The Switch from Ferric Gluconate to Ferric Carboxymaltose in Hemodialysis Patients Acts on Iron Metabolism, Erythropoietin, and Costs: A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
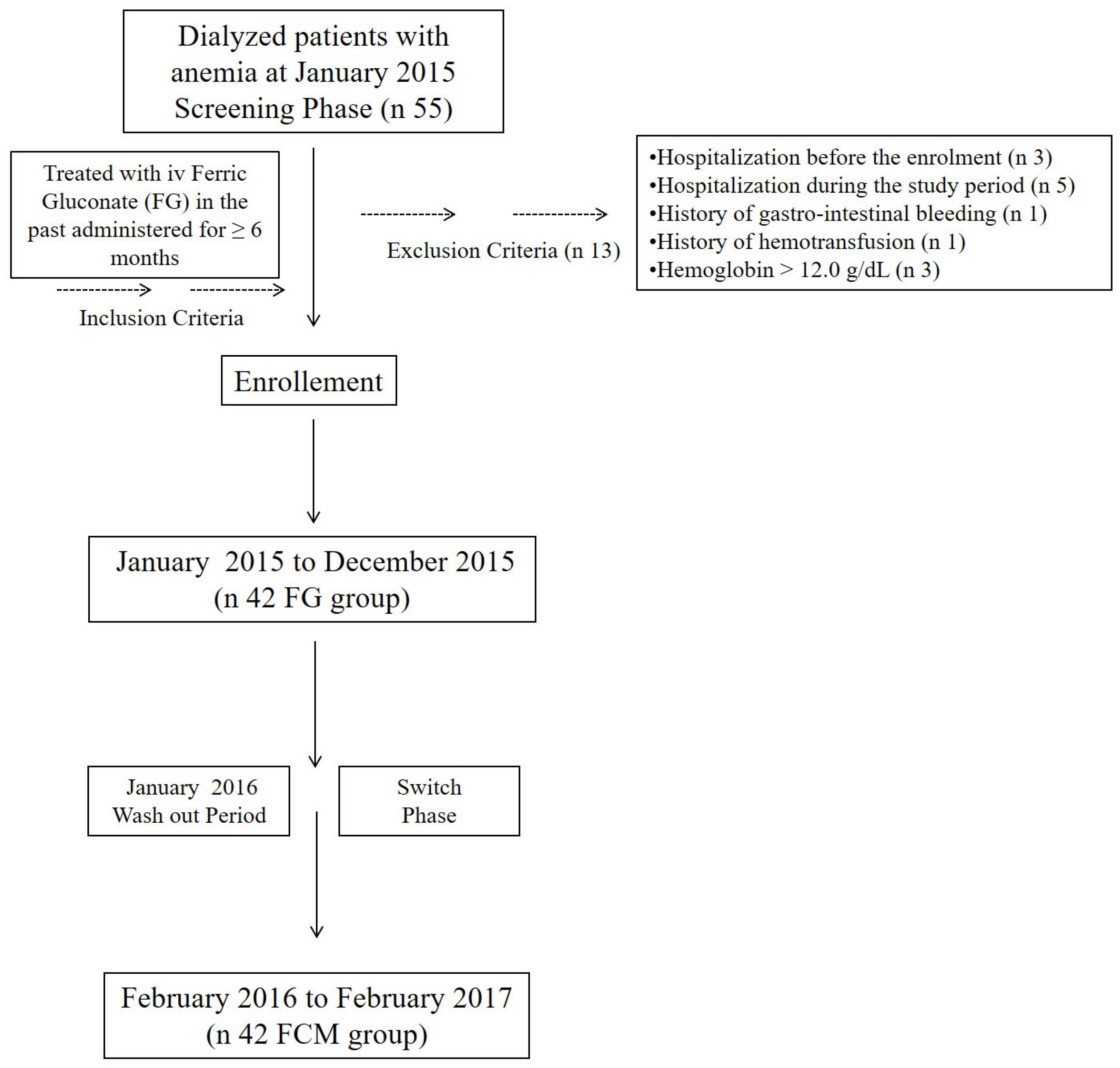
2.1. Study Design
2.2. Patients and Baseline Data
2.3. Blood Collection and Biochemical Data
2.4. Statistical Analysis
3. Results
3.1. Patients Baseline Characteristics
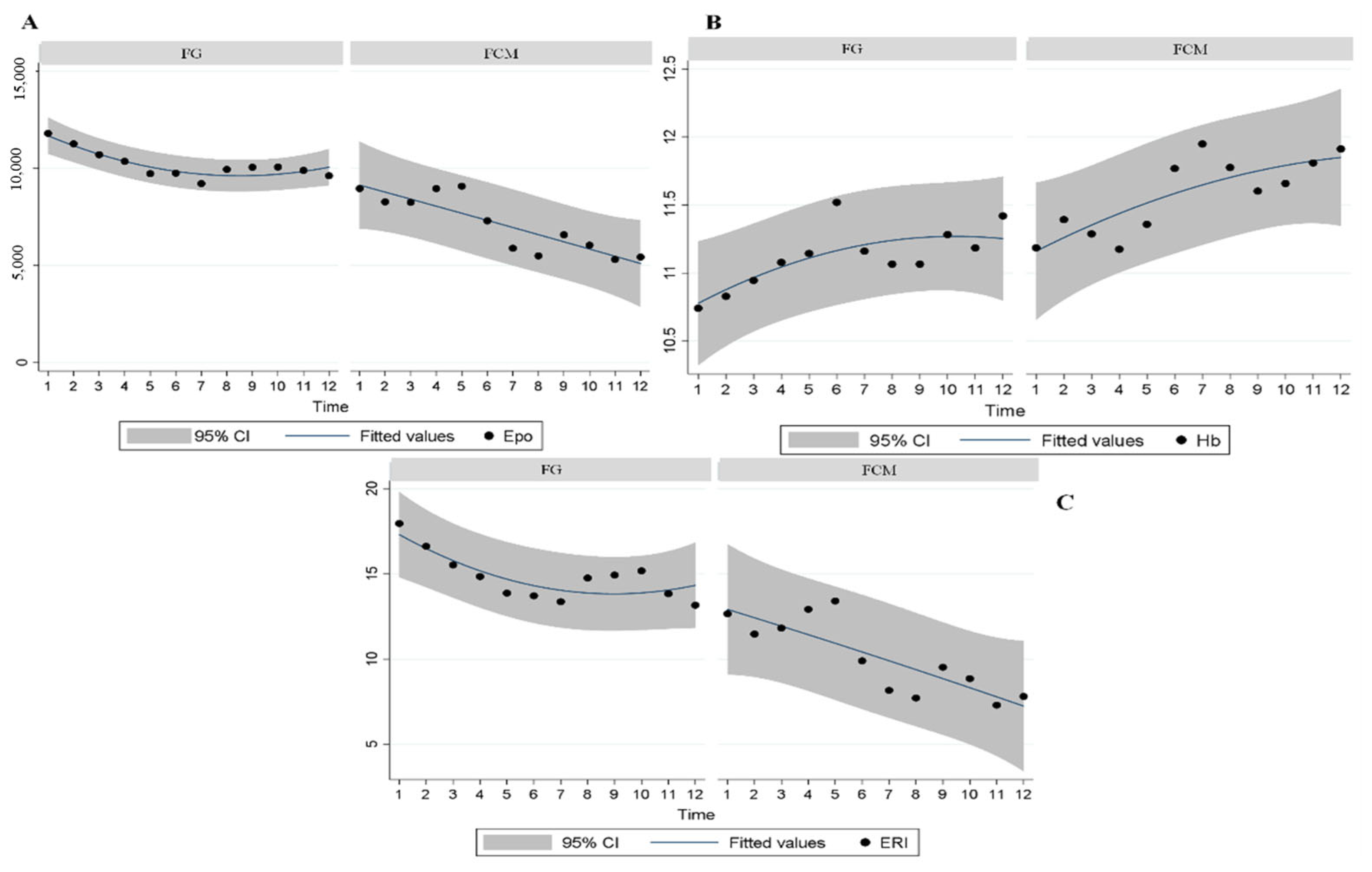
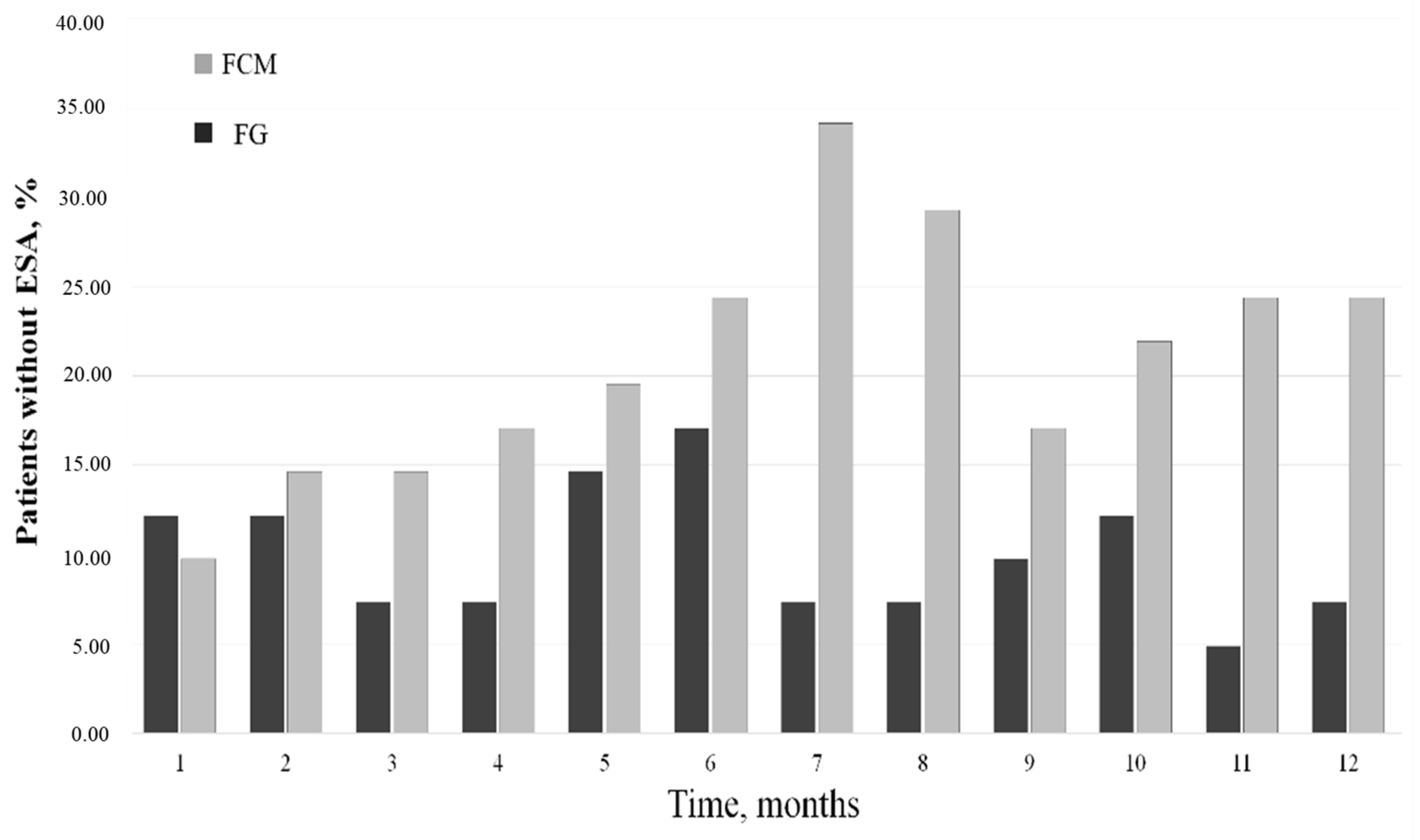
3.2. Epo and ERI Modifications
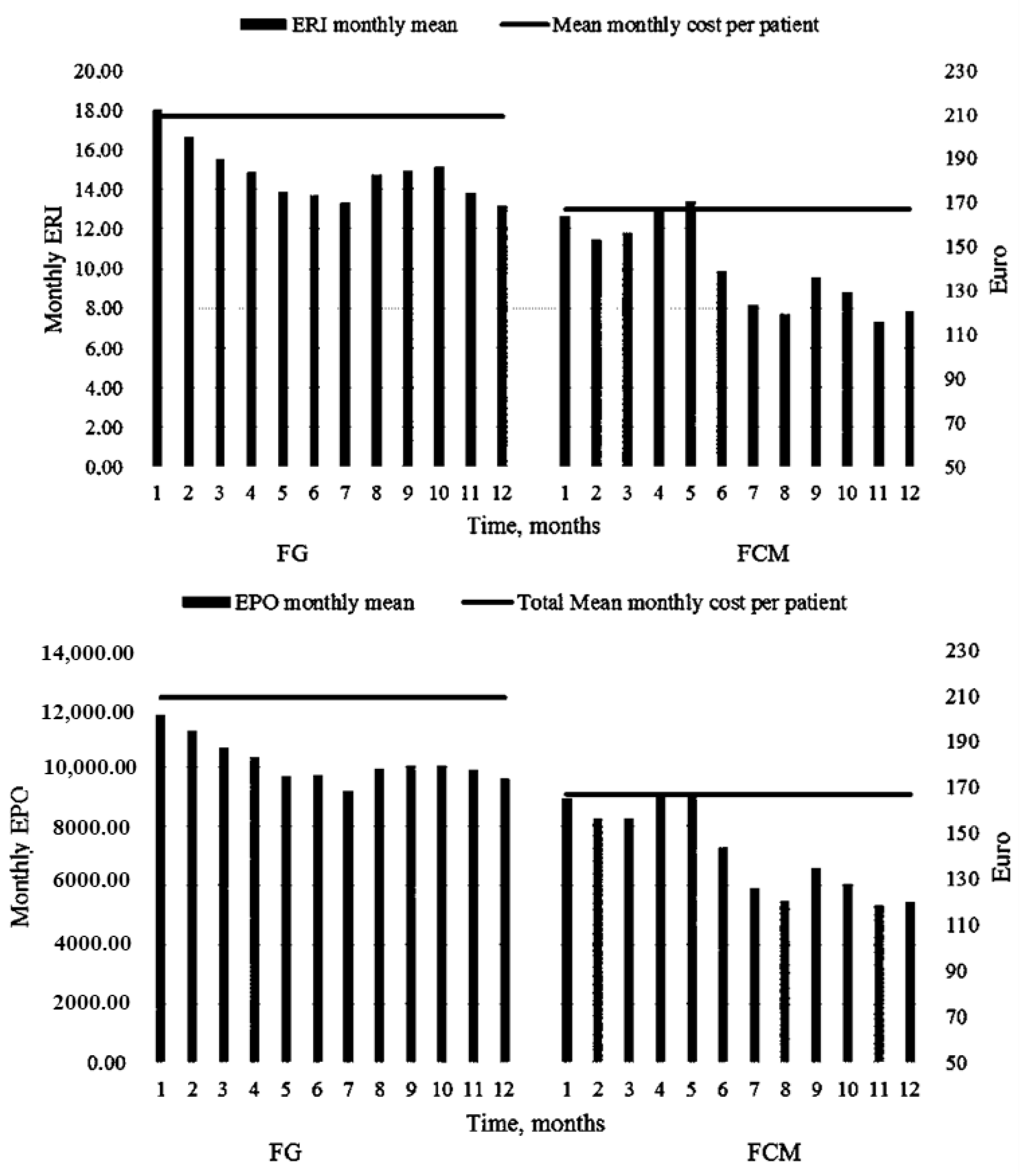
3.3. FG vs. FCM: Dosages and Costs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wish, J.B.; Aronoff, G.R.; Bacon, B.R.; Brugnara, C.; Eckardt, K.U.; Ganz, T.; Macdougall, I.C.; Núñez, J.; Perahia, A.J.; Wood, J.C. Positive iron balance in chronic kidney disease: How much is too much and how to tell? Am. J. Nephrol. 2018, 47, 72–83. [Google Scholar] [CrossRef]
- Chang, C.H.; Chang, C.C.; Chiang, S.S. Reduction in erythropoietin doses by the use of chronic intravenous iron supplementation in iron-replete hemodialysis patients. Clin. Nephrol. 2002, 57, 136–141. [Google Scholar] [CrossRef]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C. Intravenous Iron Use in the Care of Patients with Kidney Disease. Clin. J. Am. Soc. Nephrol. 2019, 14, 1528–1530. [Google Scholar] [CrossRef]
- Macdougall, I.C.; White, C.; Anker, S.D.; Bhandari, S.; Farrington, K.; Kalra, P.A.; McMurray, J.J.; Murray, H.; Tomson, C.R.; Wheeler, D.C.; et al. PIVOTAL Investigators and Committees. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N. Engl. J. Med. 2019, 380, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Brock, E.; Braunhofer, P.; Troxler, J.; Schneider, H. Budget impact of parenteral iron treatment of iron deficiency: Methodological issues raised by using real-life data. Eur. J. Health Econ. 2014, 15, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Diebold, M.; Kistler, A.D. Evaluation of iron stores in hemodialysis patients on maintenance ferric Carboxymaltose dosing. BMC Nephrol. 2019, 20, 76. [Google Scholar] [CrossRef]
