Role of Percutaneous Ablation in the Management of Intrahepatic Cholangiocarcinoma
Abstract
1. Introduction
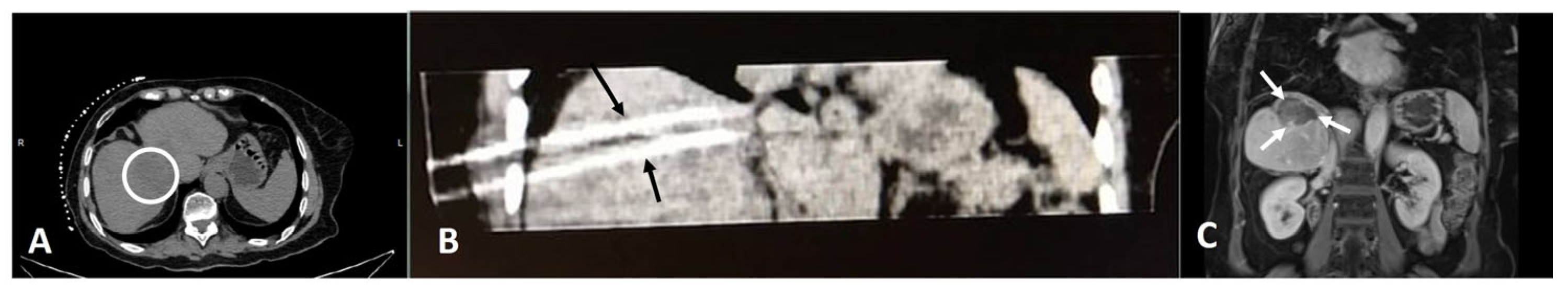
2. Interventional Ablative Treatment for iCCA
| Study | Treatment Modality | Patients | Lesions | Follow-Up (Months) | OS 1 yr | OS 2 yr | OS 3 yr | OS 4 yr | OS 5 yr | Technical Efficacy | Complication | Minor Complication Rate | Reference Number |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiou et al., 2005 | RFA | 10 | 10 | 20 (4–38) | 100% | 80% | 0% | 50% | [38] | ||||
| Carrafiello et al., 2010 | RFA | 6 | 6 | 17.5 (13–21) | 66% | 0% | 16.7% | [39] | |||||
| Kamphues et al., 2010 | RFA | 13 | 17 | 28 (12–69) | 92% | 52% | 83.3% | 66.7% | 0% | 7.6% | [40] | ||
| Giorgio et al., 2011 | RFA | 10 | 10 | 19.5 (9–64) | 100% | 83.3% | 0% | 30% | [41] | ||||
| Kim et al., 2011 | RFA | 13 | 17 | 19.5 (3.3–82.1) | 85% | 51% | 15% | 88.2% | 5.9% | 41.2% | [42] | ||
| Yu et al., 2011 | MWA | 15 | 24 | 12.8 (4–31) | 60% | 87.5% | 20% | 60% | [43] | ||||
| Fu et al., 2012 | RFA | 17 | 26 | 29 (4–122) | 84.6 | 43.3 | 28.9 | 96.2% | 3.6% | 28.6% | [44] | ||
| Haidu et al., 2012 | RFA | 11 | 36 | 35 (12–81) | 91% | 71% | 91.7% | 13% | [45] | ||||
| Xu et al., 2012 | RFA, MWA | 18 | 25 | 8.7 (1.3–86.2) | 36.3 | 30.3 | 30.3 | 92.% | 5.6% | 5.6% | [46] | ||
| Zhang et al., 2013 | RFA, MWA | 77 | 133 | 26.7 (5.0–66.7) | 69.8% | 20.5% | 94.7% | 3.9% | [47] | ||||
| Yang et al., 2015 | MWA | 26 | 39 | 19.2 (6–30) | 69.2% | 61.5% | 92.3% | 0% | 88.5% | [48] | |||
| Zhang et al., 2018 | MWA | 107 | 171 | 20.1 (2.8–63.5) | 93.5% | 39.6% | 7.9% | 92.9% | 2.8% | [49] | |||
| Giorgio et al., 2019 | RFA, MWA | 71 | 98 | 48 (8–86) | 88% | 65% | 45% | 0% | [50] | ||||
| Ni et al., 2019 | MWA | 78 | 106 | 22.7 (1–86.7) | 89.5% | 52.2% | 35% | 3.8% | 29.5% | [51] | |||
| Wu et al., 2019 | RFA | 86 | 12.3 (6.5–25.1) | 17.6% | [52] | ||||||||
| Xu et al., 2019 | MWA | 56 | 62 | 81.2% | 42.5% | 23.7% | 100% | 5.4% | [53] | ||||
| Brandi et al., 2020 | RFA | 29 | 117 | 39.9 (2–55) | 89% | 45% | 11% | 92.3% | 6.8% | 14.4% | [32] | ||
| Wang et al., 2020 | RFA, MWA | 77 | 226 | 14 (4–69) | 69.6% | 29.5% | 23.6% | 1.3% | 32.5% | [54] | |||
| Diaz-Gonzalez et al., 2020 | RFA, MWA | 27 | 33 | 26.5 (20.2–45.7) | 88.9% | 40.7% | 14.8% | 92.6% | 3.7% | 11.1% | [55] | ||
| Xiang et al., 2020 | RFA | 34 | 34 | 31 (25–34) | 89.9% | 42.4% | 23.9% | [56] | |||||
| Braunwarth et al., 2020 | RFA | 11 | 11 | 16 (1–116) | 93.8% | 71.6% | 47.7 | 90,9% | [27] | ||||
| Yang et al., 2021 | MWA | 52 | 74 | 21.2 (3.2–78.7) | 87.4% | 51.4% | 56.9% | 100% | 3.8% | 3.8% | [57] | ||
| Chu et al., 2021 | RFA | 40 | 64 | 26 (3.3–132) | 67.2% | 36.2% | 18.3 | 96.9% | 7.5% | [58] |
2.1. Radiofrequency Ablation
2.2. Microwave Ablation
2.3. Irreversible Electroporation
2.4. Electrochemotherapy
2.5. Cryoablation
3. Combining Percutaneous Ablative Techniques with Systemic Therapies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Nakeeb, A.; Pitt, H.A.; Sohn, T.A.; Coleman, J.; Abrams, R.A.; Piantadosi, S.; Hruban, R.H.; Lillemoe, K.D.; Yeo, C.J.; Cameron, J.L. Cholangiocarcinoma: A Spectrum of Intrahepatic, Perihilar, and Distal Tumors. Ann. Surg. 1996, 224, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S. Intrahepatic cholangiocarcinoma: Macroscopic type and stage classification. J. Hepato-Biliary-Pancreat. Surg. 2003, 10, 288–291. [Google Scholar] [CrossRef]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef]
