Effects of Supraglottic Airway Devices on Hemodynamic Response and Optic Nerve Sheath Diameter: Proseal LMA, LMA Supreme, and I-gel LMA
Abstract
1. Introduction
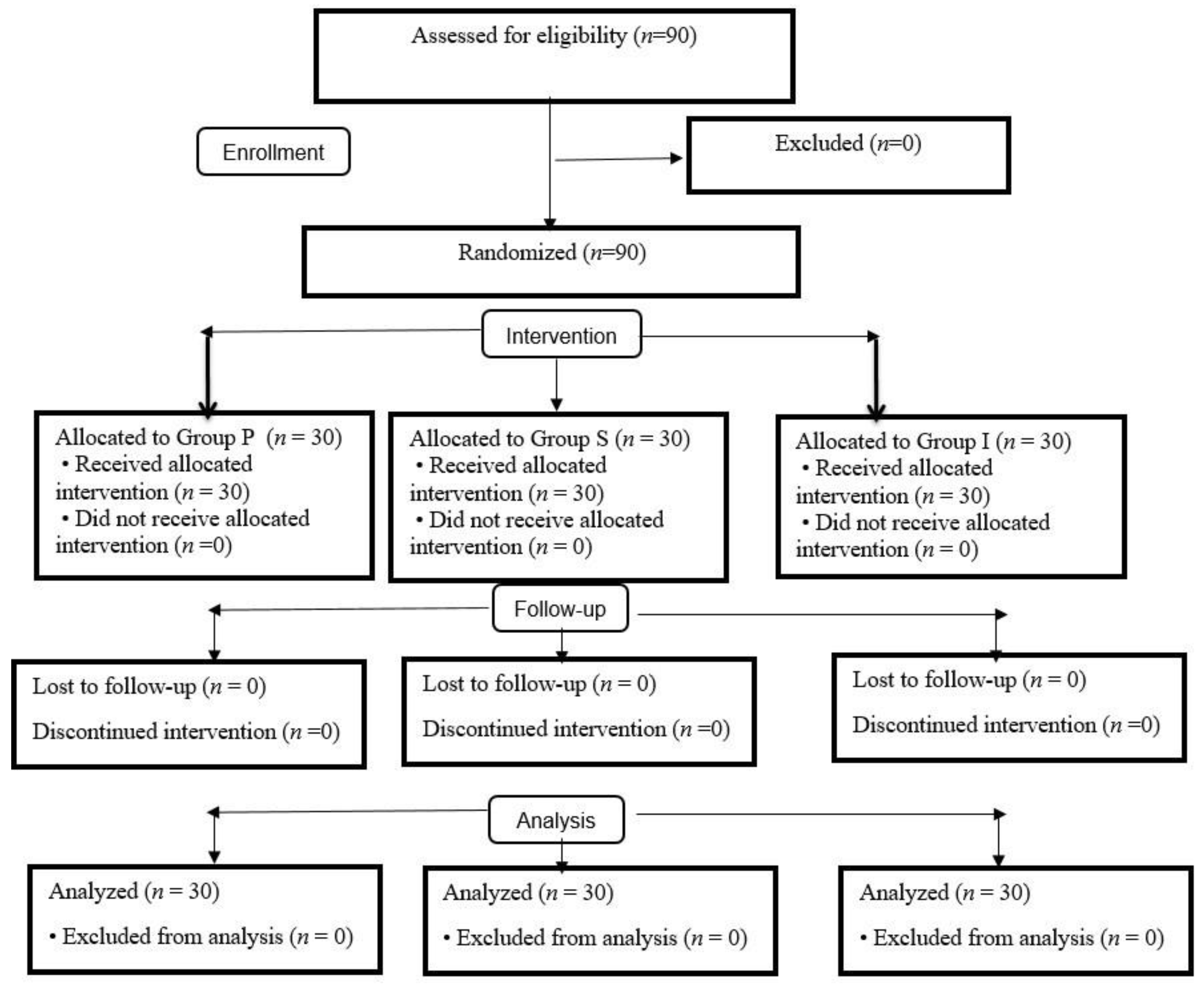
2. Materials and Methods
2.1. Compliance with Ethical Standards
2.2. Patient Population
2.3. Application of General Anesthesia and Monitoring
2.4. Data Management
2.5. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shribman, A.J.; Smith, G.; Achola, K.J. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br. J. Anaesth. 1987, 59, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Kovac, A.L. Controlling the hemodynamic response to laryngoscopy and endotracheal intubation. J. Clin. Anesth. 1996, 8, 63–79. [Google Scholar] [CrossRef]
- Obsa, M.S.; Kanche, Z.Z.; Olana, F.R.; Tura, T.S.; Adema, B.G.; Kinfe, A.A.; Kercho, M.W.; Paulos Chanko, K.; Shanka, G.M.; Lencha, A.A.; et al. Effect of Laryngeal Mask Airway Insertion on Intraocular Pressure Response: Systematic Review and Meta-Analysis. Anesthesiol. Res. Pract. 2020, 2020, 7858434. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Bisher, N.A.; Kandil, H.W.; Mowafi, H.A.; Atawia, H.A. Intraocular pressure and haemodynamic responses to insertion of the i-gel, laryngeal mask airway or endotracheal tube. Eur. J. Anaesthesiol. 2011, 28, 443–448. [Google Scholar] [CrossRef]
- Blobner, M.; Hunter, J.M. Supraglottic airway, tracheal intubation, and neuromuscular block: Will the ménage à trois endure? Br. J. Anaesth. 2021, 127, 174–177. [Google Scholar] [CrossRef] [PubMed]
- LMA Supreme™ a Second Generation SAD with and Innovative Second Seal™. 2014. Available online: https://www.lmaco.com/sites/default/files/31817-LMA-SecondSeal-A4-0214-LORES-fnl.pdf (accessed on 7 January 2022).
- Liew, G.H.; Yu, E.D.; Shah, S.S.; Kothandan, H. Comparison of the clinical performance of i-gel, LMA Supreme and LMA ProSeal in elective surgery. Singap. Med. J. 2016, 57, 432–437. [Google Scholar] [CrossRef]
- Liu, D.; Kahn, M. Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am. J. Ophthalmol. 1993, 116, 548–556. [Google Scholar] [CrossRef]
- Rajajee, V.; Vanaman, M.; Fletcher, J.J.; Jacobs, T.L. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit. Care 2011, 15, 506–515. [Google Scholar] [CrossRef]
- Geeraerts, T.; Launey, Y.; Martin, L.; Pottecher, J.; Vigué, B.; Duranteau, J.; Benhamou, D. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intens. Care Med. 2007, 33, 1704–1711. [Google Scholar] [CrossRef]
