Transient Elastography as the First-Line Assessment of Liver Fibrosis and Its Correlation with Serum Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Vibration-Controlled Transient Elastography
2.2. Laboratory Assessments
2.3. ELF Test
2.4. APRI and FIB-4
2.5. Statistical Analysis
3. Results
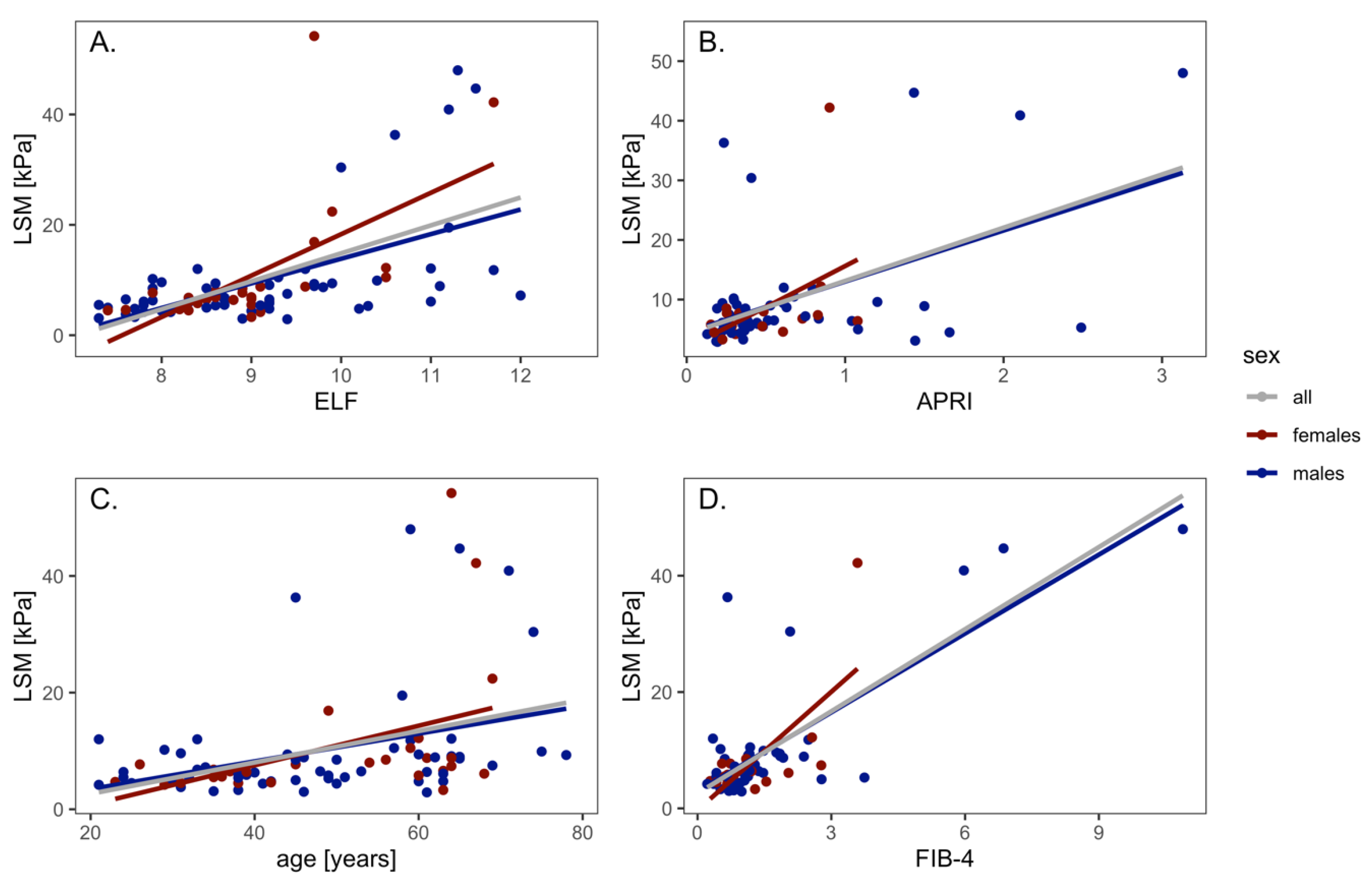
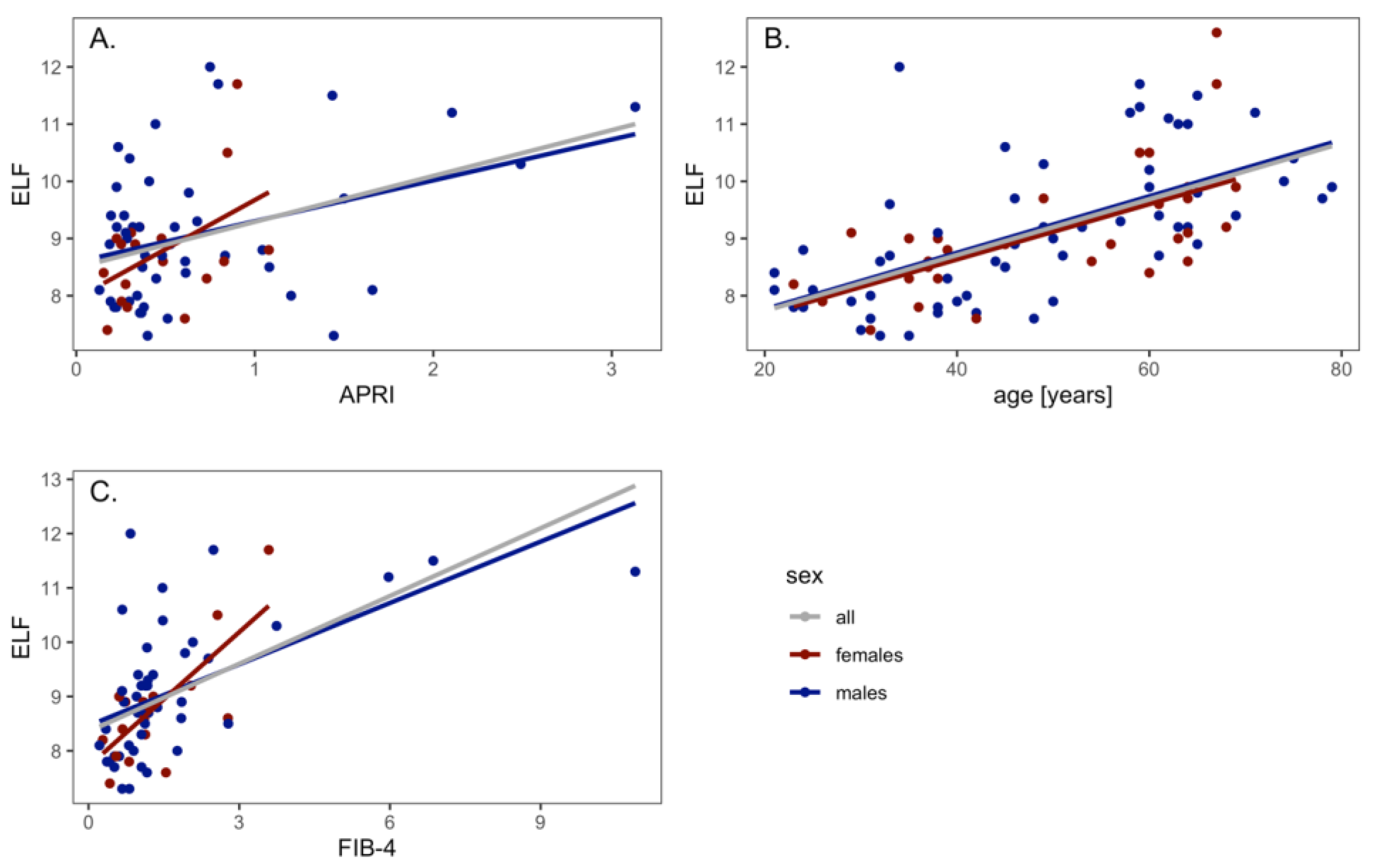
3.1. LSM and NITs Correlations
3.2. LSM and NITs Correlations According to Liver Disease Etiology
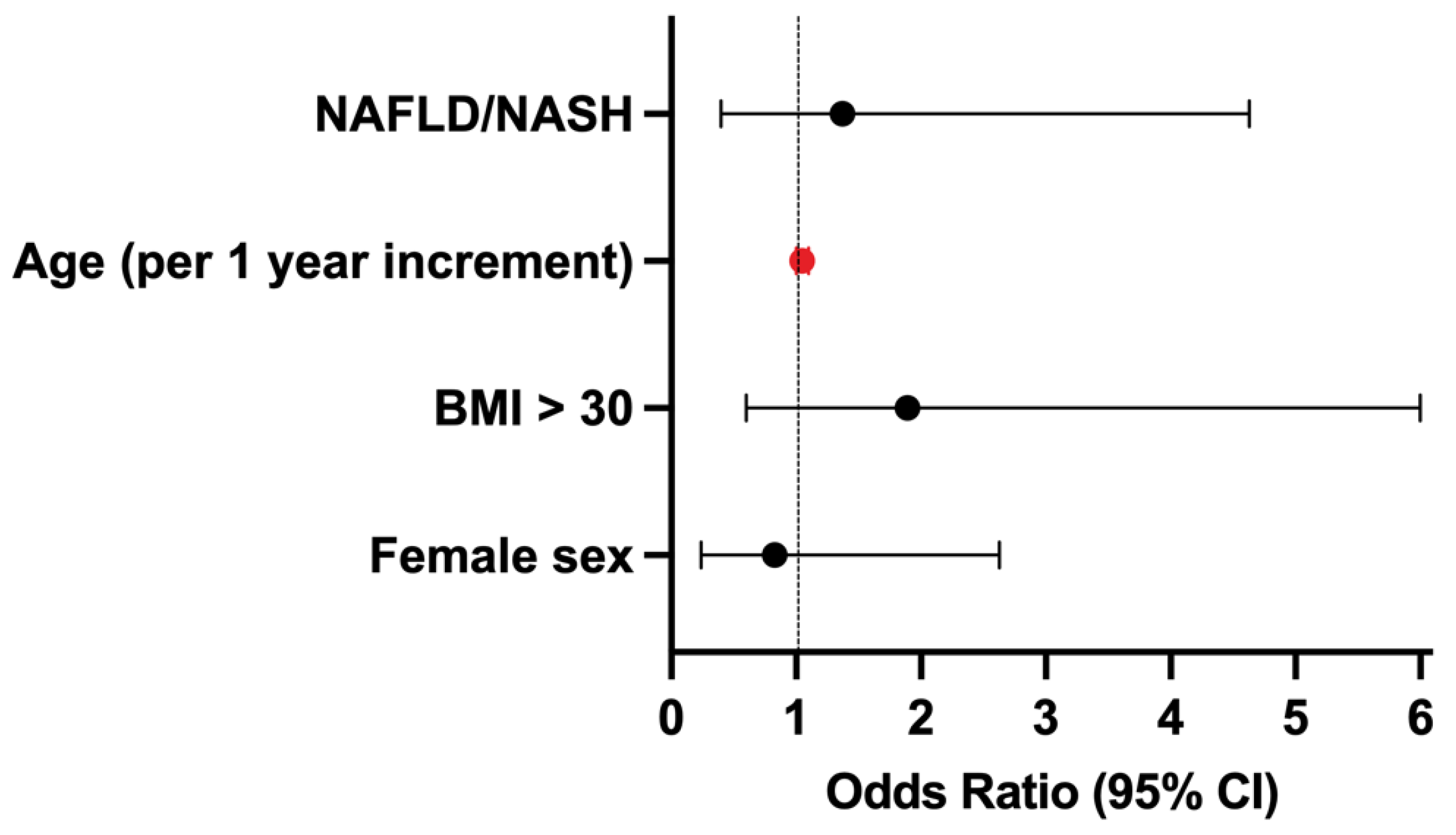
3.3. Factors Predicting Advanced Liver Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Noninvasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N.; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in non-alcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on noninvasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Dargere, D.; Paradis, V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef]
- Hytiroglou, P.; Theise, N.D. Regression of human cirrhosis: An update, 18 years after the pioneering article by Wanless et al. Virchows Arch. 2018, 473, 15–22. [Google Scholar] [CrossRef]
- Tapper, E.B.; Castera, L.; Afdhal, N.H. FibroScan (vibration-controlled transient elastography): Where does it stand in the United States practice. Clin. Gastroenterol. Hepatol. 2015, 13, 27–36. [Google Scholar] [CrossRef]
- Parkes, J.; Guha, I.N.; Roderick, P.; Rosenberg, W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J. Hepatol. 2006, 44, 462–474. [Google Scholar] [CrossRef]
- Vali, Y.; Lee, J.; Boursier, J.; Spijker, R.; Loffler, J.; Verheij, J.; Brosnan, M.J.; Böcskei, Z.; Anstee, Q.M.; LITMUS Systematic Review Team; et al. Enhanced liver fibrosis test for the noninvasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2020, 73, 252–262. [Google Scholar] [CrossRef]
