Intrauterine Growth Restriction—Prediction and Peripartum Data on Hospital Care
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
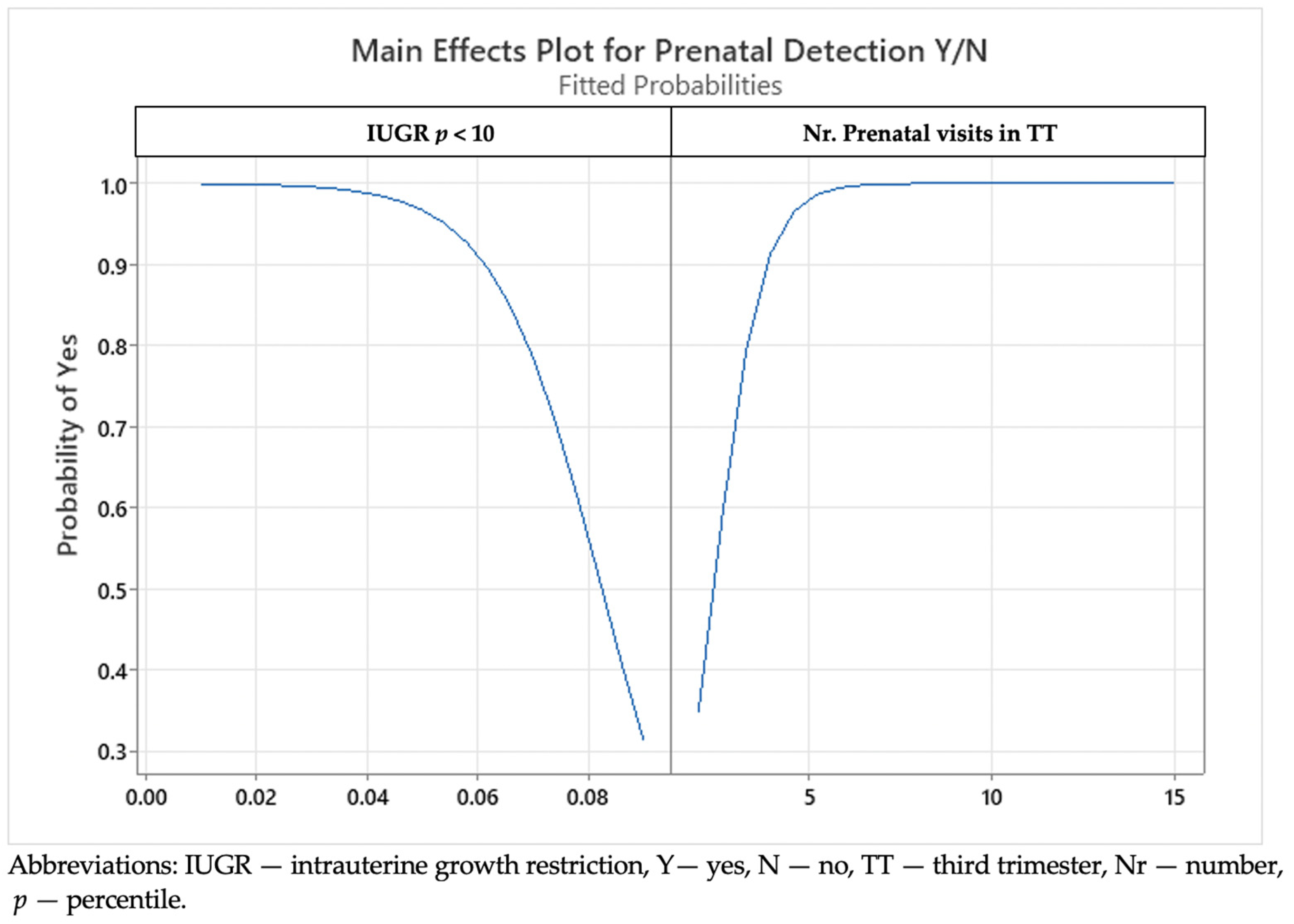
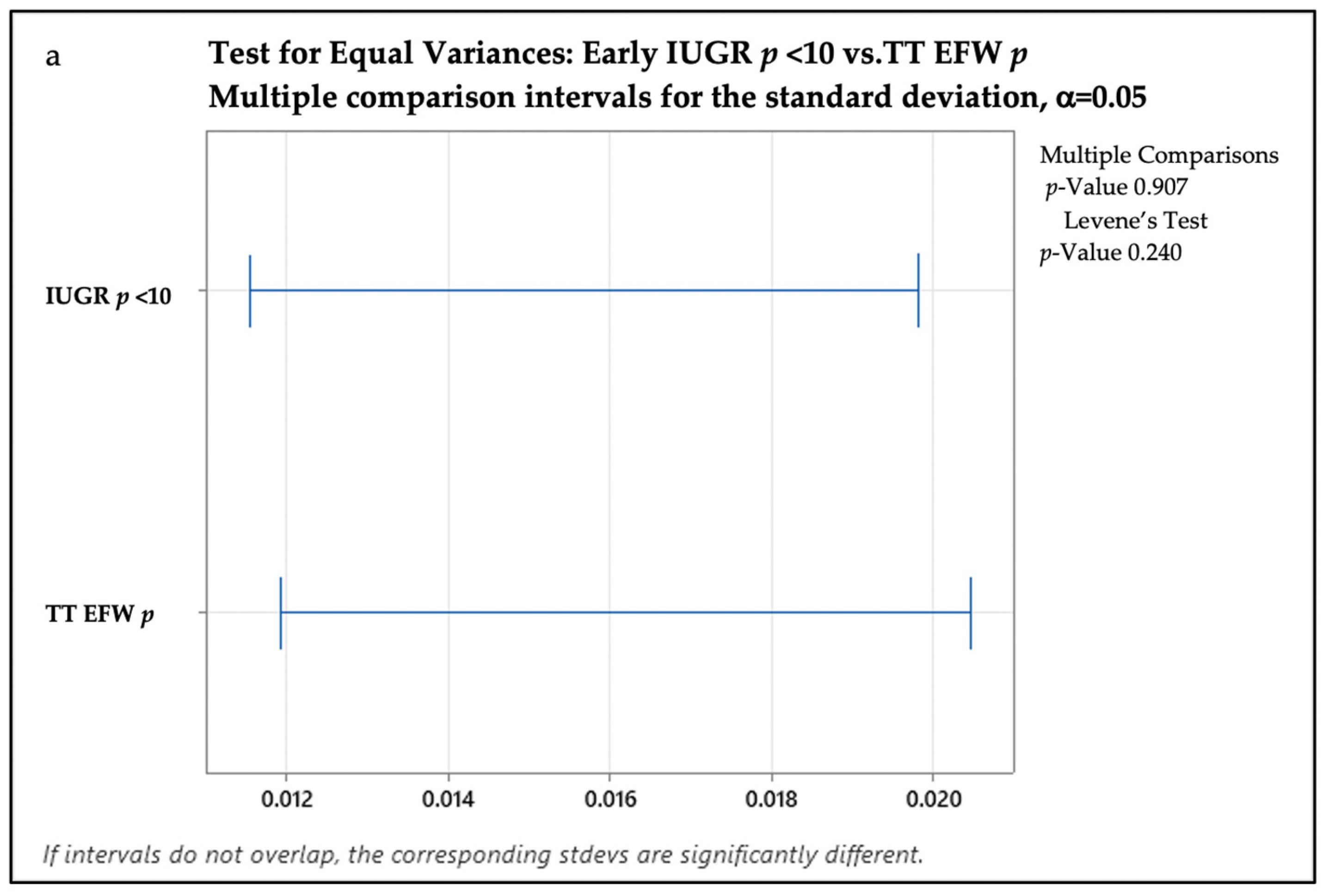
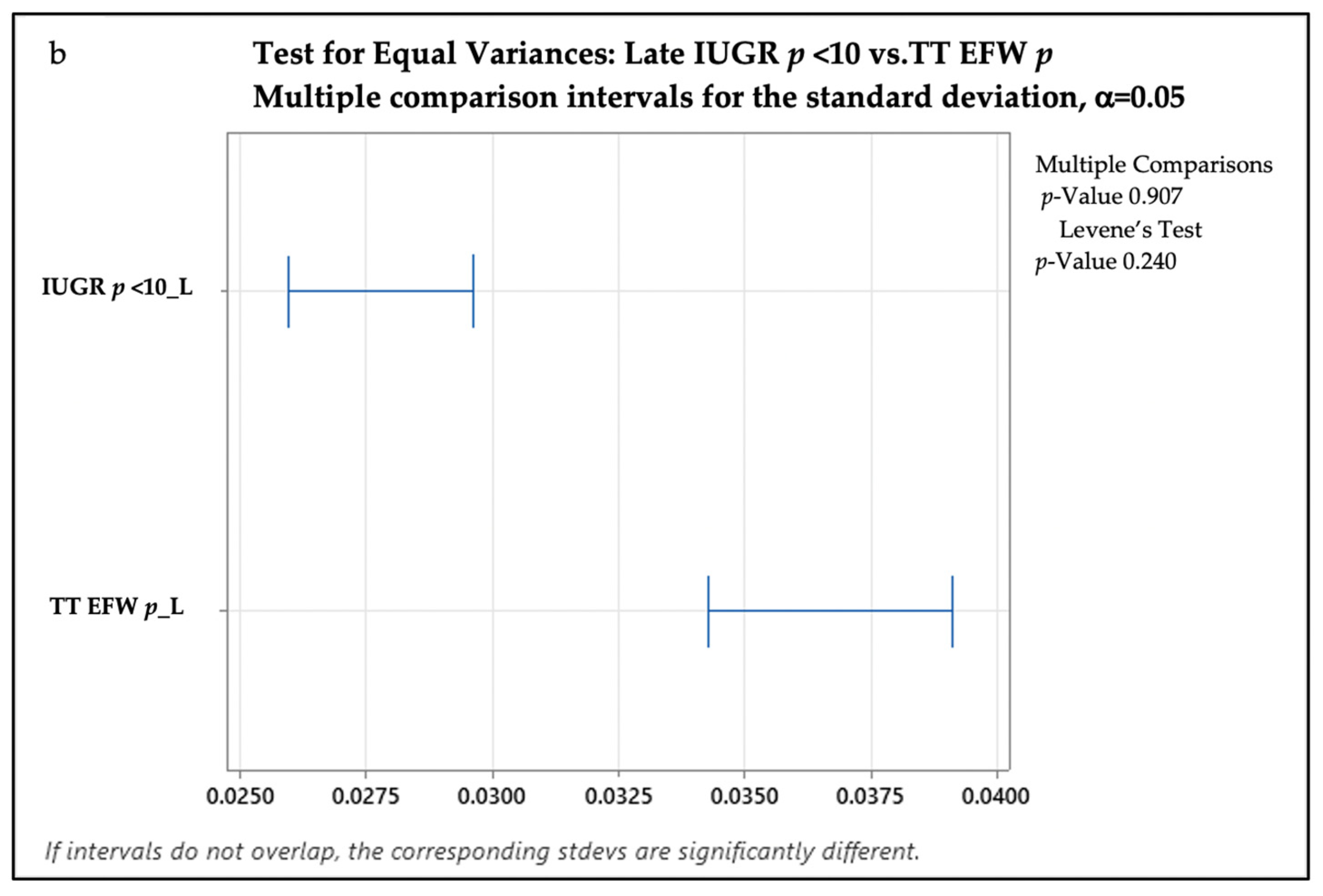
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Nardozza, L.M.M.; Caetano, A.C.R.; Zamarian, A.C.P.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.G.; Lobo, T.F.; Peixoto, A.B.; Júnior, E.A. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Gratacós, E. Update on the Diagnosis and Classification of Fetal Growth Restriction and Proposal of a Stage-Based Management Protocol. Fetal Diagn. Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef]
- Figueras, F.; Caradeux, J.; Crispi, F.; Eixarch, E.; Peguero, A.; Gratacos, E. Diagnosis and surveillance of late-onset fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S790–S802. [Google Scholar] [CrossRef] [PubMed]
