Antibiotic Use in Dental Implant Procedures: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
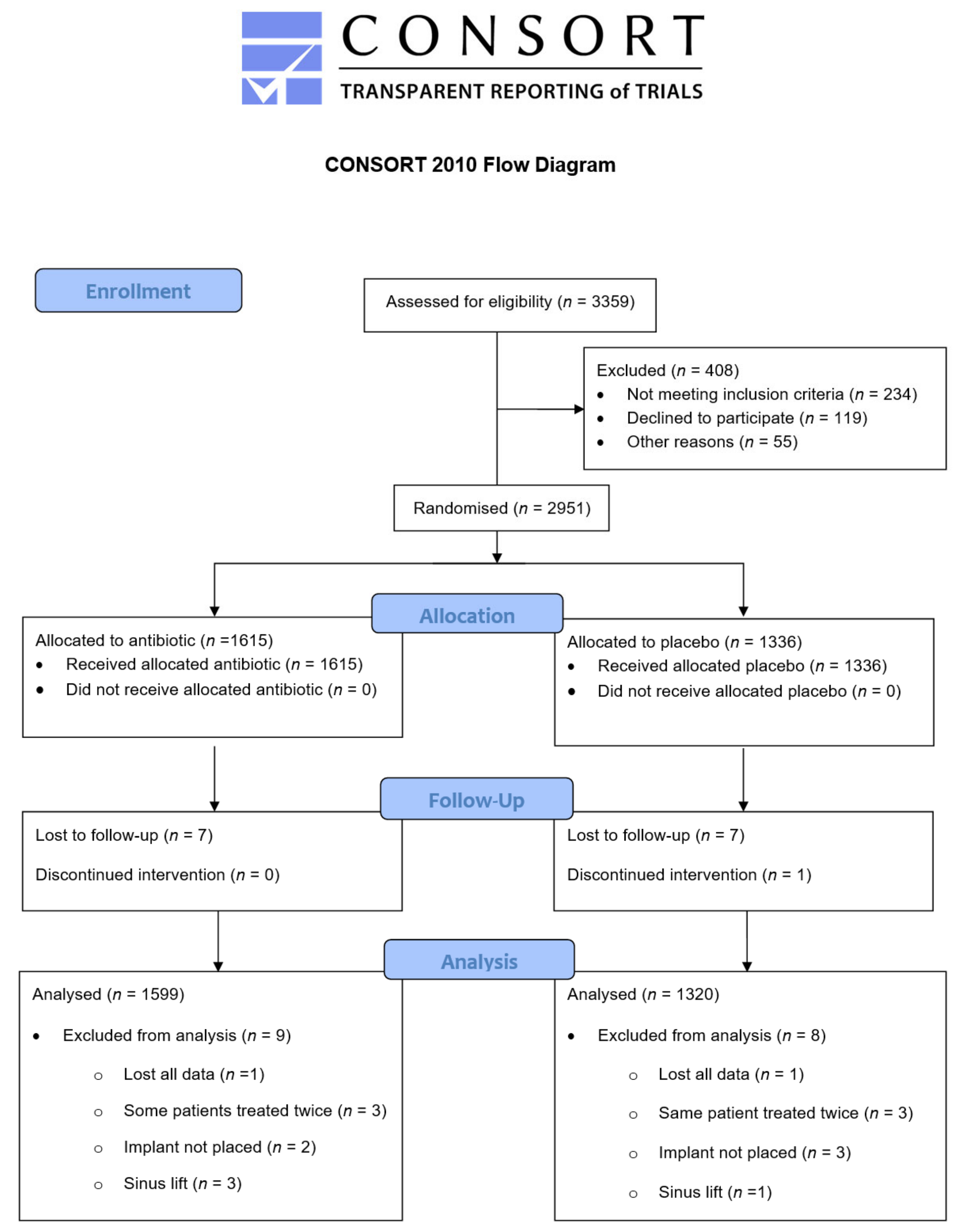
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
- Double-blind and placebo-controlled randomised clinical trial.
- Patients over 15 years of age.
- Patients undergoing DIP procedure/s.
- Only oral or systemic route of AB administration.
- Systematic and meta-analysis review.
- Studies conducted prior to the year 2000.
- Comparative AB therapy with no placebo/control.
- Trials comparing AB therapy versus another AB.