- Covic, A.; Mircescu, G. The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: A multi-centre, open-label, clinical study. Nephrol. Dial. Transplant. 2010, 25, 2722–2730. [Google Scholar] [CrossRef]
- Hofman, J.M.G.; Eisenga, M.F.; Diepenbroek, A.; Nolte, I.M.; Van Dam, B.; Westerhuis, R.; Bakker, S.J.L.; Franssen, C.F.M.; Gaillard, C.A.J.M. Switching iron sucrose to ferric carboxymaltose associates to better control of iron status in hemodialysis patients. BMC Nephrol. 2018, 19, 242. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Pasqualetti, P.; Di Tocco, T.C.; Campo, S.; Rovito, S.; Bucca, M.; Ragusa, A.; Monardo, P. Ferric carboxymaltose versus ferric gluconate in hemodialysis patients: Reduction of erythropoietin dose in 4 years of follow-up. Kidney Res. Clin. Pract. 2020, 39, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, C.; Ortalda, V.; Biasi, C.; Gambaro, G. Economic Evaluation of Ferric Carboxymaltose for the Management of Hemodialysis Patients with Iron Deficiency Anemia in Italy. Adv. Ther. 2019, 36, 3253–3264. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.; Conti, P.; Berto, P.; Molinaro, S.; Baldini, F.; Egan, C.G.; Panichi, V.; Tuscany Study Group for Ferric Carboxymaltose. Efficacy, Safety and Pharmacoeconomic Analysis of Intravenous Ferric Carboxymaltose in Anemic Hemodialysis Patients Unresponsive to Ferric Gluconate Treatment: A Multicenter Retrospective Study. J. Clin. Med. 2022, 11, 5284. [Google Scholar] [CrossRef]
- McMurray, J.; Parfrey, P.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Macdougall, I.C.; White, C.; Anker, S.D.; Bhandari, S.; Farrington, K.; Kalra, P.A.; McMurray, J.J.V.; Murray, H.; Steenkamp, R.; Tomson, C.R.V.; et al. Randomized Trial Comparing Proactive, High-Dose versus Reactive, Low-Dose Intravenous Iron Supplementation in Hemodialysis (PIVOTAL): Study Design and Baseline Data. Am. J. Nephrol. 