Validation of the CLIF-C OF Score and CLIF-C ACLF Score to Predict Transplant-Free Survival in Patients with Liver Cirrhosis and Concomitant Need for Intensive Care Unit Treatment
Abstract
1. Introduction
2. Material and Methods
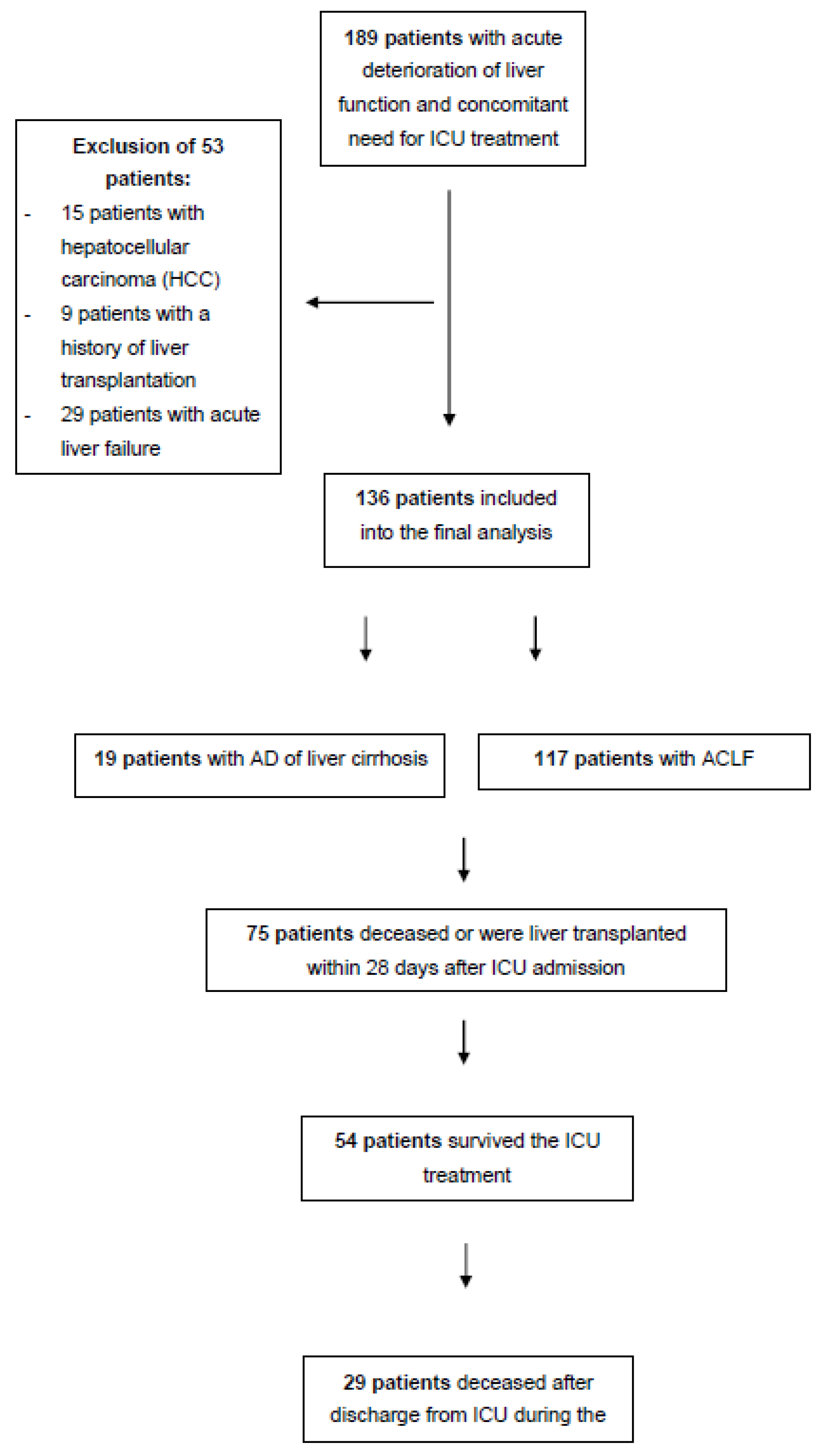
2.1. Patients
2.2. Definition of ACLF
2.3. Follow-Up
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Predictors of 28-Days Mortality
3.3. Predictors for Long-Term (90-Days and 365-Days) Mortality
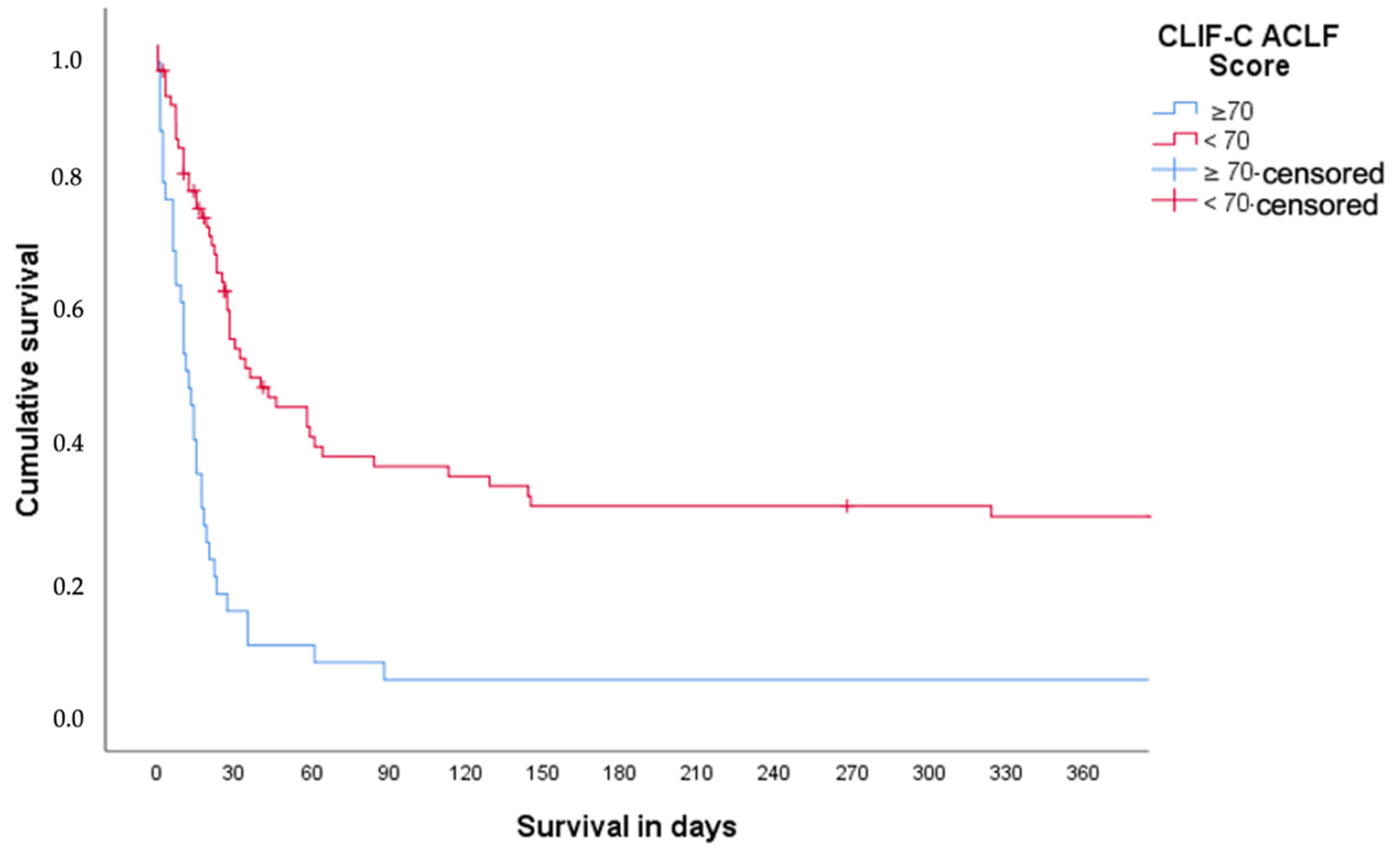
3.4. Survival Analysis in ACLF Grade 3 According to CLIF-C ACLFs
4. Discussion
5. Declaration
Availability of Supporting Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology 2013, 144, 1426–1437e9. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Moreau, R.; Jalan, R.; Ginès, P. Study, EASL-CLIF Consortium CANONIC. Acute-on-chronic liver failure: A new syndrome that will re-classify cirrhosis. J. Hepatol. 2015, 62, S131–S143. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Saliba, F.; Pavesi, M.; Amoros, A.; Moreau, R.; Ginès, P.; Levesque, E.; Durand, F.; Angeli, P.; Caraceni, P.; et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 2014, 61, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol. 2010, 53, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.S.; Pereira, R.; Alexandrino, G.; Bagulho, L. Futility of care in patients with acute-on-chronic liver failure. Hepatology 2017, 66, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Thomsen, K.L.; Zakeri, N.; Sheikh, M.; Agarwal, B.; Jalan, R.; Mookerjee, R.P. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit. Care 2018, 22, 254. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Clària, J.; Szabo, G.; Bosch, J.; Bernardi, M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 2021, 75, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Weil, D.; Levesque, E.; McPhail, M.; Cavallazzi, R.; Theocharidou, E.; Cholongitas, E.; Galbois, A.; Pan, H.C.; Karvellas, C.J.; Sauneuf, B.; et al. Prognosis of cirrhotic patients admitted to intensive care unit: A meta-analysis. Ann. Intensiv. Care 2017, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Piton, G.; Chaignat, C.; Giabicani, M.; Cervoni, J.