chiodo, V.F.; Rast, S.A.; Dugan, M.; Carter, H.A.; Rahmani, R.; Stone, J.J.; Markman, J.D. Extended-Release Gabapentin for Failed Back Surgery Syndrome: Results from a Randomized Double-Blind Cross-over Study. Pain 2019, 160, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Durand, G.; Girodon, J.; Debiais, F. Medical Management of Failed Back Surgery Syndrome in Europe: Evaluation Modalities and Treatment Proposals. Neurochirurgie 2015, 61, S57–S65. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, B.L.; Olmsted, Z.T.; Sabourin, S.; Heydari, E.; Harland, T.A.; Pilitsis, J.G. Review of the Treatments for Central Neuropathic Pain. Brain Sci. 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Dhruva, S.S.; Murillo, J.; Ameli, O.; Morin, P.E.; Spencer, D.L.; Redberg, R.F.; Cohen, K. Long-Term Outcomes in Use of Opioids, Nonpharmacologic Pain Interventions, and Total Costs of Spinal Cord Stimulators Compared with Conventional Medical Therapy for Chronic Pain. JAMA Neurol. 2023, 80, 18–29. [Google Scholar] [CrossRef]
- Patel, E.A.; Perloff, M.D. Radicular Pain Syndromes: Cervical, Lumbar, and Spinal Stenosis. Semin. Neurol. 2018, 38, 634–639. [Google Scholar] [CrossRef]
- Hershkovich, O.; Mor, Y.; Lotan, R. Intravenous Corticosteroid Therapy for Acute Lumbar Radicular Pain. J. Clin. Med. 2022, 11, 5127. [Google Scholar] [CrossRef]
- Roncoroni, C.; Baillet, A.; Durand, M.; Gaudin, P.; Juvin, R. Efficacy and Tolerance of Systemic Steroids in Sciatica: A Systematic Review and Meta-Analysis. Rheumatology 2011, 50, 1603–1611. [Google Scholar] [CrossRef]
- Galica, R.J.; Hayek, S.M.; Veizi, E.; McEwan, M.T.; Katta, S.; Ali, O.; Aziz, N.; Sondhi, N. Intrathecal Trialing of Continuous Infusion Combination Therapy with Hydromorphone and Bupivacaine in Failed Back Surgery Patients. Neuromodul. Technol. Neural Interface 2018, 21, 648–654. [Google Scholar] [CrossRef]
- Amirdelfan, K.; Webster, L.; Poree, L.; Sukul, V.; McRoberts, P. Treatment Options for Failed Back Surgery Syndrome Patients with Refractory Chronic Pain: An Evidence Based Approach. Spine 2017, 42 (Suppl. 14), S41–S52. [Google Scholar] [CrossRef]
- Khosravi, M.B.; Azemati, S.; Sahmeddini, M.A. Gabapentin versus Naproxen in the Management of Failed Back Surgery Syndrome; a Randomized Controlled Trial. Acta Anaesthesiol. Belg. 2014, 65, 31–37. [Google Scholar]
- Canos, A.; Cort, L.; Fernández, Y.; Rovira, V.; Pallares, J.; Barbera, M.; Morales-Suarez-Varela, M. Preventive Analgesia with Pregabalin in Neuropathic Pain from “Failed Back Surgery Syndrome”: Assessment of Sleep Quality and Disability. Pain Med. 2016, 17, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, A.B.; Ikizgul, O.; Karaali, C.N.; Bozkurt, S.; Konya, D.; Toktas, Z.O. Antidepressants in Spine Surgery: A Systematic Review to Determine Benefits and Risks. Asian Spine J. 2019, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, J.H.; Song, K.S.; Hong, J.Y.; Joo, Y.S.; Lee, D.H.; Hwang, C.J.; Lee, C.S. Treatment Outcomes for Patients with Failed Surgery. Pain Physician 2017, 1, E29–E43. [Google Scholar] [CrossRef]
- McClure, J.J.; Desai, B.D.; Ampie, L.; You, W.; Smith, J.S.; Buchholz, A.L. A Systematic Review of the Cost-Utility of Spinal Cord Stimulation for Persistent Low Back Pain in Patients with Failed Back Surgery Syndrome. Glob. Spine J. 2021, 11, 66S–72S. [Google Scholar] [CrossRef]
- Deer, T.R.; Krames, E.; Mekhail, N.; Pope, J.; Leong, M.; Stanton-Hicks, M.; Golovac, S.; Kapural, L.; Alo, K.; Anderson, J.; et al. The Appropriate Use of Neurostimulation: New and Evolving Neurostimulation Therapies and Applicable Treatment for Chronic Pain and Selected Disease States. Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 599–615. [Google Scholar] [CrossRef]
- Lopez, D.; Desyatnikov, D.O.; Anijar, L.; Reyes, J.; Fisher, K. Spinal Cord Stimulation Therapy for Failed Back Surgery Syndrome in a Patient with Mild Dementia Case Report. Pain Med. Case Rep. 2022, 6, 17–20. [Google Scholar] [CrossRef]
- Kashcheev, A.A.; Olegovich, G.A.; Tjurniko, V.M.; Arestov, S.O.; Vershinin, A.V.; Dmitrievich, D.M.; Poltorako, E.N.; Petrosyan, D.V. Spinal Cord Stimulation for Fail Back Surgery Syndrome: Literature Review and Clinical Study. Coluna/Columna 2018, 17, 212–215. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.H.; Song, K.S.; Hong, J.Y. Neuropathic Pain after Spinal Surgery. Asian Spine J. 2017, 11, 642–652. [Google Scholar] [CrossRef]
- Stanton, E.; Fresquez, Z.; Muehlbauer, E.J.; Wang, J.C.; Buser, Z. Onset of Mental Disorders in Patients Who Developed Failed Back Surgery Syndrome. Eur. Spine J. 2022, 31, 2612–2618. [Google Scholar] [CrossRef]
- Schoell, K.; Wang, C.; D’Oro, A.; Heindel, P.; Lee, L.; Wang, J.C.; Buser, Z. Depression Increases the Rates of Neurological Complications and Failed Back Surgery Syndrome in Patients Undergoing Lumbar Spine Surgery. Clin. Spine Surg. 2019, 32, E78–E85. [Google Scholar] [CrossRef]
- O’Sullivan, P.B.; Caneiro, J.P.; O’Keeffe, M.; Smith, A.; Dankaerts, W.; Fersum, K.; O’Sullivan, K. Cognitive Functional Therapy: An Integrated Behavioral Approach for the Targeted Management of Disabling Low Back Pain. Phys Ther. 2018, 98, 408–423. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miękisiak, G. Failed Back Surgery Syndrome: No Longer a Surgeon’s Defeat—A Narrative Review. Medicina 2023, 59, 1255. https://doi.org/10.3390/medicina59071255
Miękisiak G. Failed Back Surgery Syndrome: No Longer a Surgeon’s Defeat—A Narrative Review. Medicina. 2023; 59(7):1255. https://doi.org/10.3390/medicina59071255
Chicago/Turabian StyleMiękisiak, Grzegorz. 2023. "Failed Back Surgery Syndrome: No Longer a Surgeon’s Defeat—A Narrative Review" Medicina 59, no. 7: 1255. https://doi.org/10.3390/medicina59071255
APA StyleMiękisiak, G. (2023). Failed Back Surgery Syndrome: No Longer a Surgeon’s Defeat—A Narrative Review. Medicina, 59(7), 1255. https://doi.org/10.3390/medicina59071255