2018, 48, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.B.; Jhund, P.S.; Walters, M.R.; Petrie, M.C.; Power, A.; White, C.; Robertson, M.; Connolly, E.; Anker, S.D.; Bhandari, S.; et al. Stroke in He-modialysis Patients Randomized to Different Intravenous Iron Strategies: A Prespecified Analy-sis from the PIVOTAL Trial. Kidney360 2021, 2, 1761–1769. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bhandari, S.; White, C.; Anker, S.D.; Farrington, K.; Kalra, P.A.; Mark, P.B.; McMurray, J.J.V.; Reid, C.; Robertson, M.; et al. Intravenous Iron Dosing and Infection Risk in Patients on Hemodialysis: A Prespecified Secondary Analysis of the PIVOTAL Trial. J. Am. Soc. Nephrol. 2020, 31, 1118–1127. [Google Scholar] [CrossRef]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Małyszko, J.; Swinkels, D.W.; Tarng, D.C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef]
- Locatelli, F.; Andrulli, S.; Memoli, B.; Maffei, C.; Del Vecchio, L.; Aterini, S.; De Simone, W.; Mandalari, A.; Brunori, G.; Amato, M.; et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol. Dial. Transplant. 2006, 21, 991–998. [Google Scholar] [CrossRef]
- Pesarin, F. Multivariate Permutation Tests: With Application in “Biostatistics”; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Bielesz, B.; Lorenz, M.; Monteforte, R.; Prikoszovich, T.; Gabriel, M.; Wolzt, M.; Gleiss, A.; Hörl, W.H.; Sunder-Plassmann, G. Comparison of Iron Dosing Strategies in Patients Undergoing Long-Term Hemodialysis: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2021, 16, 1512–1521. [Google Scholar] [CrossRef]
- Cirillo, L.; Somma, C.; Allinovi, M.; Bagalà, A.; Ferro, G.; Di Marcantonio, E.; Bellelli, S.; Dallari, L.A.; Ballo, P.; Dattolo, P.C. Ferric carboxymaltose vs. ferrous sulfate for the treatment of anemia in advanced chronic kidney disease: An observational retrospective study and cost analysis. Sci. Rep. 2021, 11, 7463. [Google Scholar] [CrossRef] [PubMed]