-P.; Tamion, F.; Weiss, E.; Paugam-Burtz, C.; Capellier, G.; Di Martino, V. Prognosis of cirrhotic patients admitted to the general ICU. Ann. Intensiv. Care 2016, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Ahlbrand, C.J.; Gadban, R.; Nagel, M.; Labenz, C.; Klimpke, P.; Holtz, S.; Boedecker, S.; Michel, M.; Kremer, W.M.; et al. Applicability and safety of discontinuous ADVanced Organ Support (ADVOS) in the treatment of patients with acute-on-chronic liver failure (ACLF) outside of intensive care. PLoS ONE 2021, 16, e0249342. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Herber, A.; Franke, A.; Bruns, T.; Reuken, P.; Schiefke, I.; Zipprich, A.; Zeuzem, S.; Goeser, T.; Canbay, A.; et al. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: A multicenter randomized trial (GRAFT study). J. Hepatol. 2021, 75, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; McDiarmid, S.V.; Kamath, P.S.; Edwards, E.B.; Malinchoc, M.; Kremers, W.K.; Krom, R.A.; Kim, W.R. MELD and PELD: Application of survival models to liver allocation. Liver Transplant. 2001, 7, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Kerbert, A.J.; Gupta, S.; Alabsawy, E.; Dobler, I.; Lønsmann, I.; Hall, A.; Nielsen, S.H.; Nielsen, M.J.; Gronbaek, H.; Amoros, À.; et al. Biomarkers of extracellular matrix formation are associated with acute-on-chronic liver failure. JHEP Rep. 2021, 3, 100355. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.S.; Gu, W.; Schnitzbauer, A.A.; Trebicka, J. Liver Transplantation as a Cornerstone Treatment for Acute-On-Chronic Liver Failure. Transpl. Int. 2022, 35, 10108. [Google Scholar] [CrossRef] [PubMed]