- De Franchis, R.; Baveno, V.I.F. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Wong, V.W.; Chim, A.M.; Yiu, K.K.; Chu, S.H.; Li, M.K.; Chan, H.L.-Y. Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J. Gastroenterol. Hepatol. 2011, 26, 300–305. [Google Scholar] [CrossRef]
- Mederacke, I.; Wursthorn, K.; Kirschner, J.; Rifai, K.; Manns, M.P.; Wedemeyer, H.; Bahr, M.J. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009, 29, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Forns, X.; Alberti, A. Noninvasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 2008, 48, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Zarski, J.P.; de Ledinghen, V.; Rousselet, M.C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef]
- Papatheodoridi, M.; Hiriart, J.B.; Lupsor-Platon, M.; Bronte, F.; Boursier, J.; Elshaarawy, O.; Marra, F.; Thiele, M.; Markakis, G.; Payance, A.; et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J. Hepatol. 2021, 74, 1109–1116. [Google Scholar] [CrossRef]
- Siemens Healthcare. ELF Physician Brochure. 2016. Available online: https://marketing.webassets.siemens-healthineers.com/1800000003470658/6d7021e5e01d/elf_physician_brochure_a91dx-160435-xc1-4a00_final-03470658_1800000003470658.pdf (accessed on 20 November 2022).
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.-F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; E Congly, S.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Roulot, D.; Costes, J.L.; Buyck, J.F.; Warzocha, U.; Gambier, N.; Czernichow, S.; Le Clesiau, H.; Beaugrand, M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011, 60, 977–984. