Combination Treatment of Balloon Pulmonary Angioplasty and Direct Oral Anticoagulant in a Patient with Chronic Thromboembolic Pulmonary Hypertension Complicated by Protein S Deficiency
Abstract
1. Introduction
2. Case Presentation
2.1. Past History
2.2. Before Referral
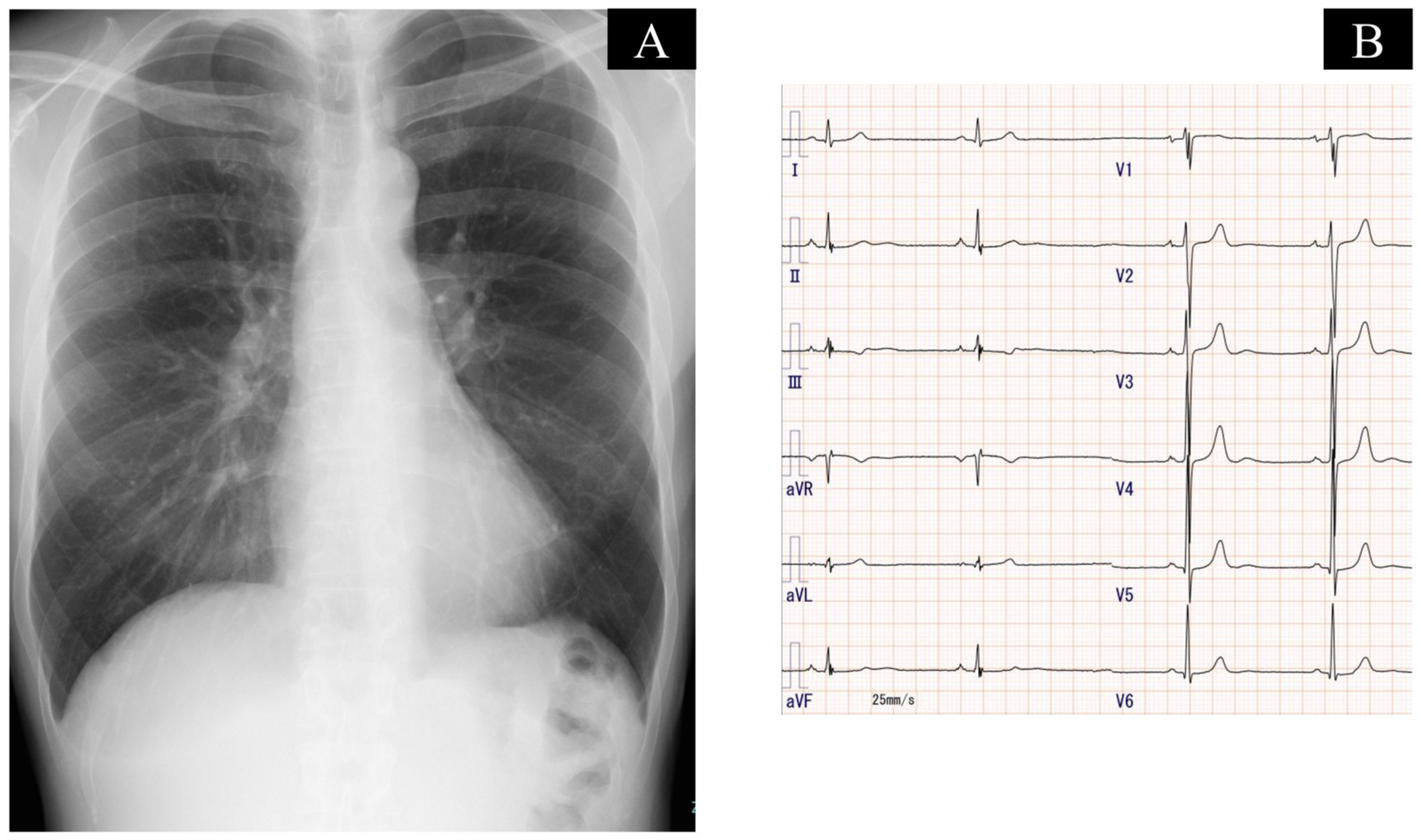
2.3. On Admission
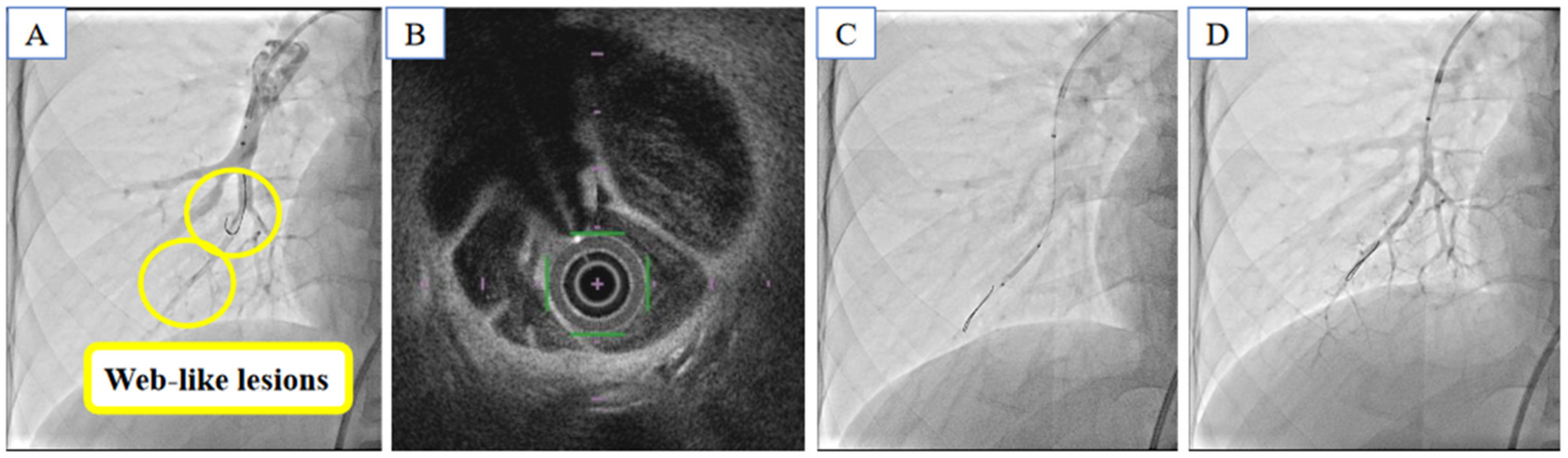
2.4. Balloon Pulmonary Angioplasty
2.5. Following Procedure
3. Discussion
3.1. Therapeutic Strategy for CTEPH and Protein S Deficiency
3.2. Angioplasty to Improve Exercise Tolerance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Ten Kate, M.K.; van der Meer, J. Protein S deficiency: A clinical perspective. Haemophilia 2008, 14, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.Y.; Liu, J.Z.; Guo, F.; Zhou, Y.P.; Wu, T.; Wang, H.; Li, J.Y.; Yan, X.X.; Peng, F.H.; Sun, K.; et al. Prevalence, Genetic Background, and Clinical Phenotype of Congenital Thrombophilia in Chronic Thromboembolic Pulmonary Hypertension. JACC Asia 2022, 2, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Takamoto, S.; Okita, Y.; Matsukawa, R.; Nakanishi, N.; Kyotani, S.; Satoh, T. Operation for chronic pulmonary thromboembolism accompanied by thrombophilia in 8 patients. Ann. Thorac. Surg. 1998, 66, 1919–1924. [Google Scholar] [CrossRef]
- Akbayrak, H.; Tekumit, H. Pulmonary thromboendarterectomy in a combined thrombophilia patient. Cardiovasc. J. Afr. 2019, 30, e4–e6. [Google Scholar] [CrossRef]
- Lindner, J.; Jansa, P.; Salaj, P.; Kunstýr, J.; Grus, T.; Maruna, P.; Bláha, J.; Rubes, D.; Ambroz, D.; Mlejnsý, F.; et al. Thrombophilia and pulmonary endarterectomy. Prague Med. Rep. 2009, 110, 51–59. [Google Scholar] [PubMed]