2.3. Selected Studies Summary
3. Results
3.1. Quality Assessment of the Selected Studies
3.2. Statistical Analysis
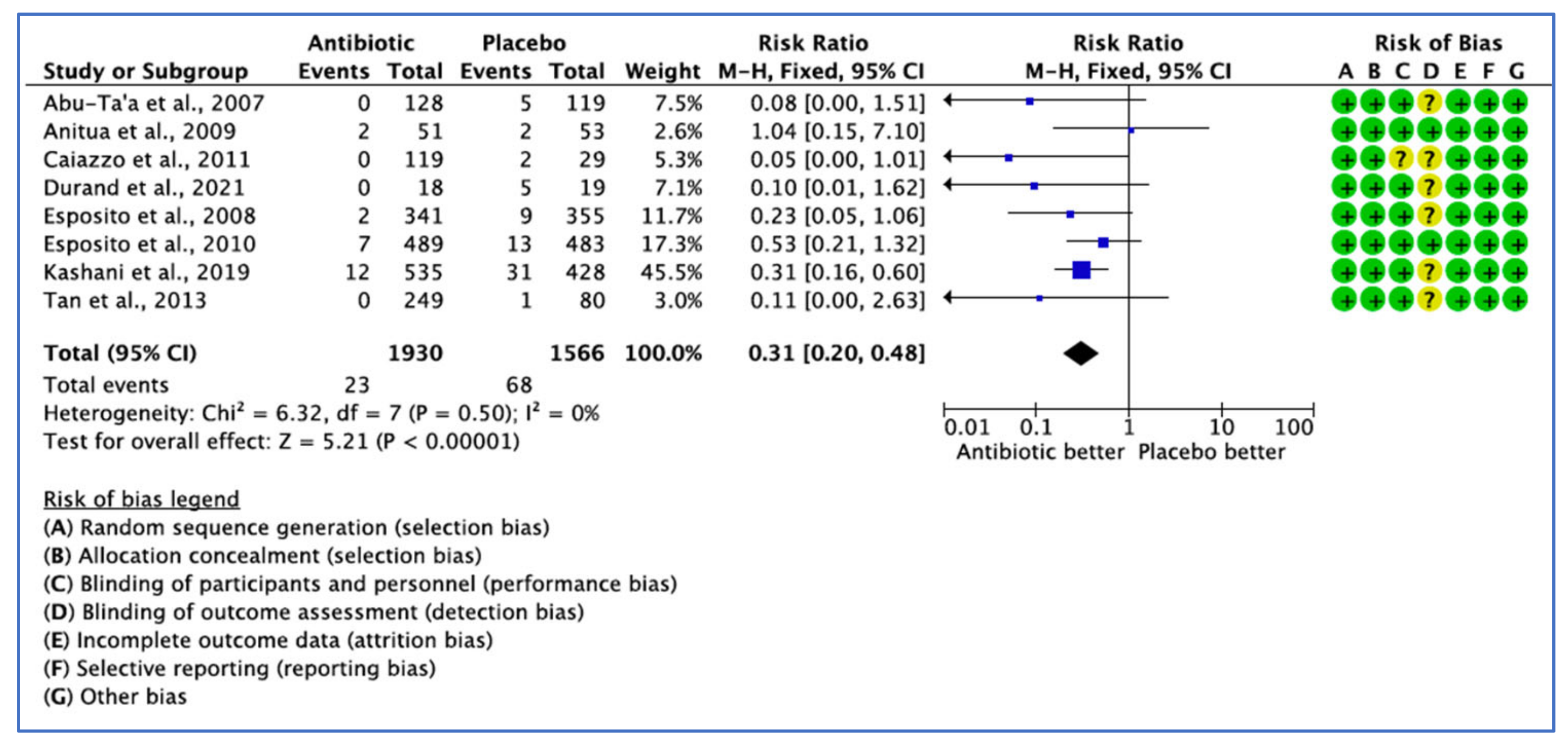
3.2.1. Implant Failure Not Prevented by the Use of ABs, by Number of Implants
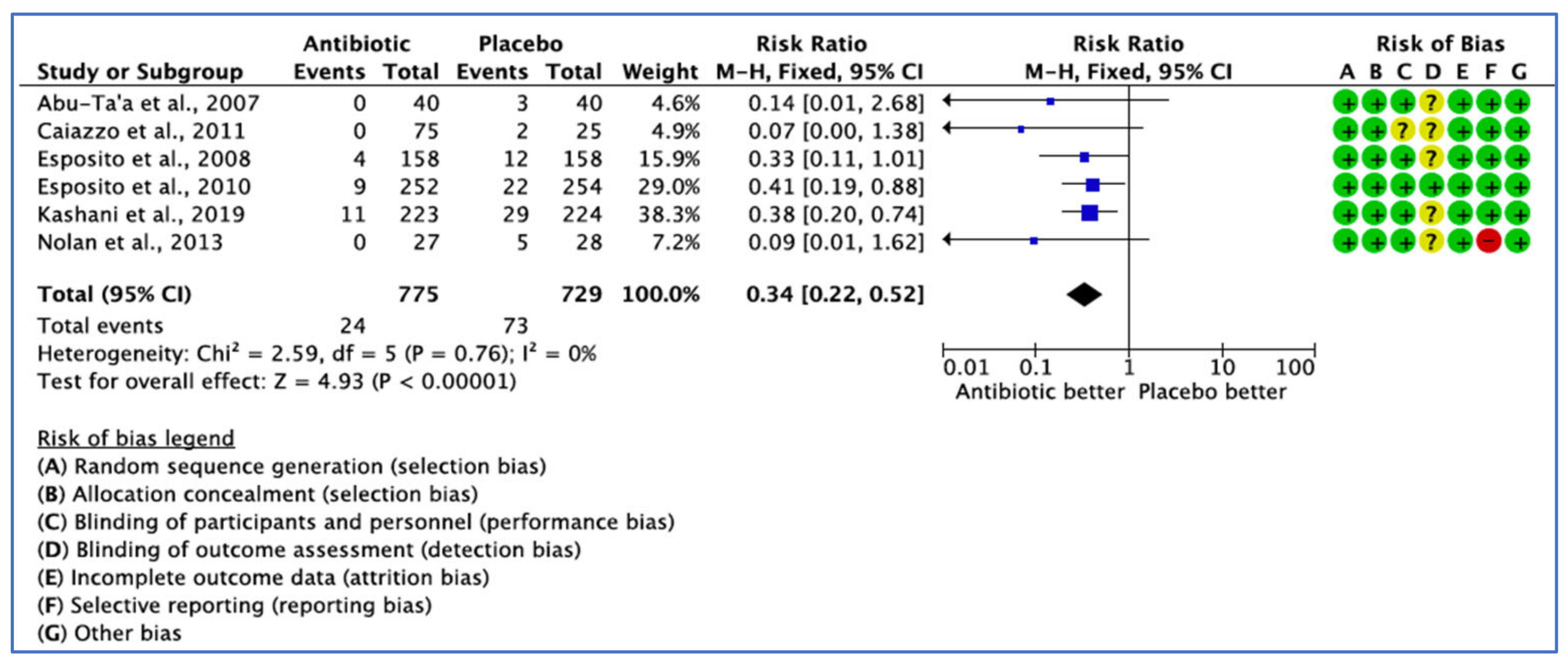
3.2.2. Implant Failure Not Prevented by the Use of ABs, by Patient
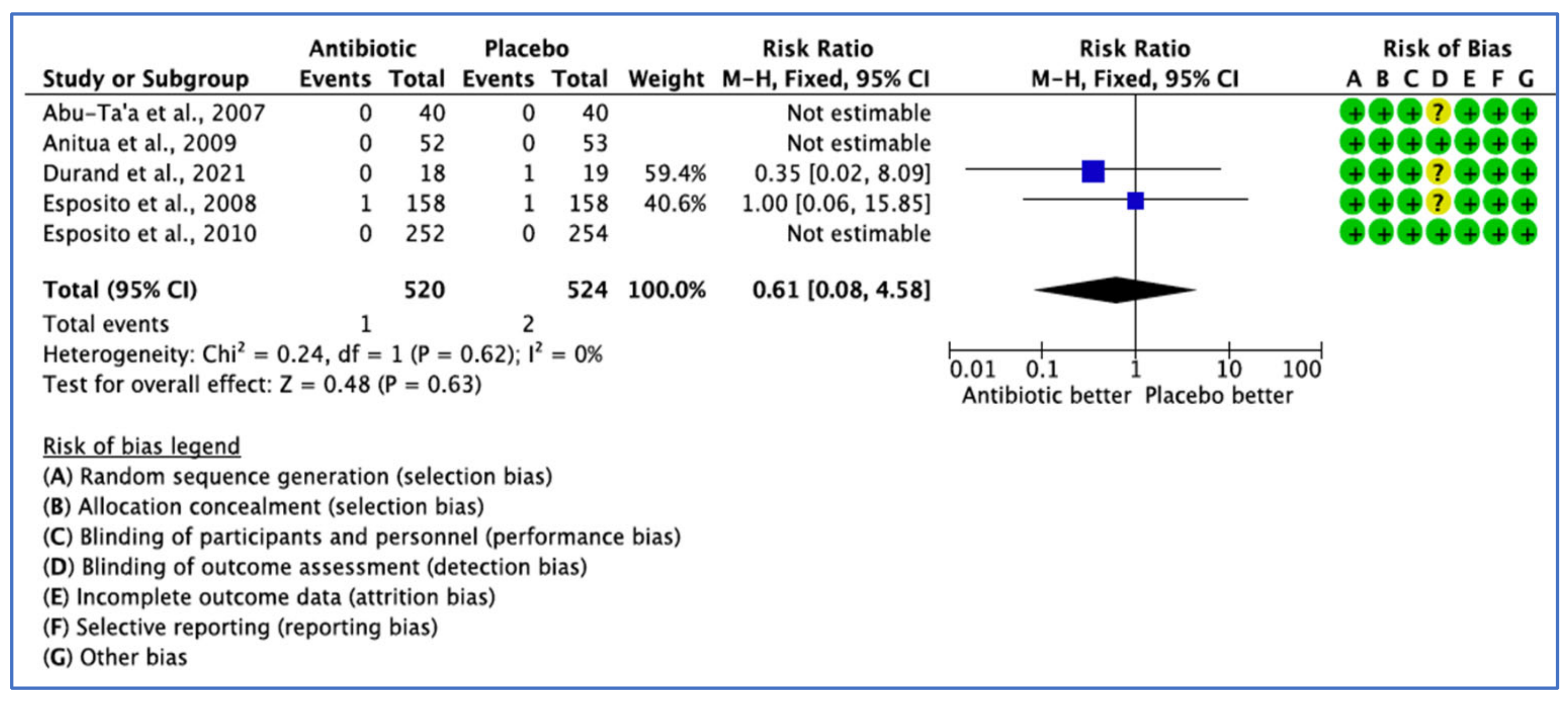
3.2.3. Postoperative Complications Analysis
3.2.4. Postoperative Antibiotic Adverse Events Analysis
3.2.5. Narrative Analysis of Other Outcomes
3.2.6. Risk of Bias
3.2.7. Number Needed to Treat Calculation
To Prevent Implant Failure Due to Infection, by Implant
To Prevent Implant Failure Due to Infection by Patient
To Prevent Complications Due to Infection
To Cause Adverse Events
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misch, C. Dental Implant Prosthetics, 2nd ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2015. [Google Scholar]
- Ho, C.K. Practical Procedures in Implant Dentistry, 2nd ed.; Wiley Online Library: Hoboken, NJ, USA, 2021. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Celik, E.; Mete, S.; Serin, F. Computational Intelligence and Soft Computing Applications in Healthcare Management Science; IGI Global: Hershey, PA, USA, 2020; p. 322. [Google Scholar]