- Shah, S.B.; Bhargava, A.K.; Choudhury, I. Noninvasive intracranial pressure monitoring via optic nerve sheath diameter for robotic surgery in steep trendelenburg position. Saudi J. Anaesth. 2015, 9, 239–246. [Google Scholar] [CrossRef]
- Butterworth, I.V.J.F.; Mackey, D.C.; Wasnick, J.D. Airway Management. In Morgan & Mikhail’s Clinical Anesthesiology, 5th ed.; Mc Graw Hill: New York, NY, USA, 2013; pp. 575–592. [Google Scholar]
- Kim, E.J.; Koo, B.N.; Choi, S.H.; Park, K.; Kim, M.S. Ultrasonographic optic nerve sheath diameter for predicting elevated intracranial pressure during laparoscopic surgery: A systematic review and meta-analysis. Surg. Endosc. 2018, 32, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Lee, J.R.; Oh, J.T.; Kim, M.S.; Jun, E.K.; An, J. Ultrasonographic Assessment of Optic Nerve Sheath Diameter during Pediatric Laparoscopy Ultrasound. Med. Biol. 2015, 41, 1241–1246. [Google Scholar]
- Whiteley, J.R.; Taylor, J.; Henry, M.; Epperson, T.I.; Hand, W.R. Detection of elevated intracranial pressure in robot-assisted laparoscopic radical prostatectomy using ultrasonography of optic nerve sheath diameter. J. Neurosurg. Anesthesiol. 2015, 27, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hong, J.H.; Park, J.Y.; Hwang, J.H.; Cho, S.S.; Kim, Y.K. Propofol attenuates the increase of sonographic optic nerve sheath diameter during robot-assisted laparoscopic prostatectomy: A randomized clinical trial. BMC Anesthesiol. 2018, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Küçükosman, G.; Aydın, B.G.; Gülçek, N.; Okyay, R.D.; Pişkin, Ö.; Ayoğlu, H. The effect of laryngoscope types on hemodynamic response and optic nerve sheath diameter. McCoy, Macintosh, and C-MAC video-laryngoscope. Saudi Med. J. 2020, 41, 930–937. [Google Scholar] [CrossRef]
- Chui, J.; Mariappan, R.; Mehta, J.; Manninen, P.; Venkatraghavan, L. Comparison of propofol and volatile agents for maintenance of anesthesia during elective craniotomy procedures: Systematic review and meta-analysis. Can. J. Anaesth 2014, 61, 347–356. [Google Scholar] [CrossRef]
- Geng, W.; Chen, C.; Sun, X.; Huang, S. Effects of sevoflurane and propofol on the optic nerve sheath diameter in patients undergoing laparoscopic gynecological surgery: A randomized controlled clinical studies. BMC Anesthesiol. 2021, 21, 30. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Lee, H.; Park, H.S.; Kim, W.J.; Baik, H.J.; Kim, D.Y. Optic nerve sheath diameter changes during gynecologic surgery in the Trendelenburg position: Comparison of propofol-based total intravenous anesthesia and sevoflurane anesthesia. Anesth. Pain Med. 2019, 14, 393–400. [Google Scholar] [CrossRef]