- Teerasarntipan, T.; Thanapirom, K.; Chirapongsathorn, S.; Suttichaimongkol, T.; Chamroonkul, N.; Bunchorntavakul, C.; Siramolpiwat, S.; Chainuvati, S.; Sobhonslidsuk, A.; Leerapun, A.; et al. Validation of prognostic scores predicting mortality in acute liver decompensation or acute-on-chronic liver failure: A Thailand multicenter study. PLoS ONE 2022, 17, e0277959. [Google Scholar] [CrossRef] [PubMed]
- de Silva, P.P.C.; Codes, L.; Rios, F.F.; Esteve, C.P.; Filho, M.T.V.; Lima, D.O.C.; Filho, G.F.d.A.; Morais, M.C.A.; Lima, B.C.; Chagas, P.B.d.O.; et al. Comparison of General and Liver-Specific Prognostic Scores in Their Ability to Predict Mortality in Cirrhotic Patients Admitted to the Intensive Care Unit. Can. J. Gastroenterol. Hepatol. 2021, 2021, 9953106. [Google Scholar]
- Solà, E.; Fernandez, J.; Ginès, P. Acute-on-Chronic Liver Failure: The Role of Precipitating Illness. Semin. Liver Dis. 2016, 36, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, Z.; Li, K.; Wang, Q.; Bai, W.; Yuan, X.; Yu, T.; Niu, J.; Yang, Z.; Zhu, X.; et al. Risk Stratification Based on Chronic Liver Failure Consortium Acute Decompensation Score in Patients With Child-Pugh B Cirrhosis and Acute Variceal Bleeding. Hepatology 2020, 73, 1478–1493. [Google Scholar] [CrossRef] [PubMed]
| Variable | All Patients | Deceased within 28 Days | Alive after 28 Days | p-Value | |
|---|---|---|---|---|---|
| N | 136 | 75 | 61 | ||
| Age (years) (IQR) | 60 (51; 67) | 59 (51; 66) | 61 (52; 69) | 0.255 | |
| Male gender, n (%) | 90 (66%) | 46 (61%) | 44 (72%) | 0.186 | |
| Etiology | alcohol, n (%) viral hepatitis, n (%) NAFLD, n (%) others, n (%) | 89 (65%) 11 (8%) 10 (7%) 17 (13%) | 51 (68%) 7 (9%) 6 (8%) 11 (15%) | 47 (77%) 4 (7%) 4 (7%) 6 (15%) | 0.855 |
| reason for decompensation of liver cirrhosis and ICU admission | Bleeding, n (%) Infection, n (%) after surgical procedure, n (%) Others, n (%) | 41 (30%) 27 (20%) 18 (13%) 50 (37%) | 20 (27%) 9 (12%) 14 (19%) 32 (42%) | 14 (23%) 13 (21%) 6 (10%) 28 (46%) | 0.873 |
ACLF at admission, n (%)