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Vasileiadi, S.; Papavdi, M.; Sveroni, E.; Antonakaki, P.; Dellaporta, E.; Koutli, E.; Michalea, S.; Manolakopoulos, S.; Koskinas, J.; et al. Liver fibrosis staging with combination of APRI and FIB-4 scoring systems in chronic hepatitis C as an alternative to transient elastography. Ann. Gastroenterol. 2019, 32, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Innes, H.; Morling, J.R.; Buch, S.; Hamill, V.; Stickel, F.; Guha, I.N. Performance of routine risk scores for predicting cirrhosis-related morbidity in the community. J. Hepatol. 2022, 77, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Abeysekera, K.W.M.; Fernandes, G.S.; Hammerton, G.; Portal, A.J.; Gordon, F.H.; Heron, J.; Hickman, M. Prevalence of steatosis and fibrosis in young adults in the UK: A population-based study. Lancet Gastroenterol. Hepatol. 2020, 5, 295–305. [Google Scholar] [CrossRef]
- Alkhouri, N.; Almomani, A.; Le, P.; Payne, J.Y.; Asaad, I.; Sakkal, C.; Vos, M.; Noureddin, M.; Kumar, P. The prevalence of alcoholic and non-alcoholic fatty liver disease in adolescents and young adults in the United States: Analysis of the NHANES database. BMC Gastroenterol. 2022, 22, 366. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Hwang, J.; Jeong, D.; Dang, N.; Kam, L.Y.; Henry, L.; Park, H.; Cheung, R.; Nguyen, M.H. Surveillance of patients with cirrhosis remains suboptimal in the United States. J. Hepatol. 2021, 75, 856–864. [Google Scholar] [CrossRef]
