Assessment of Osteoporosis and Vitamin D3 Deficiency in Patients with Idiopathic Benign Paroxysmal Positional Vertigo (BPPV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Sample Collection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Audiological Tests
2.5. Assessment of Vitamin D3 Levels
2.6. The Dix-Hallpike Maneuver
2.7. Bone Densitometry in the Lumbar Spine
2.8. Statistical Analysis
3. Results
3.1. Patient Clinical Characteristics
3.2. The Relationships between Age, Height, BMI, 25(OH) Vitamin D3 Levels and Bone Densitometry Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharyya, N.; Gubbels, S.P.; Schwartz, S.R.; Edlow, J.A.; El-Kashlan, H.; Fife, T.; Holmberg, J.M.; Mahoney, K.; Hollingsworth, D.B.; Roberts, R.; et al. Clinical practice guideline: Benign paroxysmal positional vertigo (update). Otolaryngol. Head Neck Surg. 2017, 156, S1–S47. [Google Scholar] [CrossRef] [PubMed]
- Türk, B.; Akpinar, M.; Kaya, K.S.; Korkut, A.Y.; Turgut, S. Benign Paroxysmal Positional Vertigo: Comparison of Idiopathic BPPV and BPPV Secondary to Vestibular Neuritis. Ear Nose Throat J. 2021, 100, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.H.; Kim, J.S. Update on benign paroxysmal positional vertigo. J. Neurol. 2021, 268, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Bárány, R. Diagnose von Krankheitserscheinungen im Beriche des Otolithenapparates. Acta Otolaryngol. 1921, 2, 434–437. [Google Scholar] [CrossRef]
- Imai, T.; Inohora, H. Benign paroxysmal positional vertigo. Auris Nasus Larynx 2022, 49, 737–747. [Google Scholar] [CrossRef]
- Sekine, K.; Imai, T.; Sato, G.; Ito., M.; Takeda., N. Natural history of benign paroxysmal positional vertigo and efficacy of Epley and Lempert maneuvers. Otolaryngol. Head Neck Surg. 2006, 135, 529–533. [Google Scholar] [CrossRef]
- Imai, T.; Takeda, N.; Ikezono, T.; Shigeno, K.; Asai, M.; Watanabe, Y.; Suzuki, M. Classification, diagnostic criteria and management of benign paroxysmal positional vertigo. Auris Nasus Larynx 2017, 44, 1–6. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Liu, Y.; Cao, J.; Zheng, H.; Jing, Y.; Han, L.; Ma, X.; Xia, R.; Yu, L. Risk Factors for the Recurrence of Benign Paroxysmal Positional Vertigo: A Systematic Review and Meta-Analysis. Ear Nose Throat J. 2022, 101, 112–134. [Google Scholar] [CrossRef]
- Chang, T.P.; Lin, Y.W.; Sung, P.Y.; Chuang, H.Y.; Chung, H.Y.; Liao, W.L. Benign paroxysmal positional vertigo after dental procedures: A population-based case-control study. PLoS ONE 2016, 11, e0153092. [Google Scholar] [CrossRef]
- Yuan, J.; Dai, J.; Li, W.A.; Hu, W. Factors associated with benign paroxysmal positional vertigo: A chinese case-control study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3885–3889. [Google Scholar] [CrossRef]