- Tiwari, B.; Ladha, K.; Lalit, A.; Naik, B.D. Occlusal Concepts in Full Mouth Rehabilitation: An Overview. J. Indian Prosthodont. Soc. 2014, 14, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.; Margolin, M.D.; Filippi, A.; Weiger, R.; Krastl, G. Patient assessment and diagnosis in implant treatment. Aust. Dent. J. 2008, 53, S3–S10. [Google Scholar] [CrossRef]
- Norton, M.R. Biologic and mechanical stability of single-tooth implants: 4- to 7-year follow-up. Clin. Implant. Dent. Relat. Res. 2001, 3, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Sadet, P.; Grossmann, Y. A Retrospective Evaluation of 1387 Single-Tooth Implants: A 6-Year Follow-up. J. Periodontol. 2006, 77, 2080–2083. [Google Scholar] [CrossRef]
- Buser, D.; Dmd, S.F.M.J.; Wittneben, J.; Brägger, U.; Dmd, C.A.R.; Salvi, G.E. 10-Year Survival and Success Rates of 511 Titanium Implants with a Sandblasted and Acid-Etched Surface: A Retrospective Study in 303 Partially Edentulous Patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef]
- Chappuis, V.; Buser, R.; Brägger, U.; Bornstein, M.M.; Salvi, G.E.; Buser, D. Long-Term Outcomes of Dental Implants with a Titanium Plasma-Sprayed Surface: A 20-Year Prospective Case Series Study in Partially Edentulous Patients. Clin. Implant. Dent. Relat. Res. 2013, 15, 780–790. [Google Scholar] [CrossRef]
- Busenlechner, D.; Fürhauser, R.; Haas, R.; Watzek, G.; Mailath, G.; Pommer, B. Long-term implant success at the Academy for Oral Implantology: 8-year follow-up and risk factor analysis. J. Periodontal Implant. Sci. 2014, 44, 102–108. [Google Scholar] [CrossRef]
- Krebs, M.; Schmenger, K.; Neumann, K.; Weigl, P.; Moser, W.; Nentwig, G. Long-Term Evaluation of ANKYLOS® Dental Implants, Part I: 20-Year Life Table Analysis of a Longitudinal Study of More than 12,500 Implants. Clin. Implant. Dent. Relat. Res. 2013, 17, e275–e286. [Google Scholar] [CrossRef]
- Porter, J.A.; Von Fraunhofer, J.A. Success or failure of dental implants? A literature review with treatment considerations. Gen. Dent. 2005, 53, 423. [Google Scholar]
- Levin, L.; Schwartz-Arad, D. The Effect of Cigarette Smoking on Dental Implants and Related Surgery. Implant. Dent. 2005, 14, 357–363. [Google Scholar] [CrossRef]
- Ebenezer, V.; Balakrishnan, K.; Asir, R.D.; Sragunar, B. Immediate placement of endosseous implants into the extraction sockets. J. Pharm. Bioallied Sci. 2015, 7, S234–S237. [Google Scholar] [CrossRef] [PubMed]