- Lim, J.; Singal, A.G. Surveillance and Diagnosis of Hepatocellular Carcinoma. Clin. Liver Dis. 2019, 13, 2–5. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 20, 2809–2817.e28. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of non-alcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837 e2. [Google Scholar] [CrossRef]
- Araujo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38 (Suppl. S1), 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Mark, H.E.; Villota-Rivas, M.; Palayew, A.; Carrieri, P.; Colombo, M.; Ekstedt, M.; Esmat, G.; George, J.; Marchesini, G.; et al. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J. Hepatol. 2022, 76, 771–780. [Google Scholar] [CrossRef] [PubMed]

| Variables | Females (n = 31) | Males (n = 58) | All (n = 89) | |
|---|---|---|---|---|
| Age, years (median, range) | 54 (23–69) | 47 (2179) | 49 (21–79) | |
| Weight, kg (median, range) | 76.2 (4–125) | 86.5 (58–138) | 84 (49–138) | |
| Height, cm (median, range) | 165 (150–178) | 178 (160–197) | 173 (150–197) | |
| BMI, kg/m2 (median, range) | 26.8 (18.4–39.5) | 27.7 (20.5–39) | 27.6 (18.4–39.5) | |
| Waist circumference, cm (median, range) | 87 (66–123) | 99 (78–144) | 96.5 (66–144) | |
| Liver disease etiology | ||||
| NAFLD/NASH | 11 (35.5%) | 19 (32.8%) | 30 (33.7%) | |
| Viral hepatitis (HBV, HCV, HCV/HIV) | 16 (51.6%) | 26 (44.8%) | 42 (47.2%) | |
| ALD | 2 (6.45%) | 8 (13.8%) | 10 (11.2%) | |
| Other and unknown | 2 (6.45%) | 5 (8.6%) | 7 (7.9%) | |
| Laboratory Data | ||||
| ALT, IU/L, median, range | 51.5 (16.3–161.5) | 45.2 (12.7–516.9) | 46.9 (12.6–516.9) | |
| AST, IU/L, median, range | 40.7 (16.3–87.4) | 34.9 (15.7–236.1) | 36.8 (15.7–236.1) | |
| GGT, IU/L, median, range | 51.3 (16.2–160.8) | 45.0 (12.6–514.7) | 46.8 (12.6–514.7) | |