- Han, W.; Fan, Z.; Zhou, M.; Guo, X.; Yan, W.; Lu, X.; Li, L.; Gu, C.; Chen, C.; Wu, Y. Low 25-hydroxyvitamin D levels in postmenopausal female patients with benign paroxysmal positional vertigo. Acta Oto-Laryngol. 2018, 138, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, S.; Cui, K.; Liu, C. Risk factors for benign paroxysmal positional vertigo recurrence: A systematic review and meta-analysis. J. Neurol. 2021, 268, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, J.S.; Kim, H.J.; Choi, J.Y.; Park, J.Y.; Lee, S.H.; Choi, S.Y.; Oh, S.Y.; Yang, T.H.; Park, J.H.; et al. Prevention of benign paroxysmal positional vertigo with vitamin D supplementation: A randomized trial. Neurology 2020, 95, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- He, L.L.; Li, X.Y.; Hou, M.M.; Li, X.O. Association between bone mineral density and benign paroxysmal positional vertigo: A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Choi, S.H.; Kim, J.Y.; Koo, J.W.; Kim, H.J.; Kim, J.S. Osteopenia and osteoporosis in idiopathic benign positional vertigo. Neurology 2009, 72, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef]
- Ross, P.D.; Davis, J.W.; Epstein, R.S.; Wasnich, R.D. Preexisting fractures and bone mass predict vertebral fracture incidence in women. Ann. Intern. Med. 1991, 114, 919–923. [Google Scholar] [CrossRef]
- Imamudeen, N.; Basheer, A.; Iqbal, A.M.; Manjila, N.; Haroon, N.N.; Manjila, S. Management of Osteoporosis and Spinal Fractures: Contemporary Guidelines and Evolving Paradigms. Clin. Med. Res. 2022, 20, 95–106. [Google Scholar] [CrossRef]
- Watts, N.B.; Bilezikian, J.P.; Camacho, P.M.; Greenspan, S.L.; Harris, S.T.; Hodgson, S.F.; Kleerekoper, M.; Luckey, M.M.; McClung, M.R.; Pollack, R.P.; et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr. Pract. 2010, 16, 1016. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, J.S. Impaired calcium metabolism in benign paroxysmal positional vertigo: A topical review. J. Neurol. Phys. Ther. 2019, 43, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Han, P.; Duan, M.; Chen, Z.; Hu, J.; Chen, Y.; Xu, M.; Ren, P.; Zhang, Q. Bilateral Dysfunction of Otolith Pathway in Patients With Unilateral Idiopathic BPPV Detected by ACS-VEMPs. Front. Neurol. 2022, 13, 921133. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, H.K.; von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T. Epidemiology of vestibular vertigo: A neurotologic survey of the general population. Neurology 2005, 65, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: A population based study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 710–715. [Google Scholar] [CrossRef]
- Oron, Y.; Cohen-Atsmoni, S.; Len, A.; Roth, Y. Treatment of horizontal canal BPPV: Pathophysiology, available maneuvers, and recommended treatment. Laryngoscope 2015, 125, 1959–1964. [Google Scholar] [CrossRef]
- Fife, T.D.; Iverson, D.J.; Lempert, T.; Furman, J.M.; Baloh, R.W.; Tusa, R.J.; Hain, T.C.; Herdman, S.; Morrow, M.J.; Gronseth, G.S. Practice parameter: Therapies for benign paroxysmal positional vertigo (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2008, 27, 2067–2074. [Google Scholar] [CrossRef]