- Blecha, S.; Harth, M.; Schlachetzki, F.; Zeman, F.; Blecha, C.; Flora, P.; Burger, M.; Denzinger, S.; Graf, B.M.; Helbig, H.; et al. Changes in intraocular pressure and optic nerve sheath diameter in patients undergoing robotic-assisted laparoscopic prostatectomy in steep 45° Trendelenburg position. BMC Anesthesiol. 2017, 17, 40. [Google Scholar] [CrossRef]
- Le, R.P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care. Neurocrit. Care 2014, 21, 1–26. [Google Scholar]
- Khan, M.N.; Shallwani, H.; Khan, M.U.; Shamim, M.S. Noninvasive monitoring intracranial pressure-A review of available modalities. Surg. Neurol. Int. 2017, 8, 51. [Google Scholar] [PubMed]
- Maissan, I.M.; Dirven, P.J.; Haitsma, I.K.; Hoeks, S.E.; Gommers, D.; Stolker, R.J. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J. Neurosurg. 2015, 123, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J. Clin. Epidemiol. 2010, 63, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Canac, N.; Jalaleddini, K.; Thorpe, S.G.; Thibeault, C.M.; Hamilton, R.B. Review: Pathophysiology of intracranial hypertension and noninvasive intracranial pressure monitoring. Fluids Barriers CNS 2020, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, S.A.; O’Neill, G.; Hamilton, R.; Hollman, A.S. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur. J. Ultrasound 2002, 15, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, H.H.; Shah, S.; Marill, K.; Noble, V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad. Emerg. Med. 2008, 15, 201–204. [Google Scholar] [CrossRef]
- Agrawal, D.; Raghavendran, K.; Zhao, L.; Rajajee, V. A Prospective Study of Optic Nerve Ultrasound for the Detection of Elevated Intracranial Pressure in Severe Traumatic Brain Injury. Crit. Care Med. 2020, 48, e1278–e1285. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, U.; Kumar, M.; Mishra, P.K. Comparative Study of Hemodynamic Responses to Airway Maintenance Devices: Proseal LMA V/S IGEL Airway. JMSCR 2014, 2, 1320–1328. [Google Scholar]
- Wood, M.L.; Forrest, E.T. The haemodynamic response to the insertion of the laryngeal mask airway: A comparison with laryngoscopy and tracheal intubation. Acta Anaesthesiol. Scand. 1994, 38, 510–513. [Google Scholar] [CrossRef]