| ALP, IU/L, median, range | 71.4 (32.4–159.6) | 75.0 (39.0–330.5) | 72.9 (32.4–330.6) | |
| Total bilirubin, mg/dL, median, range | 0.45 (0.2–1.0) | 0.39 (0.3–1.9) | 0.51 (0.2–1.8) | |
| Platelets [×109/L], median, range | 224 (122–398) | 218 (66–483) | 224 (66–483) | |
| Noninvasive Serum Tests | ||||
| APRI score | 0.35 (0.15–1.08) | 0.48 (0.13–3.13) | 0.40 (0.13–3.13) | |
| FIB-4 score | 1.1 (0.28–3.54) | 1.12 (0.21–10.75) | 1.11 (0.21–10.75) | |
| ELF score | 9 (7.4–12.6) | 8.95 (7.3–12) | 9 (7.3–12.6) | |
| VCTE Assessment (Fibroscan®) | ||||
| LSM [kPa] | 6.9 (3.3–8.5) | 5.5 (2.9–48) | 6.7 (2.9–54.2) | |
| Variable | Viral Hepatitis | NAFLD/ NASH | ALD | Other | p |
|---|---|---|---|---|---|
| Age, years (median, range) | 49 (21–79) | 49 (21–75) | 59.5 (45–63) | 60 (32–61) | N.S. |
| Weight, kg (median, range) | 79.3 (49–118) | 88 (72–125) | 81.5 (61–138) | 87 (77–102) | 0.04 |
| Height, cm (median, range) | 172 (156–192) | 176 (150–197) | 178 (170–186) | 172 (165–193) | N.S. |
| BMI, kg/m2 (median, range) | 26.0 (18.7–39) | 29.0 (21–39) | 27.4 (18.4–39.0) | 26.9 (23.8–30.1) | 0.03 |
| Waist circumference, | 90 (66–126) | 102 (90–123) | 95 (85–144) | 91 (90–103) | N.S. |
| cm (median, range) | |||||
| Laboratory data | |||||
| ALT, IU/L, median, range | 45 (19–356) | 60 (18–516) | 24 (18–42) | 52 (12–81) | 0.002 |
| AST, IU/L, median, range | 36 (16–236) | 36 (15–130) | 33 (20–75) | 46 (23–53) | N.S. |
| GGT, IU/L, median, range | 51 (9–1130) | 49 (17–404) | 90 (24–519) | 77 (19–107) | 0.01 |
| ALP, IU/L, median, range | 71 (32–169) | 77 (44–212) | 75 (60–124) | 60 (60–331) | N.S. |
| Total bilirubin, mg/dl, median, range | 0.50 (0.18–1.9) | 0.58 (0.18–1.35) | 0.50 (0.35–1.69) | 0.52 (0.35–0.84) | 0.003 |