- Lewit, E.M.; Baker, L.S.; Corman, H.; Shiono, P.H. The Direct Cost of Low Birth Weight. Futur. Child. 1995, 5, 35. [Google Scholar] [CrossRef]
- Neefjes, C.; Akker, E.V.D.; Jacod, B. Impact of suspected late-onset fetal growth restriction on obstetrical interventions and perinatal outcomes at term; a retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 272, 69–72. [Google Scholar] [CrossRef]
- Atlass, J.H.; Rogan, S.; Himes, K.P. Accuracy of estimated fetal weight in extremely preterm infants and the impact of prepregnancy body mass index. Am. J. Obstet. Gynecol. MFM 2022, 4, 100615. [Google Scholar] [CrossRef]
- Marien, M.; Perron, S.; Bergeron, A.-M.; Singbo, N.; Demers, S. Comparison of the Accuracy of INTERGROWTH-21 and Hadlock Ultrasound Formulae for Fetal Weight Prediction. J. Obstet. Gynaecol. Can. 2021, 43, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Badiu, A.M.; Hodorog, A.D.; Stancioi-Cismaru, A.F.; Gheonea, M.; Capitanescu, R.G.; Sirbu, O.C.; Tanase, F.; Bernad, E.; Tudorache, S. Early Onset Intrauterine Growth Restriction—Data from a Tertiary Care Center in a Middle-Income Country. Medicina 2023, 59, 17. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Villar, J.; Kennedy, S.H.; Papageorghiou, A.T. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 52, 430–441. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Carpenter, R.J.; Deter, R.L.; Park, S.K. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 1984, 150, 535–540. [Google Scholar] [CrossRef]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Śmiałek, D. Birth Weight Percentile Calculator. Available online: https://www.omnicalculator.com/health/birthweight-percentile (accessed on 26 February 2023).