- Theodoraki, K.; Fassoulaki, A. Cardiovascular responses to laryngoscopy and tracheal intubation are not accompanied by ST-segment changes. Eur. J. Anaesthesiol. 2009, 26, 520–522. [Google Scholar] [CrossRef]
- Allahyari, E.; Azimi, A.; Zarei, H.; Bamdad, S. Comparison of endotracheal intubation, laryngeal mask airway, and I-gel in children undergoing strabismus surgery. J. Res. Med. Sci. 2021, 28, 9. [Google Scholar]
- Ozhan, M.O.; Eskin, M.B.; Atik, B.; Suzer, M.A.; Capalar, C.O. Laryngeal mask airway for general anesthesia in interventional neuroradiology procedures. Saudi Med. J. 2019, 40, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Karwacki, Z.; Witkowska, M.; Niewiadomski, S.; Wiatr, A.; Bukowski, P.; Wierzchowska, J.; Zapaśnik, A. Anaesthetic management for endovascular treatment of unruptured intracranial aneurysms. Anaesthesiol. Intensive 2013, 45, 145–148. [Google Scholar] [CrossRef]
- Süzer, M.A.; Özhan, M.Ö.; Çaparlar, C.Ö.; Eşkin, M.B.; Atik, B. Airway management in general anesthesia for endovascular treatment of cerebral arteriovenous malformation: A retrospective observational study. Braz. J. Anesthesiol. 2022, 72, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Kucukosman, G.; Aydın, B.G. Are there predictive tests that determine the difficulty in Laryngeal Mask Airway Insertion? J. Pak. Med. Assoc. 2021, 71, 434–439. [Google Scholar] [PubMed]
- Mukadder, S.; Zekine, B.; Erdogan, K.G.; Ulku, O.; Muharrem, U.; Saim, Y.; Mahmut, D. Comparison of the proseal, supreme, and i-gel SAD in gynecological laparoscopic surgeries. Sci. World J. 2015, 2015, 634320. [Google Scholar] [CrossRef]
| Group P (n = 30) | Group S (n = 30) | Group I (n = 30) | p | |
|---|---|---|---|---|
| Female/Male (n) | 24/6 | 19/11 | 20/10 | 0.329 |
| Age (years) | 42.7 ± 10 | 43.6 ± 11.5 | 39.6 ± 12.9 | 0.386 |
| Length (cm) | 164.2 ± 8.5 | 167.5 ± 9.3 | 168.2 ± 9.3 | 0.184 |
| Weight (kg) | 66.6 ± 7.5 | 66.6 ± 6.2 | 69.2 ± 7.4 | 0.248 |
| ASA (I/II) | 5/25 | 8/22 | 5/25 | 0.535 |
| Mallampati (I/II) | 10/20 | 9/21 | 13/17 | 0.532 |
| SAD insertion time (s) | 9.9 ± 3.3 | 8.2 ± 5.4 | 7.5 ± 3.9 | 0.087 |
| Time (min) | Group P (n = 30) | Group S (n = 30) | Group I (n = 30) | p * |
|---|---|---|---|---|
| T0 | 76 (64–136) a | 81.5 (53–116) a | 77 (60–132) a | 0.783 |
| T1 | 73.5 (55–135) a | 74 (52–115) a | 77.5 (59–121) a | 0.726 |
| T5 | 71.5 (51–105) b | 71 (51–108) b | 71 (52–110) b | 0.976 |