- Puebla-Aldama, D.; Cueto-Robledo, G.; Barragan-Martinez, M.D.; Roldan-Valadez, E.; Navarro-Vergara, D.I.; Garcia-Cesar, M.; Heredia-Flores, K.L.; Torres-Rojas, M.B.; Garcia-Treminio, C.F.; Cueto-Romero, H.D. Review of Functional Status and Hemodynamic Parameters in Patients Diagnosed with Chronic Thromboembolic Pulmonary Hypertension (CTEPH) with and without Antiphospholipid Syndrome (APLS). Curr. Probl. Cardiol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Dahlbäck, B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood 2008, 112, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Skelley, J.W.; White, C.W.; Thomason, A.R. The use of direct oral anticoagulants in inherited thrombophilia. J. Thromb. Thrombolysis. 2017, 43, 24–30. [Google Scholar] [CrossRef]
- Aoki, T.; Sugimura, K.; Tatebe, S.; Miura, M.; Yamamoto, S.; Yaoita, N.; Suzuki, H.; Sato, H.; Kozu, K.; Konno, R.; et al. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo-embolic pulmonary hypertension: Long-term effects and procedure-related complications. Eur. Heart J. 2017, 38, 3152–3159. [Google Scholar] [CrossRef] [PubMed]
- Shinkura, Y.; Nakayama, K.; Yanaka, K.; Kinutani, H.; Tamada, N.; Tsuboi, Y.; Satomi-Kobayashi, S.; Otake, H.; Shinke, T.; Emoto, N.; et al. Extensive revascularisation by balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension beyond haemodynamic normalisation. EuroIntervention 2018, 13, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Inami, T.; Kataoka, M.; Kikuchi, H.; Goda, A.; Satoh, T. Balloon pulmonary angioplasty for symptomatic chronic thromboembolic disease without pulmonary hypertension at rest. Int. J. Cardiol. 