- Cappell, K.A.; Shreay, S.; Cao, Z.; Varker, H.V.; Paoli, C.J.; Gitlin, M. Red blood cell (RBC) transfusion rates among US chronic dialysis patients during changes to Medicare end-stage renal disease (ESRD) reimbursement systems and erythropoiesis stimulating agent (ESA) labels. BMC Nephrol. 2014, 15, 116. [Google Scholar] [CrossRef]
- Li, X.; Kshirsagar, A.V.; Brookhart, M.A. Safety of intravenous iron in hemodialysis patients. Hemodial. Int. 2017, 21, S93–S103. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Frei, G.L.; Maesaka, J. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am. J. Kidney Dis. 1995, 26, 41–46. [Google Scholar] [CrossRef]
- Besarab, A.; Amin, N.; Ahsan, M.; Vogel, S.E.; Zazuwa, G.; Frinak, S.; Zazra, J.J.; Anandan, J.V.; Gupta, A. Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J. Am. Soc. Nephrol. 2000, 11, 530–538. [Google Scholar] [CrossRef]
- Roger, S.D.; Gaillard, C.A.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Van Wyck, D.B.; Cronin, M.; Meier, Y.; Larroque, S.; Macdougall, I.C.; et al. Safety of intravenous ferric carboxymaltose versus oral iron in patients with nondialysis-dependent CKD: An analysis of the 1-year FIND-CKD trial. Nephrol. Dial. Transplant. 2017, 32, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Coyne, D.W.; Kapoian, T.; Suki, W.; Singh, A.K.; Moran, J.E.; Dahl, N.V.; Rizkala, A.R.; the DRIVE Study Group. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J. Am. Soc. Nephrol. 2007, 18, 975–984. [Google Scholar] [CrossRef]
- Fishbane, S.; Spinowitz, B. Update on Anemia in ESRD and Earlier Stages of CKD: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 423–435. [Google Scholar] [CrossRef]