| Platelets [×109/L], median, range | 216 (11–373) | 257 (223–267) | 300 (66–483) | 234 (159–431) | N.S. |
| Noninvasive serum tests | |||||
| APRI score | 0.37 (0.13–2.49) | 0.55 (0.15–1.44) | 0.24 (0.19–3.13) | 0.42 (0.22–0.79) | N.S. |
| FIB-4 score | 1.04 (0.2–5.9) | 1.10 (0.34–2.75) | 1.13 (0.66–10.75) | 1.11 (0.42–2.46) | N.S. |
| ELF score | 9 (7.4–11.7) | 8.7 (7.3–10.5) | 9.4 (9.0–11.5) | 9.9 (7.8–12.6) | N.S. |
| VCTE assessment (Fibroscan®) | |||||
| LSM | 6.7 (4.2–42.2) | 6.5 (3.1–54.2) | 8.5 (3–44.7) | 6.3 (6.1–11.8) | N.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzlova, N.; Mnozil Stridova, K.; Merta, D.; Rychlik, I.; Frankova, S. Transient Elastography as the First-Line Assessment of Liver Fibrosis and Its Correlation with Serum Markers. Medicina 2023, 59, 752. https://doi.org/10.3390/medicina59040752
Uzlova N, Mnozil Stridova K, Merta D, Rychlik I, Frankova S. Transient Elastography as the First-Line Assessment of Liver Fibrosis and Its Correlation with Serum Markers. Medicina. 2023; 59(4):752. https://doi.org/10.3390/medicina59040752
Chicago/Turabian StyleUzlova, Nikola, Katerina Mnozil Stridova, Dusan Merta, Ivan Rychlik, and Sona Frankova. 2023. "Transient Elastography as the First-Line Assessment of Liver Fibrosis and Its Correlation with Serum Markers" Medicina 59, no. 4: 752. https://doi.org/10.3390/medicina59040752
APA StyleUzlova, N., Mnozil Stridova, K., Merta, D., Rychlik, I., & Frankova, S. (2023). Transient Elastography as the First-Line Assessment of Liver Fibrosis and Its Correlation with Serum Markers. Medicina, 59(4), 752. https://doi.org/10.3390/medicina59040752