| T10 | 69 (49–100) b | 71.5 (50–112) b | 67.5 (48–121) b | 0.882 |
| p ** | <0.001 | <0.001 | <0.001 |
| Table 30. | Group P (n = 30) | Group S (n = 30) | Group I (n = 30) | p * |
|---|---|---|---|---|
| T0 | 100 (66–136) a | 105 (80–137) a | 101.5 (73–157) a | 0.594 |
| T1 | 92 (59–120) a | 85.5 (61–141) a | 85 (66–122) a | 0.742 |
| T5 | 71 (45–105) b | 72.5 (60–125) b | 74 (56–122) b | 0.641 |
| T10 | 74 (53–121) b | 72 (54–98) b | 75 (54–110) b | 0.360 |
| p ** | <0.001 | <0.001 | <0.001 |
| Time (min) | Group P (n = 30) | Group S (n = 30) | Group I (n = 30) | p * |
|---|---|---|---|---|
| T0 | 3.4 (2.7–4.5) | 3.7 (2.4–4.7) | 3.5 (2.7–5.2) | 0.167 |
| T1 | 4 (3.3–4.9) & | 3.9 (2.7–5) & | 3.8 (3–5.5) & | 0.982 |
| T5 | 3.4 (2.8–4.7) | 3.6 (2.7–4.7) | 3.7 (2.8–5.3) | 0.220 |
| T10 | 3.6 (2.8–4.5) | 3.6 (2.5–5.2) | 3.5 (3–5.1) | 0.845 |
| p ** | <0.001 | <0.001 | <0.001 |
| Time (min) | Group P (n = 30) | Group S (n = 30) | Group I (n = 30) | p * |
|---|---|---|---|---|
| T0 | 3.5 (2.5–4.3) | 3.6 (2.1–4.9) | 3.6 (2.8–5.3) | 0.303 |
| T1 | 3.9 (3.2–5) & | 4 (2.8–5) & | 4 (3.1–5.3) & | 0.948 |
| T5 | 3.6 (2.8–4.7) | 3.5 (2.3–5.7) | 3.8 (3–5.1) | 0.088 |
| T10 | 3.7 (2.8–4.3) | 3.6 (2.8–4.7) | 3.7 (3–5.1) | 0.707 |
| p ** | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okyay, R.D.; Küçükosman, G.; Köksal, B.G.; Pişkin, Ö.; Ayoğlu, H. Effects of Supraglottic Airway Devices on Hemodynamic Response and Optic Nerve Sheath Diameter: Proseal LMA, LMA Supreme, and I-gel LMA. Medicina 2023, 59, 753. https://doi.org/10.3390/medicina59040753
Okyay RD, Küçükosman G, Köksal BG, Pişkin Ö, Ayoğlu H. Effects of Supraglottic Airway Devices on Hemodynamic Response and Optic Nerve Sheath Diameter: Proseal LMA, LMA Supreme, and I-gel LMA. Medicina. 2023; 59(4):753. https://doi.org/10.3390/medicina59040753
Chicago/Turabian StyleOkyay, Rahşan Dilek, Gamze Küçükosman, Bengü Gülhan Köksal, Özcan Pişkin, and Hilal Ayoğlu. 2023. "Effects of Supraglottic Airway Devices on Hemodynamic Response and Optic Nerve Sheath Diameter: Proseal LMA, LMA Supreme, and I-gel LMA" Medicina 59, no. 4: 753. https://doi.org/10.3390/medicina59040753
APA StyleOkyay, R. D., Küçükosman, G., Köksal, B. G., Pişkin, Ö., & Ayoğlu, H. (2023). Effects of Supraglottic Airway Devices on Hemodynamic Response and Optic Nerve Sheath Diameter: Proseal LMA, LMA Supreme, and I-gel LMA. Medicina, 59(4), 753. https://doi.org/10.3390/medicina59040753