| 19 (14%) 18 (13%) 34 (25%) 65 (48%) | 6 (8%) 10 (13%) 17 (23%) 42 (56%) | 13 (21%) 9 (15%) 17 (28%) 23 (38%) | 0.072 | |
| MELD (IQR) | 28 (20; 36) | 33 (27; 36) | 21 (16; 28) | <0.001 | |
| Infection at ICU admission, n (%) | 38 (28%) | 21 (28%) | 17 (28%) | 0.975 | |
| Renal replacement therapy during ICU treatment, n (%) | 78 (57%) | 58 (77%) | 20 (33%) | <0.001 | |
| Mechanical ventilation during ICU treatment, n (%) | 75 (55%) | 50 (67%) | 25 (41%) | <0.003 | |
| Vasopressor therapy during ICU treatment, n (%) | 117 (86%) | 69 (92%) | 48 (79%) | <0.026 | |
| CLIF-C OF score (IQR) CLIF-C ACLF score (IQR) | 11 (9; 13) 56 (51; 66) | 12 (10; 14) 61 (53; 71) | 10 (8; 12) 53 (49; 58) | <0.001 <0.001 | |
| Bilirubin (mg/dL) (IQR) | 4 (1.6; 9.9) | 6.7 (2.6; 19.6) | 2.2 (1.1; 4.8) | <0.001 | |
| Sodium (mmol/L)(IQR) | 136 (131; 141) | 137 (131; 140) | 136 (131; 141) | 0.613 | |
| Platelets (/nL) (IQR) | 94 (53; 165) | 88 (51; 144) | 114 (67; 177) | 0.035 | |
| INR (IQR) | 1.6 (1.3; 2.1) | 1.9 (1.5; 2.2) | 1.3 (1.2; 1.8) | <0.001 | |
| Creatinine (mg/dL) (IQR) | 2.1 (1.3; 3.2) | 2.4 (1.4; 3.3) | 1.9 (1.1; 3.0) | 0.167 | |
| WBC (/nL) (IQR) | 10 (7; 15.4) | 11.7 (8.3; 17) | 9 (5.8; 15) | 0.051 | |
| CRP (mg/L) (IQR) | 29 (12; 71) | 41 (16; 105) | 19 (8; 43) | 0.005 | |
| 28-Days Mortality AUROC (95% CI) | AUROC (90-Days Mortality) | AUROC (365-Days Mortality) | |
|---|---|---|---|
| CLIF_C OF score (total cohort) | 0.687 (0.599–0.774) | 0.713 (0.613–0.812) | 0.689 (0.581–0.796) |
| CLIF-C OF score (patients with ACLF) | 0.652 (0.554–0.750) | 0.666 (0.551–0.781) | 0.678 (0.552–0.804) |
| CLIF-C ACLF score (patients with ACLF) | 0.717 (0.626–0.809) | 0.710 (0.598–0.821) | 0.675 (0.550–0.800) |
| AD score (patients without ACLF) | 0.792 (0.560–1.000) | 0.713 (0.527–0.899) | 0.653 (0.425–0.880) |
| MELD score (total cohort) | 0.795 (0.719–0.870) | 0.837 (0.756–0.918) | 0.756 (0.655–0.857) |
| MELD score (patients with ACLF) | 0.767 (0.680–0.854) | 0.844 (0.758–0.931) | 0.784 (0.679–0.890) |