- Romero, R.; Kalache, K.; Kadar, N. Timing the delivery of the preterm severely growth-restricted fetus: Venous Doppler, cardiotocography or the biophysical profile? Ultrasound Obstet. Gynecol. 2002, 19, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Society for Maternal-Fetal Medicine (SMFM); Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics and the Society for Maternal-Fetal Medicin. ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019, 133, e97–e109. [Google Scholar] [CrossRef]
- American Institute of Ultrasound in Medicine. AIUM Practice Parameter for the Performance of Detailed Second- and Third-Trimester Diagnostic Obstetric Ultrasound Examinations. J. Ultrasound Med. 2019, 38, 3093–3100. [Google Scholar] [CrossRef]
- Lees, C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Thilaganathan, B. Ultrasound fetal weight estimation at term may do more harm than good. Ultrasound Obstet. Gynecol. 2018, 52, 5–8. [Google Scholar] [CrossRef]
- Mattioli, K.P.; Sanderson, M.; Chauhan, S.P. Inadequate identification of small-for-gestational-age fetuses at an urban teaching hospital. Int. J. Gynecol. Obstet. 2010, 109, 140–143. [Google Scholar] [CrossRef]
- Sovio, U.; White, I.R.; Dacey, A.; Pasupathy, D.; Smith, G.C.S. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: A prospective cohort study. Lancet 2015, 386, 2089–2097. [Google Scholar] [CrossRef]
- Monier, I.; Blondel, B.; Ego, A.; Kaminiski, M.; Goffinet, F.; Zeitlin, J. Poor effectiveness of antenatal detection of fetal growth restriction and consequences for obstetric management and neonatal outcomes: A French national study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.G.; Molin, J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol. 2005, 25, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.; Formuso, C.; Syngelaki, A.; Akolekar, R.; Nicolaides, K.H. Prediction of small-for-gestational-age neonates at 35–37 weeks’ gestation: Contribution of maternal factors and growth velocity between 20 and 36 weeks. Ultrasound Obstet. Gynecol. 2019, 53, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Caradeux, J.; Martinez-Portilla, R.J.; Peguero, A.; Sotiriadis, A.; Figueras, F. Diagnostic performance of third-trimester ultrasound for the prediction of late-onset fetal growth restriction: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 220, 449–459.e19. [Google Scholar] [CrossRef]
- Fadigas, C.; Guerra, L.; Larroca, S.G.-T.; Poon, L.C.; Nicolaides, K.H. Prediction of small-for-gestational-age neonates: Screening by uterine artery Doppler and mean arterial pressure at 35–37 weeks. Ultrasound Obstet. Gynecol. 2015, 45, 715–721. [Google Scholar] [CrossRef]
- Stephens, K.; Al-Memar, M.; Beattie-Jones, S.; Dhanjal, M.; Mappouridou, S.; Thorne, E.; Lees, C. Comparing the relation between ultrasound-estimated fetal weight and birthweight in cohort of small-for-gestational-age fetuses. Acta Obstet. Gynecol. Scand. 2019, 98, 1435–1441. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, B.; Gomez, A.R.; Moreno, B.S.J.; de Alba, C.; Galindo, A.; Villalain, C.; Pallás, C.; Herraiz, I. Smoking influence on early and late fetal growth. J. Périnat. Med. 2021, 50, 200–206. [Google Scholar] [CrossRef]