- Liao, P.; Cao, L.; Chen, H.; Pang, S.Z. Analysis of metastasis and survival between extrahepatic and intrahepatic cholangiocarcinoma: A large population-based study. Medicine 2021, 100, e25635. [Google Scholar] [CrossRef]
- Gad, M.M.; Saad, A.M.; Faisaluddin, M.; Gaman, M.A.; Ruhban, I.A.; Jazieh, K.A.; Al-Husseini, M.J.; Simons-Linares, C.R.; Sonbol, M.B.; Estfan, B.N. Epidemiology of Cholangiocarcinoma; United States Incidence and Mortality Trends. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 885–893. [Google Scholar] [CrossRef]
- Renzulli, M.; Ramai, D.; Singh, J.; Sinha, S.; Brandi, N.; Ierardi, A.M.; Albertini, E.; Sacco, R.; Facciorusso, A.; Golfieri, R. Locoregional Treatments in Cholangiocarcinoma and Combined Hepatocellular Cholangiocarcinoma. Cancers 2021, 13, 3336. [Google Scholar] [CrossRef]
- Welzel, T.M.; Graubard, B.I.; El-Serag, H.B.; Shaib, Y.H.; Hsing, A.W.; Davila, J.A.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based case-control study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2007, 5, 1221–1228. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Wozniak, A.; Cook, P.; Adaniel, C.; Acevedo, J.; Azócar, L.; Hsing, A.W.; Roa, J.C.; Pasetti, M.F.; Miquel, J.F.; et al. Salmonella enterica serovar Typhi and gallbladder cancer: A case-control study and meta-analysis. Cancer Med. 2016, 5, 3235–3310. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.M.; Wu, Z.X.; Han, B.; Mao, Y.Q.; Chen, H.L.; Han, S.F.; Xia, J.L.; Wang, L.S. The association between BMI and gallbladder cancer risk: A meta-analysis. Oncotarget 2016, 7, 43669–43679. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, S.; Tovoli, F.; Mazzotta, A.; Vasuri, F.; Edeline, J.; Malvi, D.; Boudjema, K.; Renzulli, M.; Jeddou, H.; D’Errico, A.; et al. Non-Alcoholic Steatohepatitis as a Risk Factor for Intrahepatic Cholangiocarcinoma and Its Prognostic Role. Cancers 2020, 12, 3182. [Google Scholar] [CrossRef]
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Madoff, D.C.; Abi-Jaoudeh, N.; Braxton, D.; Goyal, L.; Jain, D.; Odisio, B.C.; Salem, R.; Schattner, M.; Sheth, R.; Li, D. An Expert, Multidisciplinary Perspective on Best Practices in Biomarker Testing in Intrahepatic Cholangiocarcinoma. Oncologist 2022, 27, 884–891. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- Squires, M.H.; Cloyd, J.M.; Dillhoff, M.; Schmidt, C.; Pawlik, T.M. Challenges of surgical management of intrahepatic cholangiocarcinoma. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 671–681. [Google Scholar] [CrossRef]
- Aljiffry, M.; Walsh, M.J.; Molinari, M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990–2009. World J. Gastroenterol. 2009, 15, 4240–4262. [Google Scholar] [CrossRef]
- Entezari, P.; Riaz, A. Intrahepatic Cholangiocarcinoma. Semin. Interv. Radiol. 2020, 37, 475–483. [Google Scholar] [CrossRef]
- Guglielmi, A.; Ruzzenente, A.; Campagnaro, T.; Pachera, S.; Valdegamberi, A.; Nicoli, P.; Cappellani, A.; Malfermoni, G.; Iacono, C. Intrahepatic cholangiocarcinoma: Prognostic factors after surgical resection. World J. Surg. 2009, 33, 1247–1254. [Google Scholar] [CrossRef]