| MELD score (patients without ACLF) | 0.851 (0.657–1.000) | 0.722 (0.480–0.965) | 0.494 (0.222–0.766) |
| Sensitivity | Specificity | Positive Predictive Value (PPV) | Negative Predictive Value (NPV) | |
|---|---|---|---|---|
CLIF-C ACLF score *
| 25.7 38.6 54.3 | 97.8 87.2 85.1 | 94.7 81.8 84.4 | 6.9 48.8 55.6 |
CLIF-C OF score
| 19.7 34.2 39.5 | 96.7 95.1 86.9 | 88.2 89.7 78.9 | 49.2 53.7 53.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagel, M.; Westphal, R.; Hilscher, M.; Galle, P.R.; Schattenberg, J.M.; Schreiner, O.; Labenz, C.; Wörns, M.A. Validation of the CLIF-C OF Score and CLIF-C ACLF Score to Predict Transplant-Free Survival in Patients with Liver Cirrhosis and Concomitant Need for Intensive Care Unit Treatment. Medicina 2023, 59, 866. https://doi.org/10.3390/medicina59050866
Nagel M, Westphal R, Hilscher M, Galle PR, Schattenberg JM, Schreiner O, Labenz C, Wörns MA. Validation of the CLIF-C OF Score and CLIF-C ACLF Score to Predict Transplant-Free Survival in Patients with Liver Cirrhosis and Concomitant Need for Intensive Care Unit Treatment. Medicina. 2023; 59(5):866. https://doi.org/10.3390/medicina59050866
Chicago/Turabian StyleNagel, Michael, Ruben Westphal, Max Hilscher, Peter R. Galle, Jörn M. Schattenberg, Oliver Schreiner, Christian Labenz, and Marcus Alexander Wörns. 2023. "Validation of the CLIF-C OF Score and CLIF-C ACLF Score to Predict Transplant-Free Survival in Patients with Liver Cirrhosis and Concomitant Need for Intensive Care Unit Treatment" Medicina 59, no. 5: 866. https://doi.org/10.3390/medicina59050866
APA StyleNagel, M., Westphal, R., Hilscher, M., Galle, P. R., Schattenberg, J. M., Schreiner, O., Labenz, C., & Wörns, M. A. (2023). Validation of the CLIF-C OF Score and CLIF-C ACLF Score to Predict Transplant-Free Survival in Patients with Liver Cirrhosis and Concomitant Need for Intensive Care Unit Treatment. Medicina, 59(5), 866. https://doi.org/10.3390/medicina59050866