- Surapaneni, H.; Yalamanchili, P.; Basha, M.; Potluri, S.; Elisetti, N.; Kumar, M.K. Antibiotics in dental implants: A review of literature. J. Pharm. Bioallied Sci. 2016, 8, S28–S31. [Google Scholar] [CrossRef]
- Resnick, R.; Misch, C.E. Avoiding Complications in Oral Implantology; Elsevier: St. Louis, MI, USA, 2017. [Google Scholar]
- Hupp, J.; Ferneini, E. Head, Neck, and Orofacial Infections, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Papaspyridakos, P.; Chen, C.-J.; Singh, M.; Weber, H.-P.; Gallucci, G. Success Criteria in Implant Dentistry: A systematic review. J. Dent. Res. 2011, 91, 242–248. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- The UK Faculty of Public Health. Numbers Needed to Treat (NNTs)—Calculation, Interpretation, Advantages and Disadvantages. Available online: https://www.healthknowledge.org.uk/public-health-textbook/research-methods/1a-epidemiology/nnts (accessed on 21 January 2023).
- Centre for Evidence-Based Medicine (CEBM), UK. Number Needed to Treat. Available online: https://www.cebm.ox.ac.uk/resources/ebm-tools/number-needed-to-treat-nnt (accessed on 23 January 2023).