- Hyder, O.; Marques, H.; Pulitano, C.; Marsh, J.W.; Alexandrescu, S.; Bauer, T.W.; Gamblin, T.C.; Sotiropoulos, G.C.; Paul, A.; Barroso, E.; et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: An Eastern and Western experience. JAMA Surg. 2014, 149, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.C.; Cassera, M.A.; Vetto, J.T.; Orloff, S.L.; Hansen, P.D.; Billingsley, K.G. Surgical treatment of intrahepatic cholangiocarcinoma: Outcomes and predictive factors. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2011, 13, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Hatzaras, I.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L.; et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013, 153, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Fondevila, C.; Donadon, M.; Gringeri, E.; Mocchegiani, F.; Schlitt, H.J.; Ijzermans, J.N.M.; Vivarelli, M.; Zieniewicz, K.; Olde Damink, S.W.M.; et al. Surgery for cholangiocarcinoma. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39 (Suppl. 1), 143–155. [Google Scholar] [CrossRef] [PubMed]
- Braunwarth, E.; Schullian, P.; Kummann, M.; Reider, S.; Putzer, D.; Primavesi, F.; Stättner, S.; Öfner, D.; Bale, R. Aggressive local treatment for recurrent intrahepatic cholangiocarcinoma-Stereotactic radiofrequency ablation as a valuable addition to hepatic resection. PLoS ONE 2022, 17, e0261136. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Ma, J.; Meng, X.; Liu, Y.; Yin, C.; Zhang, T.; Wang, P.; Park, Y.K.; Jung, H.W. Effects of a rhizome aqueous extract of Dioscorea batatas and its bioactive compound, allantoin in high fat diet and streptozotocin-induced diabetic mice and the regulation of liver, pancreas and skeletal muscle dysfunction. J. Ethnopharmacol. 2020, 259, 112926. [Google Scholar] [CrossRef]
- Tritripmongkol, P.; Plengsuriyakarn, T.; Tarasuk, M.; Na-Bangchang, K. In vitro cytotoxic and toxicological activities of ethanolic extract of Kaempferia galanga Linn. and its active component, ethyl-p-methoxycinnamate, against cholangiocarcinoma. J. Integr. Med. 2020, 18, 326–333. [Google Scholar] [CrossRef]
- Ben Khaled, N.; Jacob, S.; Rössler, D.; Bösch, F.; De Toni, E.N.; Werner, J.; Ricke, J.; Mayerle, J.; Seidensticker, M.; Schulz, C.; et al. Current State of Multidisciplinary Treatment in Cholangiocarcinoma. Dig. Dis. 2022, 40, 581–595. [Google Scholar] [CrossRef]
- Brandi, G.; Rizzo, A.; Dall’Olio, F.G.; Felicani, C.; Ercolani, G.; Cescon, M.; Frega, G.; Tavolari, S.; Palloni, A.; De Lorenzo, S.; et al. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: A retrospective single-center experience. Int. J. Hyperth. 2020, 37, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Groot Koerkamp, B.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef]
- Iezzi, R.; Gangi, A.; Posa, A.; Pua, U.; Liang, P.; Santos, E.; Kurup, A.N.; Tanzilli, A.; Tenore, L.; De Leoni, D.; et al. Emerging Indications for Interventional Oncology: Expert Discussion on New Locoregional Treatments. Cancers 2023, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.S.; Chen, D.; Lin, H. Laparoscopic hepatectomy versus radiofrequency ablation for hepatocellular carcinoma: A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 5711–5724. [Google Scholar] [CrossRef]
- Chiou, Y.Y.; Hwang, J.I.; Chou, Y.H.; Wang, H.K.; Chiang, J.H.; Chang, C.Y. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J. Med. Sci. 2005, 21, 304–309. [Google Scholar] [CrossRef]