- Modungo, G.C.; Pirodda, A.; Ferri, G.G.; Rasciti, L.; Ceroni, A.R. A relationship between autoimmune thyroiditis and benign paroxysmal positional vertigo? Med. Hypotheses 2000, 54, 614–615. [Google Scholar]
- Shu, Y.; Liao, N.; Fang, F.; Shi, Q.; Yan, N.; Hu, Y. The relationship between psychological conditions and recurrence of benign paroxysmal positional vertigo: A retrospective cohort study. BMC Neurol. 2023, 23, 137. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, B.; Luo, J.; Ma, Y.; Li, J.; Zhang, T.; Yu, G. Global trends in the research on benign paroxysmal positional vertigo: A 20-year bibliometric and visualization analysis. Front. Neurol. 2022, 17, 1046257. [Google Scholar] [CrossRef]
- De Stefano, A.; Dispenza, F.; Suarez, H.; Perez-Fernandez, N.; Manrique-Huarte, R.; Ban, J.H.; Kim, M.B.; Strupp, M.; Feil, K.; Oliveira, C.A.; et al. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo. Auris Nasus Larynx 2014, 41, 31–36. [Google Scholar] [CrossRef]
- Zhu, C.T.; Zhao, X.Q.; Ju, Y.; Wang, Y.; Chen, M.M.; Cui, Y. Clinical Characteristics and Risk Factors for the Recurrence of Benign Paroxysmal Positional Vertigo. Front. Neurol. 2019, 13, 1190. [Google Scholar] [CrossRef]
- Fu, C.Y.; Zhang, Z.Z.; Chen, J.; Jaiswal, S.K.; Yan, F.L. Unhealthy Lifestyle Is an Important Risk Factor of Idiopathic BPPV. Front. Neurol. 2020, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, G.; Nicastro, L.; Gatto, A.; Tofanelli, M. Repeated canalith repositioning procedure in BPPV: Effects on recurrence and dizziness prevention. Am. J. Otolaryngol. 2017, 38, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Talaat, H.S.; Abuhadied, G.; Talaat, A.S.; Abdelaal, M.S.S. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, S.; Attanasio, G.; Ralli, M.; Marcelli, V.; de Vincentiis, M.; Greco, A.; Gallo, A. Does cervical range of motion affect the outcomes of canalith repositioning procedures for posterior canal benign positional paroxysmal vertigo? Am. J. Otolaryngol. 2019, 40, 494–498. [Google Scholar] [CrossRef]
- Talaat, H.S.; Kabel, A.M.H.; Khaliel, L.H.; Abuhadied, G.; Abo El-Naga, H.A.E.-R.; Talaat, A.S. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx 2016, 43, 237–241. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.A.; Fahim, D.F.M.; Bazeed, S.E.S.; Alemam, M.F.; Aref, Z.F. Relation between vitamin D deficiency and benign paroxysmal positional vertigo. Sci. Rep. 2021, 19, 16855. [Google Scholar] [CrossRef]
- Yang, H.; Gu, H.; Sun, W.; Li, Y.; Wu, H.; Burnee, M.; Zhuang, J. Estradiol deficiency is a risk factor for idiopathic benign paroxysmal positional vertigo in postmenopausal female patients. Laryngoscope 2018, 128, 948–953. [Google Scholar] [CrossRef]
- Yang, L.; Xu, L.; Xu, Y.; Zhang, Y.; Vijayakumar, S.; Jones, S.M.; Lundberg, Y.W. Deficiency on Otoconia. J. Assoc. Res. Otolaryngol. 2018, 19, 353–362. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Lv, X. Predictive values of serum estradiol, calcium, and 25-hydroxyvitamin D levels for recurrence of benign paroxysmal positional vertigo in postmenopausal women. Turk. J. Phys. Med. Rehabil. 2022, 68, 30–36. [Google Scholar] [CrossRef]