- Mikhail, A.; Brown, C.; Williams, J.A.; Mathrani, V.; Shrivastava, R.; Evans, J.; Isaac, H.; Bhandari, S. Renal association clinical practice guideline on Anaemia of Chronic Kidney Disease. BMC Nephrol. 2017, 18, 345. [Google Scholar] [CrossRef]
- Erdem, E.; Karatas, A.; Ecder, T. The Relationship between Serum Ferritin Levels and 5-Year All-Cause Mortality in Hemodialysis Patients. Blood Purif. 2022, 51, 55–61. [Google Scholar] [CrossRef]
- Faria, B.; da Costa, M.G.; Poppelaars, F.; Franssen, C.F.M.; Pestana, M.; Berger, S.P.; Daha, M.R.; Gaillard, C.A.J.M.; Seelen, M.A. Administration of Intravenous Iron Formulations Induces Complement Activation in-vivo. Front. Immunol. 2019, 10, 1885. [Google Scholar] [CrossRef] [PubMed]
- Righini, M.; Dalmastri, V.; Capelli, I.; Orsi, C.; Donati, G.; Pallotti, M.G.; Pedone, C.; Casella, G.; Chieco, P.; La Manna, G. Intravenous Iron Replacement Therapy Improves Cardiovascular Outcomes in Hemodialysis Patients. In Vivo 2021, 35, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Fukuma, S.; Yamaguchi, T.; Hashimoto, S.; Nakai, S.; Iseki, K.; Tsubakihara, Y.; Fukuhara, S. Erythropoiesis-stimulating agent responsiveness and mortality in hemodialysis patients: Results from a cohort study from the dialysis registry in Japan. Am. J. Kidney Dis. 2012, 59, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Minutolo, R.; Borzumati, M.; Sposini, S.; Abaterusso, C.; Carraro, G.; Santoboni, A.; Mura, C.; Filiberti, O.; Santoro, D.; Musacchio, R.; et al. Dosing penalty of erythropoiesis-stimulating agents after switching from originator to biosimilar preparations in stable hemodialysis patients. Am. J. Kidney Dis. 2016, 68, 170–172. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Burdmann, E.A.; Chen, C.-Y.; Cooper, M.E.; de Zeeuw, D.; Eckardt, K.-U.; Feyzi, J.M.; Ivanovich, P.; Kewalramani, R.; Levey, A.S.; et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 2009, 361, 2019–2032. [Google Scholar] [CrossRef]