- Grivell, R.M.; Wong, L.; Bhatia, V. Regimens of fetal surveillance for impaired fetal growth. Cochrane Database Syst. Rev. 2012, 2012, CD007113. [Google Scholar] [CrossRef]
- Talmor, A.; Daemen, A.; Murdoch, E.; Missfelder-Lobos, H.; Timmerman, D.; Bourne, T.; Giussani, D.; Lees, C. Defining the relationship between fetal Doppler indices, abdominal circumference and growth rate in severe fetal growth restriction using functional linear discriminant analysis. J. R. Soc. Interface 2013, 10, 20130376. [Google Scholar] [CrossRef]
- Bilardo, C.M.; Hecher, K.; Visser, G.H.A.; Papageorghiou, A.T.; Marlow, N.; Thilaganathan, B.; Van Wassenaer-Leemhuis, A.; Todros, T.; Marsal, K.; Frusca, T.; et al. Severe fetal growth restriction at 26-32 weeks: Key messages from the TRUFFLE study. Ultrasound Obstet. Gynecol. 2017, 50, 285–290. [Google Scholar] [CrossRef]
- Demirci, O.; Selçuk, S.; Kumru, P.; Asoğlu, M.R.; Mahmutoğlu, D.; Boza, B.; Türkyılmaz, G.; Bütün, Z.; Arısoy, R.; Tandoğan, B. Maternal and fetal risk factors affecting perinatal mortality in early and late fetal growth restriction. Taiwan. J. Obstet. Gynecol. 2015, 54, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Say, R.; Robson, S.; Kleijnen, J.; Khan, K. Systematic review and meta-analysis of middle cerebral artery Doppler to predict perinatal wellbeing. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 141–155. [Google Scholar] [CrossRef]
- Akolekar, R.; Syngelaki, A.; Gallo, D.M.; Poon, L.C.; Nicolaides, K.H. Umbilical and fetal middle cerebral artery Doppler at 35-37 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet. Gynecol. 2015, 46, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Vedmedovska, N.; Rezeberga, D.; Teibe, U.; Melderis, I.; Donders, G.G. Placental pathology in fetal growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 36–40. [Google Scholar] [CrossRef]
- Oliveira, L.H.; Xavier, C.C.; Lana, A.M.A. Changes in placental morphology of small for gestational age newborns. J. Pediatr. 2002, 78, 397–402. [Google Scholar] [CrossRef]
- Dittkrist, L.; Vetterlein, J.; Henrich, W.; Ramsauer, B.; Schlembach, D.; Abou-Dakn, M.; Gembruch, U.; Schild, R.L.; Duewal, A.; Schaefer-Graf, U.M. Percent error of ultrasound examination to estimate fetal weight at term in different categories of birth weight with focus on maternal diabetes and obesity. BMC Pregnancy Childbirth 2022, 22, 241. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.; Arezina, J. The accuracy of ultrasound estimation of fetal weight in comparison to birth weight: A systematic review. Ultrasound 2018, 26, 32–41. [Google Scholar] [CrossRef]

| Feature/Association | Eo-IUGR | Lo-IUGR | p |
|---|---|---|---|
| Age | 28.17 (19–37) | 29 (17–42) | 0.0976 |
| BMI | 24.2 (19–28) | 23 (18–35) | 0.205 |
| Smoking | 65.38% | 33.9% | <0.01 |
Gestational hypertension
| 75% 44.24% 0 5.78% | 25.44% 15.26% 3.4% 1.7% | <0.01 <0.01 <0.01 0.557 |
| GDM | 17.31% | 5.1% | 0.007 |
| Characteristics | Eo-IUGR | Lo-IUGR | p |
|---|---|---|---|
| ST placental UtA centile | 98.5 (98–100) | 66 (56–100) | <0.01 |
| ST non-placental UtA centile | 96.5 (94–100) | 51 (28–100) | <0.01 |