- Jang, Y.S.; Kang, M.K. Relationship between bone mineral density and clinical features in women with idiopathic benign paroxysmal positional vertigo. Otol. Neurotol. 2009, 30, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Han, W.W.; Wu, Y.Q.; Fan, Z.Y.; Yang, X.Y.; Guan, Q.F.; Yan, W.; Lu, X.X.; Liu, X.X.; Zhou, M.; Li, L.; et al. Characteristics of bone metabolism in postmenopausal female patients with different types of idiopathic benign paroxysmal positional vertigo: A single-centre retrospective study. Am. J. Otolaryngol. 2021, 42, 103149. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, F.; Cheng, Z.; Wang, Q. Association between osteoporosis and benign paroxysmal positional vertigo: A systematic review. BMC Neurol. 2014, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.J.; Min, C.; Choi, H.G. Association between benign paroxysmal positional vertigo and osteoporosis: Two nested case-control studies. Osteoporos. Int. 2020, 31, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Han, S.H.; Kim, Y.K.; Park, M.H. Clinical features of recurrence and osteoporotic changes in benign paroxysmal positional vertigo. Auris Nasus Larynx 2017, 44, 156–161. [Google Scholar] [CrossRef]
- Yang, C.J.; Kim, Y.; Lee, H.S.; Park, H.J. Bone mineral density and serum 25-hydroxyvitamin D in patients with idiopathic benign paroxysmal positional vertigo. J. Vestib. Res. 2018, 27, 287–294. [Google Scholar] [CrossRef]
- Chan, K.-C.; Tsai, Y.-T.; Yang, Y.-H.; Chen, P.-C.; Chang, P.-H. Osteoporosis is associated with increased risk for benign paroxysmal positional vertigo: A nationwide population-based study. Arch. Osteoporos. 2017, 25, 106. [Google Scholar] [CrossRef]
- Tabor, E.; Grodzki, A.; Pluskiewicz, W. Higher education and better knowledge of osteoporosis improve bone health in Polish postmenopausal women. Endokrynol. Pol. 2022, 73, 831–836. [Google Scholar] [CrossRef]
- Pluskiewicz, W.; Adamczyk, P.; Drozdzowska, B. Impaired Functional Status Increases Fracture Incidence in 10-year Follow-Up: The Results from RAC-OST-POL Study. J. Clin. Densitom. 2023, 26, 104–108. [Google Scholar] [CrossRef]
- Wilk, R.; Adamczyk, P.; Pluskiewicz, W.; Skrzypek, M.; Hajzyk, M.; Koczy, B. One year of the COVID-19 pandemic in Poland-the incidence of osteoporotic forearm, arm, and hip fractures. Arch. Osteoporos. 2022, 17, 38. [Google Scholar] [CrossRef]
- Jiang, X.; Gruner, M.; Trémollieres, F.; Pluskiewicz, W.; Sornay-Rendu, E.; Adamczyk, P.; Schantz, P.F. Diagnostic accuracy of FRAX in predicting the 10-year risk of osteoporotic fractures using the USA treatment thresholds: A systematic review and meta-analysis. Bone 2017, 99, 20–25. [Google Scholar] [CrossRef] [PubMed]
| M | Me | SD | Sk. | Kurt. | Min. | Max. | W | p | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 49 | 50 | 10.45 | −0.35 | −1.04 | 29 | 65 | 0.94 | 0.134 |
| Weight (kg) | 68.08 | 63.65 | 14.11 | 0.73 | −0.09 | 47 | 102.3 | 0.94 | 0.122 |