- Carrafiello, G.; Laganà, D.; Cotta, E.; Mangini, M.; Fontana, F.; Bandiera, F.; Fugazzola, C. Radiofrequency ablation of intrahepatic cholangiocarcinoma: Preliminary experience. Cardiovasc. Interv. Radiol. 2010, 33, 835–839. [Google Scholar] [CrossRef]
- Kamphues, C.; Seehofer, D.; Eisele, R.M.; Denecke, T.; Pratschke, J.; Neumann, U.P.; Neuhaus, P. Recurrent intrahepatic cholangiocarcinoma: Single-center experience using repeated hepatectomy and radiofrequency ablation. J. Hepato-Biliary-Pancreat. Sci. 2010, 17, 509–515. [Google Scholar] [CrossRef]
- Giorgio, A.; Calisti, G.; De Stefano, G.; Farella, N.; Di Sarno, A.; Amendola, F.; Scognamiglio, U.; Giorgio, V. Radiofrequency ablation for intrahepatic cholangiocarcinoma: Retrospective analysis of a single centre experience. Anticancer Res. 2011, 31, 4575–4580. [Google Scholar]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, K.A.; Kim, P.N. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR. Am. J. Roentgenol. 2011, 196, W205–W209. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.A.; Liang, P.; Yu, X.L.; Cheng, Z.G.; Han, Z.Y.; Liu, F.Y.; Yu, J. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur. J. Radiol. 2011, 80, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, W.; Wu, W.; Yan, K.; Xing, B.C.; Chen, M.H. Radiofrequency ablation for postoperative recurrences of intrahepatic cholangiocarcinoma. Chin. J. Cancer Res. 2011, 23, 295–300. [Google Scholar] [CrossRef]
- Haidu, M.; Dobrozemsky, G.; Schullian, P.; Widmann, G.; Klaus, A.; Weiss, H.; Margreiter, R.; Bale, R. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: A retrospective study. Cardiovasc. Interv. Radiol. 2012, 35, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Wang, Y.; Lu, M.D.; Liu, L.N. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br. J. Radiol. 2012, 85, 1078–1084. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hu, P.; Wang, N.; Shen, Q.; Sun, A.X.; Kuang, M.; Qian, G.J. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2013, 20, 3596–3602. [Google Scholar] [CrossRef]
- Yang, G.W.; Zhao, Q.; Qian, S.; Zhu, L.; Qu, X.D.; Zhang, W.; Yan, Z.P.; Cheng, J.M.; Liu, Q.X.; Liu, R.; et al. Percutaneous microwave ablation combined with simultaneous transarterial chemoembolization for the treatment of advanced intrahepatic cholangiocarcinoma. Onco Targets Ther. 2015, 8, 1245–1250. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, J.; Yu, X.; Han, Z.; Cheng, Z.; Liu, F.; Liang, P. Clinical and survival outcomes of percutaneous microwave ablation for intrahepatic cholangiocarcinoma. Int. J. Hyperth. 2018, 34, 292–297. [Google Scholar] [CrossRef]
- Giorgio, A.; Gatti, P.; Montesarchio, L.; Santoro, B.; Dell’Olio, A.; Crucinio, N.; Coppola, C.; Scarano, F.; Biase, F.; Ciracì, E.; et al. Intrahepatic Cholangiocarcinoma and Thermal Ablation: Long-term Results of An Italian Retrospective Multicenter Study. J. Clin. Transl. Hepatol. 2019, 7, 287–292. [Google Scholar] [CrossRef]