2019, 289, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Sugimura, K.; Terui, Y.; Tatebe, S.; Fukui, S.; Miura, M.; Yamamoto, S.; Yaoita, N.; Suzuki, H.; Sato, H.; et al. Beneficial effects of riociguat on hemodynamic responses to exercise in CTEPH patients after balloon pulmonary angioplasty—A randomized controlled study. Int. J. Cardiol. Heart Vasc. 2020, 29, 100579. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumida, T.; Imamura, T.; Ushijima, R.; Kinugawa, K. Combination Treatment of Balloon Pulmonary Angioplasty and Direct Oral Anticoagulant in a Patient with Chronic Thromboembolic Pulmonary Hypertension Complicated by Protein S Deficiency. Medicina 2023, 59, 909. https://doi.org/10.3390/medicina59050909
Izumida T, Imamura T, Ushijima R, Kinugawa K. Combination Treatment of Balloon Pulmonary Angioplasty and Direct Oral Anticoagulant in a Patient with Chronic Thromboembolic Pulmonary Hypertension Complicated by Protein S Deficiency. Medicina. 2023; 59(5):909. https://doi.org/10.3390/medicina59050909
Chicago/Turabian StyleIzumida, Toshihide, Teruhiko Imamura, Ryuichi Ushijima, and Koichiro Kinugawa. 2023. "Combination Treatment of Balloon Pulmonary Angioplasty and Direct Oral Anticoagulant in a Patient with Chronic Thromboembolic Pulmonary Hypertension Complicated by Protein S Deficiency" Medicina 59, no. 5: 909. https://doi.org/10.3390/medicina59050909
APA StyleIzumida, T., Imamura, T., Ushijima, R., & Kinugawa, K. (2023). Combination Treatment of Balloon Pulmonary Angioplasty and Direct Oral Anticoagulant in a Patient with Chronic Thromboembolic Pulmonary Hypertension Complicated by Protein S Deficiency. Medicina, 59(5), 909. https://doi.org/10.3390/medicina59050909