- Singh, A.K.; Szczech, L.; Tang, K.L.; Barnhart, H.; Sapp, S.; Wolfson, M.; Reddan, D. Correction of anemia with epoetin alfa in chronic kidney disease. N. Engl. J. Med. 2006, 355, 2085–2098. [Google Scholar] [CrossRef]
- Del Vecchio, L.; Locatelli, F. An overview on safety issues related to erythropoiesis-stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin. Drug Saf. 2016, 15, 1021–1030. [Google Scholar] [CrossRef]
- Perez-Garcia, R.; Varas, J.; Cives, A.; Martin-Malo, A.; Aljama, P.; Ramos, R.; Pascual, J.; Stuard, S.; Canaud, B.; Merello, J.I.; et al. Increased mortality in haemodialysis patients administered high doses of erythropoiesis-stimulating agents: A propensity score-matched analysis. Nephrol. Dial. Transplant. 2018, 33, 690–699. [Google Scholar] [CrossRef]
| F/U | FG Treatment | FCM Treatment | |||
|---|---|---|---|---|---|
| Time | Mean ± SD | 95% CI | Mean ± SD | 95% CI | p-Value |
| 1 | 17.9 ± 2.0 | 14.0–21.9 | 12.6 ± 1.5 | 9.6–15.7 | 0.036 |
| 2 | 16.6 ± 1.8 | 12.9–20.3 | 11.4 ± 1.5 | 8.4–14.5 | 0.037 |
| 3 | 15.5 ± 1.3 | 12.8–18.2 | 11.8 ± 1.7 | 8.4–15.1 | 0.094 |
| 4 | 14.8 ± 1.5 | 11.8–17.8 | 12.9 ± 1.5 | 9.9–15.9 | 0.380 |
| 5 | 13.8 ± 1.8 | 10.1–17.5 | 13.4 ± 1.9 | 9.5–17.3 | 0.865 |
| 6 | 13.7 ± 2.0 | 9.7–17.7 | 9.9 ± 1.6 | 6.7–13.0 | 0.154 |
| 7 | 13.3 ± 1.5 | 10.2–16.5 | 8.1 ± 1.2 | 5.7–10.6 | 0.011 |
| 8 | 14.7 ± 1.8 | 11.1–18.3 | 7.7 ± 1.2 | 5.2–10.2 | 0.001 |
| 9 | 14.9 ± 1.8 | 11.2–18.6 | 9.5 ± 1.4 | 6.7–12.3 | 0.022 |
| 10 | 15.1 ± 2.0 | 11.1–19.2 | 8.8 ± 1.3 | 6.2–11.4 | 0.012 |
| 11 | 13.8 ± 1.2 | 11.3–16.3 | 7.3 ± 1.0 | 5.3–9.3 | <0.001 |
| 12 | 13.1 ± 1.5 | 10.1–16.2 | 7.8 ± 1.6 | 4.6–11.0 | 0.014 |
| F/U | FG Treatment | FCM Treatment | |||
|---|---|---|---|---|---|
| Time | Mean ± SD | 95% CI | Mean ± SD | 95% CI | p-Value |
| 1 | 10.7 ± 0.2 | 10.2–11.2 | 11.1 ± 0.2 | 10.7–11.6 | 0.185 |
| 2 | 10.8 ± 0.2 | 10.3–11.2 | 11.3 ± 0.2 | 11.0–11.7 | 0.068 |
| 3 | 10.9 ± 0.2 | 10.5–11.3 | 11.2 ± 0.2 | 10.8–11.7 | 0.251 |