- Ni, J.Y.; An, C.; Zhang, T.Q.; Huang, Z.M.; Jiang, X.Y.; Huang, J.H. Predictive value of the albumin-bilirubin grade on long-term outcomes of CT-guided percutaneous microwave ablation in intrahepatic cholangiocarcinoma. Int. J. Hyperth. 2019, 36, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tsilimigras, D.I.; Farooq, A.; Hyer, J.M.; Merath, K.; Paredes, A.Z.; Mehta, R.; Sahara, K.; Shen, F.; Pawlik, T.M. Potential survival benefit of radiofrequency ablation for small solitary intrahepatic cholangiocarcinoma in nonsurgically managed patients: A population-based analysis. J. Surg. Oncol. 2019, 120, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, L.; Xu, W.; Du, C.; Yang, L.; Tong, J.; Yi, Y. Ultrasound-guided percutaneous microwave ablation versus surgical resection for recurrent intrahepatic cholangiocarcinoma: Intermediate-term results. Int. J. Hyperth. 2019, 36, 351–358. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Z.; Liu, B.; Chen, H.; Yin, T.; Zheng, J.; Li, W. Long-term outcome and prognostic nomogram for intrahepatic cholangiocarcinoma after thermal ablation: A retrospective study. Transl. Cancer Res. 2020, 9, 6743–6754. [Google Scholar] [CrossRef]
- Díaz-González, Á.; Vilana, R.; Bianchi, L.; García-Criado, Á.; Rimola, J.; Rodríguez de Lope, C.; Ferrer, J.; Ayuso, C.; Da Fonseca, L.G.; Reig, M.; et al. Thermal Ablation for Intrahepatic Cholangiocarcinoma in Cirrhosis: Safety and Efficacy in Non-Surgical Patients. J. Vasc. Interv. Radiol. 2020, 31, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Hu, D.; Jin, Z.; Liu, P.; Lin, H. Radiofrequency Ablation vs. Surgical Resection for Small Early-Stage Primary Intrahepatic Cholangiocarcinoma. Front. Oncol. 2020, 10, 540662. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, Z.; Han, Z.; Liu, F.; Yu, X.; Yu, J.; Liang, P. Assessment of the Outcomes of Intrahepatic Cholangiocarcinoma after Ultrasound-Guided Percutaneous Microwave Ablation Based on Albumin-Bilirubin Grade. Cardiovasc. Interv. Radiol. 2021, 44, 261–270. [Google Scholar] [CrossRef]
- Chu, H.H.; Kim, J.H.; Shin, Y.M.; Won, H.J.; Kim, P.N. Percutaneous Radiofrequency Ablation for Recurrent Intrahepatic Cholangiocarcinoma after Curative Resection: Multivariable Analysis of Factors Predicting Survival Outcomes. AJR Am. J. Roentgenol. 2021, 217, 426–432. [Google Scholar] [CrossRef]
- Lucatelli, P.; Argirò, R.; Crocetti, L.; Rocco, B.; Bozzi, E.; Gasparrini, F.; Tanzilli, A.; Catalano, C.; Iezzi, R. Percutaneous Thermal Segmentectomy: Proof of Concept. Cardiovasc. Interv. Radiol. 2022, 45, 665–676. [Google Scholar] [CrossRef]
- Garnon, J.; Delmas, L.; De Marini, P.; Dalili, D.; Koch, G.; Auloge, P.; Cazzato, R.L.; Gangi, A. Triple-Antenna Microwave Ablation with Repositioning for the Creation of a Reliable 6-cm Ablation Zone in the Liver. Cardiovasc. Interv. Radiol. 2021, 44, 1291–1295. [Google Scholar] [CrossRef]
- Hong, K.; Georgiades, C. Radiofrequency ablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. 2010, 21, S179–S186. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, S.A.; Liu, Z.; Lobo, S.M.; Ahmed, M.; Hines-Peralta, A.U.; Lenkinski, R.E.; Goldberg, S.N. Radiofrequency ablation: Importance of background tissue electrical conductivity--an agar phantom and computer modeling study. Radiology 2005, 236, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.N.; Gazelle, G.S.; Solbiati, L.; Livraghi, T.; Tanabe, K.K.; Hahn, P.F.; Mueller, P.R. Ablation of liver tumors using percutaneous RF therapy. AJR Am. J. Roentgenol. 