| TT placental UtA centile | 96 (95–100) | 65 (52–100) | <0.01 |
| TT non placental UtA centile | 92.3 (91–100) | 53 (22–99) | <0.01 |
| TT placental notch | 80.5% | 9% | <0.01 |
| TT non-placental notch | 69.44% | 11% | <0.01 |
| TT CPR centile | 1 (1–5) | 14 (1–39) | <0.01 |
| ST AC centile | 9% (1–12%) | 35% (10–55%) | <0.01 |
| TT AC centile | 1% (1–7%) | 20% (1–30%) | <0.01 |
| ST EFW centile | 10% (5–15%) | 36% (10–56%) | <0.01 |
| TT EFW centile | 2% (1–6%) | 6% (1–15%) | <0.01 |
| Parameter | Eo-IUGR | Lo-IUGR | p |
|---|---|---|---|
| GA at delivery | 31.7 (28–34) | 37 (34–38) | <0.01 |
| Placental weight (g) | 312 +/− 109 | 403 +/− 133 | <0.01 |
| Total nr of prenatal visits | 11.5 (10–30) | 9 (3–18) | <0.01 |
| Nr of prenatal visits in TT | 6 (5–15) | 4 (2–10) | <0.01 |
| Characteristic/Complications | Eo-IUGR | Lo-IUGR | p |
|---|---|---|---|
| Hospitalization Days | 38 (22–90) | 3.8 (2–32) | <0.01 |
| Apgar Score | 5.3 (1–8) | 8.1 (2–10) | <0.01 |
| Resuscitation | 19 (36.53%) | 7 (11.86%) | <0.01 |
| Birth Percentile | 2.3% (1–10%) | 7% (3–10%) | <0.01 |
| NICU Days | 10.8 (0–60) | 2,3 (0–31) | <0.01 |
| Respiratory Distress Syndrome | 20 (38.4%) | 3 (5.08%) | <0.01 |
| Bronchopulmonary Dysplasia | 1 (1.92%) | 0 | - |
| Transient Apnea | 49 (94.23%) | 5 (8.47%) | <0.01 |
| Hypotension | 6 (11.53%) | 3 (5.08%) | <0.01 |
| PDA | 16 (30.76%) | 1 (1.69%) | <0.01 |
| IVH | 6 (11.53%) | 2 (3.38%) | <0.01 |
| PVL | 2 (3.84%) | 0 | - |
| Hypothermia | 0 | 0 | - |
| Immature GI System | 40 (76.92%) | 14 (23.72%) | <0.01 |
| NEC | 1 (1.92%) | 0 | - |
| Anemia | 52 (100%) | 6 (10.16%) | <0.01 |
| Jaundice | 15 (28.84%) | 5 (8.47%) | <0.01 |
| Transient Hypoglycemia | 22 (42.3%) | 3 (5.08%) | <0.01 |
| Infection | 12 (23.07%) | 1 (1.69%) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, M.; Stancioi-Cismaru, A.F.; Gheonea, M.; Luciu, E.D.; Aron, R.M.; Pana, R.C.; Marinas, C.M.; Degeratu, S.; Sorop-Florea, M.; Carp-Veliscu, A.; et al. Intrauterine Growth Restriction—Prediction and Peripartum Data on Hospital Care. Medicina 2023, 59, 773. https://doi.org/10.3390/medicina59040773
Dinu M, Stancioi-Cismaru AF, Gheonea M, Luciu ED, Aron RM, Pana RC, Marinas CM, Degeratu S, Sorop-Florea M, Carp-Veliscu A, et al. Intrauterine Growth Restriction—Prediction and Peripartum Data on Hospital Care. Medicina. 2023; 59(4):773. https://doi.org/10.3390/medicina59040773
Chicago/Turabian StyleDinu, Marina, Andreea Florentina Stancioi-Cismaru, Mihaela Gheonea, Elinor Dumitru Luciu, Raluca Maria Aron, Razvan Cosmin Pana, Cristian Marius Marinas, Stefan Degeratu, Maria Sorop-Florea, Andreea Carp-Veliscu, and et al. 2023. "Intrauterine Growth Restriction—Prediction and Peripartum Data on Hospital Care" Medicina 59, no. 4: 773. https://doi.org/10.3390/medicina59040773
APA StyleDinu, M., Stancioi-Cismaru, A. F., Gheonea, M., Luciu, E. D., Aron, R. M., Pana, R. C., Marinas, C. M., Degeratu, S., Sorop-Florea, M., Carp-Veliscu, A., Hodorog, A. D., & Tudorache, S. (2023). Intrauterine Growth Restriction—Prediction and Peripartum Data on Hospital Care. Medicina, 59(4), 773. https://doi.org/10.3390/medicina59040773