| 4 | 11.0 ± 0.1 | 10.7–11.4 | 11.1 ± 0.1 | 10.8–11.5 | 0.688 |
| 5 | 11.1 ± 0.1 | 10.8–11.4 | 11.3 ± 0.2 | 10.8–11.8 | 0.454 |
| 6 | 11.5 ± 0.1 | 11.1–11.8 | 11.7 ± 0.1 | 11.4–12.0 | 0.290 |
| 7 | 11.1 ± 0.1 | 10.8–11.5 | 11.9 ± 0.1 | 11.6–12.2 | 0.001 |
| 8 | 11.0 ± 0.1 | 10.7–11.3 | 11.7 ± 0.1 | 11.4–12.0 | 0.002 |
| 9 | 11.0 ± 0.1 | 10.6–11.4 | 11.6 ± 0.1 | 11.2–11.9 | 0.033 |
| 10 | 11.2 ± 0.1 | 10.9–11.6 | 11.6 ± 0.1 | 11.3–11.9 | 0.138 |
| 11 | 11.1 ± 0.1 | 10.8–11.5 | 11.8 ± 0.1 | 11.4–12.2 | 0.013 |
| 12 | 11.4 ± 0.1 | 11.1–11.7 | 11.9 ± 0.6 | 11.5–12.2 | 0.049 |
| F/U | FG Treatment | FCM Treatment | |||
|---|---|---|---|---|---|
| Time | Mean ± SD | 95% CI | Mean ± SD | 95% CI | p-Value |
| 1 | 11,804.8 ± 1240.1 | 9371.1–14,238.6 | 8963.4 ± 1044.2 | 6914.2–11,012.5 | 0.090 |
| 2 | 11,268.2 ± 1255.2 | 8805.0–13,731.5 | 8280.4 ± 1068.1 | 6184.4–10,376.5 | 0.076 |
| 3 | 10,707.3 ± 1020.0 | 8705.5–12,709.0 | 8256.1 ± 1061.6 | 6172.6–10,339.5 | 0.107 |
| 4 | 10,365.8 ± 1028.1 | 8348.3–12,383.4 | 8963.4 ± 1006.7 | 6987.8–10,939.0 | 0.348 |
| 5 | 9731.7 ± 1164.5 | 7446.4–12,016.9 | 9085.3 ± 1148.4 | 6831.7–11,339.0 | 0.701 |
| 6 | 9756.1 ± 1288.3 | 7227.9–12,284.3 | 7304.8 ± 1061.0 | 5222.6–9387.1 | 0.164 |
| 7 | 9219.5 ± 965.1 | 7325.6–11,113.4 | 5890.2 ± 865.9 | 4190.9–7589.5 | 0.011 |
| 8 | 9951.2 ± 1044.2 | 7901.9–12,000.4 | 5500.0 ± 802.4 | 3925.3–7074.6 | <0.001 |
| 9 | 10,060.9 ± 1047.0 | 8006.1–12,115.7 | 6585.3 ± 837.3 | 4942.1–8228.6 | 0.011 |
| 10 | 10,073.1 ± 1102.3 | 7909.9–12,236.3 | 6048.7 ± 830.0 | 4419.9–7677.6 | 0.004 |
| 11 | 9900.0 ± 819.4 | 8291.8–11,508.1 | 5317.0 ± 667.5 | 4007.0–6627.1 | <0.001 |
| 12 | 9625.0 ± 998.5 | 7665.5–11,584.4 | 5439.0 ± 842.5 | 3785.70–7092.3 | 0.001 |
| Resource | Unit cost | |
|---|---|---|
| FCM * | EUR 3.876/mL (100 mg) | |
| FG * | EUR 0.38431/fL (62.5 mg) | |
| Syringe * | EUR 0.034 | |
| Infusion set * | EUR 0.093 | |
| Physiological solution 100 mL * | EUR 0.396 | |