1998, 170, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; El-Haddad, G.; Sheth, R.A.; Parikh, N.S.; Ganguli, S.; Shyn, P.B.; Choi, J.; Brown, K.T. Liver-Directed Therapies for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control J. Moffitt Cancer Cent. 2017, 24, 1073274817729244. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Hahn, P.F.; Tanabe, K.K.; Mueller, P.R.; Schima, W.; Athanasoulis, C.A.; Compton, C.C.; Solbiati, L.; Gazelle, G.S. Percutaneous radiofrequency tissue ablation: Does perfusion-mediated tissue cooling limit coagulation necrosis? J. Vasc. Interv. Radiol. 1998, 9, 101–111. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, P.H.; Kim, J.H.; Kim, P.N.; Won, H.J.; Shin, Y.M.; Choi, S.H. Thermal ablation in the treatment of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Eur. Radiol. 2022, 32, 1205–1215. [Google Scholar] [CrossRef]
- Han, K.; Ko, H.K.; Kim, K.W.; Won, H.J.; Shin, Y.M.; Kim, P.N. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. J. Vasc. Interv. Radiol. 2015, 26, 943–948. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Hemming, A.W.; Zendejas, I.; George, T.; Nelson, D.R.; Soldevila-Pico, C.; Firpi, R.J.; Morelli, G.; Clark, V.; Cabrera, R. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol. Rep. 2013, 29, 1259–1267. [Google Scholar] [CrossRef]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, P.N.; Lee, S.G.; Hwang, S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur. J. Radiol. 2011, 80, e221–e225. [Google Scholar] [CrossRef]
- Laimer, G.; Jaschke, N.; Gottardis, M.; Schullian, P.; Putzer, D.; Sturm, W.; Bale, R. Stereotactic Radiofrequency Ablation of an Unresectable Intrahepatic Cholangiocarcinoma (ICC): Transforming an Aggressive Disease into a Chronic Condition. Cardiovasc. Interv. Radiol. 2020, 43, 791–796. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave ablation: Principles and applications. Radiographics 2005, 25 (Suppl. 1), S69–S83. [Google Scholar] [CrossRef]
- Andreano, A.; Brace, C.L. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc. Interv. Radiol. 2013, 36, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, A.; Kim, J.U.; Eliahoo, J.; Taylor-Robinson, S.D.; Khan, S.A. Ablative Therapy for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Clin. Exp. Hepatol. 2019, 9, 740–748. [Google Scholar] [CrossRef]
- Kim-Fuchs, C.; Candinas, D.; Lachenmayer, A. The Role of Conventional and Stereotactic Microwave Ablation for Intrahepatic Cholangiocarcinoma. J. Clin. Med. 2021, 10, 2963. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Velonakis, G.; Mazioti, A.; Tsochatzis, A.; Vrachliotis, T.; Kelekis, A.; Kelekis, N. Percutaneous Navigation under Local Anesthesia for Computed Tomography-Guided Microwave Ablation of Malignant Liver Lesions Located in the Hepatic Dome. Medicina 2021, 57, 1056. [Google Scholar] [CrossRef] [PubMed]
- Tarek, M. Membrane electroporation: A molecular dynamics simulation. Biophys. J. 2005, 88, 4045–4053. [Google Scholar] [CrossRef]
- Delemotte, L.; Tarek, M. Molecular dynamics simulations of lipid membrane electroporation. J. Membr. Biol. 2012, 245, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Ball, C.; Thomson, K.R.; Kavnoudias, H. Irreversible electroporation: A new challenge in “out of operating theater” anesthesia. Anesth. Analg. 2010, 110, 1305–1309. [Google Scholar] [CrossRef]