| Height reported by the patient (cm) | 163.43 | 162 | 7.23 | 0.36 | −0.85 | 152 | 178 | 0.96 | 0.325 |
| Height measured by the technician (cm) | 162.54 | 161.85 | 7.18 | 0.27 | −1.19 | 151.4 | 174.4 | 0.93 | 0.079 |
| BMI (kg/m2) | 25.70 | 25.32 | 4.66 | 0.78 | 1.14 | 17.81 | 39.22 | 0.96 | 0.292 |
| 25(OH) Vit. D (ng/mL) | 32.63 | 29.21 | 12.70 | 1.34 | 1.64 | 14.48 | 66.86 | 0.86 | 0.001 |
| Total BMD (g/cm2) | 0.96 | 0.94 | 0.12 | 0.42 | −0.55 | 0.75 | 1.21 | 0.96 | 0.345 |
| Total Z-score | 0.05 | −0.05 | 1.08 | 0.36 | −0.74 | −1.60 | 2.40 | 0.96 | 0.397 |
| Total T-score | −1.16 | −0.95 | 2.54 | −3.91 | 18.59 | −13 | 1.5 | 0.58 | <0.001 |
| M | Me | SD | Sk. | Kurt. | Min. | Max. | W | p | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 36.86 | 41 | 9.87 | −0.92 | −1.20 | 23 | 45 | 0.78 | 0.026 |
| Weight (kg) | 97.63 | 101 | 14.97 | −0.44 | −0.90 | 74 | 1140.6 | 0.95 | 0.696 |
| Height reported by the patient (cm) | 180.43 | 178 | 7.11 | 0.59 | −0.78 | 172 | 192 | 0.94 | 0.666 |
| Height measured by the technician (cm) | 178.66 | 176.50 | 7.97 | 0.59 | −0.77 | 169.5 | 1910.7 | 0.94 | 0.636 |
| BMI (kg/m2) | 30.50 | 30.36 | 3.71 | 0.60 | 0.07 | 25.76 | 360.79 | 0.97 | 0.929 |
| 25(OH) Vit. D (ng/mL) | 25.03 | 23.87 | 5.09 | 0.71 | 1.79 | 17.73 | 340.31 | 0.94 | 0.683 |
| Total BMD (g/cm2) | 1.06 | 1.07 | 0.08 | 0 | −1.56 | 0.96 | 10.16 | 0.95 | 0.697 |
| Total Z-score | -0.13 | 0 | 0.69 | −0.23 | −0.61 | −1.20 | 00.80 | 0.98 | 0.944 |
| Total T-score | -0.26 | −0.20 | 0.71 | 0.79 | 1.59 | −1.20 | 00.70 | 0.94 | 0.650 |
| Age (Years) | Height Measured by the Technician (cm) | BMI (kg/m2) | Vit. D (ng/mL) | |
|---|---|---|---|---|
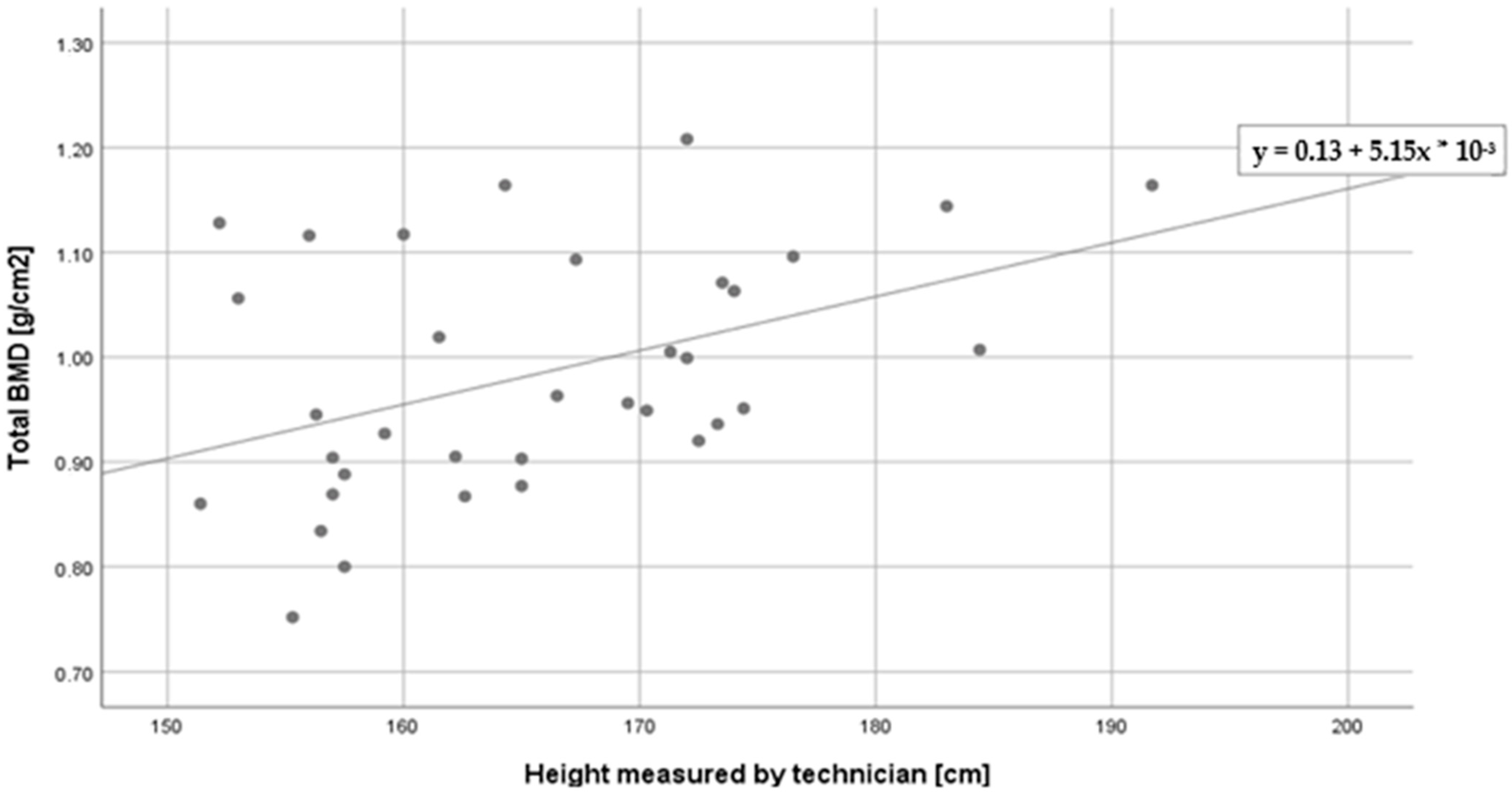
| Total BMD (g/cm2) | −0.30 ^ | 0.44 ** | 0.20 | 0.03 |
| Total Z-score | 0.29 ^ | 0.15 | 0.25 | 0.25 |
| Total T-score | 0.27 | 0.13 | 0.18 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miśkiewicz-Orczyk, K.; Pluskiewicz, W.; Kos-Kudła, B.; Misiołek, M. Assessment of Osteoporosis and Vitamin D3 Deficiency in Patients with Idiopathic Benign Paroxysmal Positional Vertigo (BPPV). Medicina 2023, 59, 862. https://doi.org/10.3390/medicina59050862
Miśkiewicz-Orczyk K, Pluskiewicz W, Kos-Kudła B, Misiołek M. Assessment of Osteoporosis and Vitamin D3 Deficiency in Patients with Idiopathic Benign Paroxysmal Positional Vertigo (BPPV). Medicina. 2023; 59(5):862. https://doi.org/10.3390/medicina59050862
Chicago/Turabian StyleMiśkiewicz-Orczyk, Katarzyna, Wojciech Pluskiewicz, Beata Kos-Kudła, and Maciej Misiołek. 2023. "Assessment of Osteoporosis and Vitamin D3 Deficiency in Patients with Idiopathic Benign Paroxysmal Positional Vertigo (BPPV)" Medicina 59, no. 5: 862. https://doi.org/10.3390/medicina59050862
APA StyleMiśkiewicz-Orczyk, K., Pluskiewicz, W., Kos-Kudła, B., & Misiołek, M. (2023). Assessment of Osteoporosis and Vitamin D3 Deficiency in Patients with Idiopathic Benign Paroxysmal Positional Vertigo (BPPV). Medicina, 59(5), 862. https://doi.org/10.3390/medicina59050862