| Epo-z 2000 UI * | EUR 28.09 | |
| Epo-z 3000 UI * | EUR 42.15 | |
| Epo-z 4000 UI * | EUR 56.23 | |
| Epo-z 5000 UI * | EUR 70.28 | |
| Epo-z 6000 UI * | EUR 84.30 | |
| Epo-z 8000 UI * | EUR 112.34 | |
| Epo-z 10,000 UI * | EUR 140.58 | |
| Personal time—physician (1 h) # | EUR 41.50 | |
| Personal time—nurse (1 h) # | EUR 15.70 | |
| Cost item in EUR | FG | FCM |
| Iron treatment | 1528 | 5213.2 |
| Epo-z | 73,349.4 | 50,746.1 |
| Infusion material | 2079.4 | 703.4 |
| Personnel | 28,433.3 | 27,517.9 |
| Total | 105,390.2 | 84,180.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacquaniti, A.; Gargano, R.; Campo, S.; Casuscelli di Tocco, T.; Schifilliti, S.; Monardo, P. The Switch from Ferric Gluconate to Ferric Carboxymaltose in Hemodialysis Patients Acts on Iron Metabolism, Erythropoietin, and Costs: A Retrospective Analysis. Medicina 2023, 59, 1071. https://doi.org/10.3390/medicina59061071
Lacquaniti A, Gargano R, Campo S, Casuscelli di Tocco T, Schifilliti S, Monardo P. The Switch from Ferric Gluconate to Ferric Carboxymaltose in Hemodialysis Patients Acts on Iron Metabolism, Erythropoietin, and Costs: A Retrospective Analysis. Medicina. 2023; 59(6):1071. https://doi.org/10.3390/medicina59061071
Chicago/Turabian StyleLacquaniti, Antonio, Romana Gargano, Susanna Campo, Teresa Casuscelli di Tocco, Silvia Schifilliti, and Paolo Monardo. 2023. "The Switch from Ferric Gluconate to Ferric Carboxymaltose in Hemodialysis Patients Acts on Iron Metabolism, Erythropoietin, and Costs: A Retrospective Analysis" Medicina 59, no. 6: 1071. https://doi.org/10.3390/medicina59061071
APA StyleLacquaniti, A., Gargano, R., Campo, S., Casuscelli di Tocco, T., Schifilliti, S., & Monardo, P. (2023). The Switch from Ferric Gluconate to Ferric Carboxymaltose in Hemodialysis Patients Acts on Iron Metabolism, Erythropoietin, and Costs: A Retrospective Analysis. Medicina, 59(6), 1071. https://doi.org/10.3390/medicina59061071