- Cannon, R.; Ellis, S.; Hayes, D.; Narayanan, G.; Martin, R.C., 2nd. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J. Surg. Oncol. 2013, 107, 544–549. [Google Scholar] [CrossRef]
- Silk, M.T.; Wimmer, T.; Lee, K.S.; Srimathveeravalli, G.; Brown, K.T.; Kingham, P.T.; Fong, Y.; Durack, J.C.; Sofocleous, C.T.; Solomon, S.B. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J. Vasc. Interv. Radiol. 2014, 25, 112–118. [Google Scholar] [CrossRef]
- Tian, G.; Zhao, Q.; Chen, F.; Jiang, T.; Wang, W. Ablation of hepatic malignant tumors with irreversible electroporation: A systematic review and meta-analysis of outcomes. Oncotarget 2017, 8, 5853–5860. [Google Scholar] [CrossRef] [PubMed]
- Mafeld, S.; Wong, J.J.; Kibriya, N.; Stenberg, B.; Manas, D.; Bassett, P.; Aslam, T.; Evans, J.; Littler, P. Percutaneous Irreversible Electroporation (IRE) of Hepatic Malignancy: A Bi-institutional Analysis of Safety and Outcomes. Cardiovasc. Interv. Radiol. 2019, 42, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, M.P.; Reginelli, A.; Maggialetti, N.; Carbone, M.; Giovine, S.; Laporta, A.; Urraro, F.; Nardone, V.; Grassi, R.; Cappabianca, S.; et al. Preliminary results in unresectable cholangiocarcinoma treated by CT percutaneous irreversible electroporation: Feasibility, safety and efficacy. Med. Oncol. 2020, 37, 45. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; D’Alessio, V.; Simonetti, I.; Grassi, F.; Silvestro, L.; Palaia, R.; Belli, A.; Patrone, R.; Piccirillo, M.; et al. Percutanous Electrochemotherapy (ECT) in Primary and Secondary Liver Malignancies: A Systematic Review. Diagnostics 2023, 13, 209. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Torre, M.L.; Perteghella, S.; Ansaloni, L.; Maestri, M.; Giunchedi, P. Electrochemotherapy of Deep-Seated Tumors: State of Art and Perspectives as Possible “EPR Effect Enhancer” to Improve Cancer Nanomedicine Efficacy. Cancers 2021, 13, 4437. [Google Scholar] [CrossRef]
- Tarantino, L.; Busto, G.; Nasto, A.; Nasto, R.A.; Tarantino, P.; Fristachi, R.; Cacace, L.; Bortone, S. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A feasibility study. Eur. J. Surg. Oncol. 2018, 44, 1603–1609. [Google Scholar] [CrossRef]
- Iezzi, R.; Posa, A.; Caputo, C.T.; De Leoni, D.; Sbaraglia, F.; Rossi, M.; Tortora, G.; Tagliaferri, L.; Valentini, V.; Colosimo, C. Safety and Feasibility of Analgosedation for Electrochemotherapy of Liver Lesions. Life 2023, 13, 631. [Google Scholar] [CrossRef]
- Rubinsky, B.; Lee, C.Y.; Bastacky, J.; Onik, G. The process of freezing and the mechanism of damage during hepatic cryosurgery. Cryobiology 1990, 27, 85–97. [Google Scholar] [CrossRef]
- Seifert, J.K.; France, M.P.; Zhao, J.; Bolton, E.J.; Finlay, I.; Junginger, T.; Morris, D.L. Large volume hepatic freezing: Association with significant release of the cytokines interleukin-6 and tumor necrosis factor a in a rat model. World J. Surg. 2002, 26, 1333–1341. [Google Scholar] [CrossRef]
- Stewart, G.J.; Preketes, A.; Horton, M.; Ross, W.B.; Morris, D.L. Hepatic cryotherapy: Double-freeze cycles achieve greater hepatocellular injury in man. Cryobiology 1995, 32, 215–219. [Google Scholar] [CrossRef]
- Seifert, J.K.; Morris, D.L. World survey on the complications of hepatic and prostate cryotherapy. World J. Surg. 1999, 23, 109–113, discussion 113–104. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Bai, W.; Dong, Z.; Wang, C.; Lu, Y.; Zeng, Z.; Qu, J.; Lou, M.; Wang, H.; Gao, X.; et al. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS ONE 2015, 10, e0123065. [Google Scholar] [CrossRef] [PubMed]
- Glazer, D.I.; Tatli, S.; Shyn, P.B.; Vangel, M.G.; Tuncali, K.; Silverman, S.G. Percutaneous Image-Guided Cryoablation of Hepatic Tumors: Single-Center Experience With Intermediate to Long-Term Outcomes. AJR Am. J. Roentgenol. 2017, 209, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.; Parikh, N.; El-Haddad, G.; Kis, B. Ablation of Intrahepatic Cholangiocarcinoma. Semin. Interv. Radiol. 2019, 36, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Ross, P.; Wasan, H.S.; Hubner, R.A.; McNamara, M.G.; Lopes, A.; Manoharan, P.; Palmer, D.; Bridgewater, J.; Valle, J.W. Advanced Intrahepatic Cholangiocarcinoma: Post Hoc Analysis of the ABC-01, -02, and -03 Clinical Trials. J. Natl. Cancer Inst. 2020, 112, 200–210. [Google Scholar] [CrossRef]
- Yamagata, M.; Nakajima, T.; Aihara, T.; Nishijima, N.; Tomoo, Y.; Matsuki, G.; Fujikawa, M.; Ichise, N.; Kasai, M.; Okamoto, R.; et al. Two Cases of Elderly Patients with Giant Intrahepatic Cholangiocarcinoma Treated with Multidisciplinary Therapy including Ablation Therapy. Gan Kagaku Ryoho Cancer Chemother. 2022, 49, 1559–1561. [Google Scholar]
- Zhang, A.; Liu, B.; Xu, D.; Sun, Y. Advanced intrahepatic cholangiocarcinoma treated using anlotinib and microwave ablation: A case report. Medicine 2019, 98, e18435. [Google Scholar] [CrossRef]
- Yan, X.; Zhuang, L.P.; Ning, Z.Y.; Wang, P.; Meng, Z.Q. Addition of thermal ablation to systemic chemotherapy for the treatment of unresectable intrahepatic cholangiocarcinoma: A propensity score matching analysis. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 81–88. [Google Scholar] [CrossRef]
- Seidensticker, R.; Seidensticker, M.; Doegen, K.; Mohnike, K.; Schütte, K.; Stübs, P.; Kettner, E.; Pech, M.; Amthauer, H.; Ricke, J. Extensive Use of Interventional Therapies Improves Survival in Unresectable or Recurrent Intrahepatic Cholangiocarcinoma. Gastroenterol. Res. Pract. 2016, 2016, 8732521. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charalampopoulos, G.; Iezzi, R.; Tsitskari, M.; Mazioti, A.; Papakonstantinou, O.; Kelekis, A.; Kelekis, N.; Filippiadis, D. Role of Percutaneous Ablation in the Management of Intrahepatic Cholangiocarcinoma. Medicina 2023, 59, 1186. https://doi.org/10.3390/medicina59071186
Charalampopoulos G, Iezzi R, Tsitskari M, Mazioti A, Papakonstantinou O, Kelekis A, Kelekis N, Filippiadis D. Role of Percutaneous Ablation in the Management of Intrahepatic Cholangiocarcinoma. Medicina. 2023; 59(7):1186. https://doi.org/10.3390/medicina59071186
Chicago/Turabian StyleCharalampopoulos, Georgios, Roberto Iezzi, Maria Tsitskari, Argyro Mazioti, Olympia Papakonstantinou, Alexis Kelekis, Nikolaos Kelekis, and Dimitrios Filippiadis. 2023. "Role of Percutaneous Ablation in the Management of Intrahepatic Cholangiocarcinoma" Medicina 59, no. 7: 1186. https://doi.org/10.3390/medicina59071186
APA StyleCharalampopoulos, G., Iezzi, R., Tsitskari, M., Mazioti, A., Papakonstantinou, O., Kelekis, A., Kelekis, N., & Filippiadis, D. (2023). Role of Percutaneous Ablation in the Management of Intrahepatic Cholangiocarcinoma. Medicina, 59(7), 1186. https://doi.org/10.3390/medicina59071186