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Caiazzo, A.; Casavecchia, P.; Barone, A.; Brugnami, F. A Pilot Study to Determine the Effectiveness of Different Amoxicillin Regimens in Implant Surgery. J. Oral Implant. 2011, 37, 691–696. [Google Scholar] [CrossRef]
- Anitua, E.; Aguirre, J.J.; Gorosabel, A.; Barrio, P.; Errazquin, J.M.; Román, P.; Pla, R.; Carrete, J.; de Petro, J.; Orive, G. A mul-ticentre placebo-controlled randomised clinical trial of antibiotic prophylaxis for placement of single dental implants. Eur. J. Oral. Implantol. 2009, 2, 283–292. [Google Scholar]
- Tan, W.C.; Ong, M.; Han, J.; Mattheos, N.; Pjetursson, B.E.; Tsai, A.Y.-M.; Sanz, I.; Wong, M.C.; Lang, N.P.; on Behalf of the ITI Antibiotic Study Group. Effect of systemic antibiotics on clinical and patient-reported outcomes of implant therapy—A multicenter randomized controlled clinical trial. Clin. Oral Implant. Res. 2013, 25, 185–193. [Google Scholar] [CrossRef]
- Payer, M.; Tan, W.C.; Han, J.; Ivanovski, S.; Mattheos, N.; Pjetursson, B.E.; Zhuang, L.; Fokas, G.; Wong, M.C.M.; Acham, S.; et al. The effect of systemic antibiotics on clinical and patient-reported outcome measures of oral implant therapy with simultaneous guided bone regeneration. Clin. Oral Implant. Res. 2020, 31, 442–451. [Google Scholar] [CrossRef]
- Durand, R.; Kersheh, I.; Marcotte, S.; Boudrias, P.; Schmittbuhl, M.; Cresson, T.; Rei, N.; Rompré, P.H.; Voyer, R. Do postoperative antibiotics influence one-year peri-implant crestal bone remodelling and morbidity? A double-blinded randomized clinical trial. Clin. Oral Implant. Res. 2021, 32, 1318–1327. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Esposito, M.; Cannizzaro, G.; Bozzoli, P.; Checchi, L.; Ferri, V.; Landriani, S.; Leone, M.; Todisco, M.; Torchio, C.; Testori, T.; et al. Effectiveness of prophylactic antibiotics at placement of dental implants: A pragmatic multicentre placebo-controlled randomised clinical trial. Eur. J. Oral Implant. 2010, 3, 135–143. [Google Scholar]
- Abu-Ta’A, M.; Quirynen, M.; Teughels, W.; Van Steenberghe, D. Asepsis during periodontal surgery involving oral implants and the usefulness of peri-operative antibiotics: A prospective, randomized, controlled clinical trial. J. Clin. Periodontol. 2007, 35, 58–63. [Google Scholar] [CrossRef]
- Nolan, R.; Kemmoona, M.; Polyzois, I.; Claffey, N. The influence of prophylactic antibiotic administration on post-operative morbidity in dental implant surgery. A prospective double blind randomized controlled clinical trial. Clin. Oral Implant. Res. 2013, 25, 252–259. [Google Scholar] [CrossRef]
- Kashani, H.; Hilon, J.; Rasoul, M.H.; Friberg, B. Influence of a single preoperative dose of antibiotics on the early implant failure rate. A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 278–283. [Google Scholar] [CrossRef]
- Khoury, S.B.; Thomas, L.; Walters, J.D.; Sheridan, J.F.; Leblebicioglu, B. Early Wound Healing Following One-Stage Dental Implant Placement With and without Antibiotic Prophylaxis: A Pilot Study. J. Periodontol. 2008, 79, 1904–1912. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Coulthard, P.; Oliver, R.; Worthington, H.V. The efficacy of antibiotic prophylaxis at placement of dental implants: A Cochrane systematic review of randomised controlled clinical trials. Eur. J. Oral Implant. 2008, 1, 95–103. [Google Scholar]
- Laskin, D.M.; Dent, C.D.; Morris, H.F.; Ochi, S.; Olson, J.W. The Influence of Preoperative Antibiotics on Success of Endosseous Implants at 36 Months. Ann. Periodontol. 2000, 5, 166–174. [Google Scholar] [CrossRef]
- Thomas, B.; Ciliska, D.; Dobbins, M.; Micucci, S. A Process for Systematically Reviewing the Literature: Providing the Research Evidence for Public Health Nursing Interventions. Worldviews Evid.-Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.M., Welch, V., Eds.; John Wiley and Sons: Chichester, UK, 2022. [Google Scholar]
- Ata-Ali, J.; Ata-Ali, F. Do antibiotics decrease implant failure and postoperative infections? A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2014, 43, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for replacing missing teeth: Antibiotics at dental implant placement to prevent complications. Cochrane Database Syst. Rev. 2013, 2013, CD004152. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Klinge, B.; Quirynen, M. The 4th EAO Consensus Conference 11–14 February 2015, Pfäffikon, Schwyz, Switzerland. Clin. Oral Implant. Res. 2015, 26, iii–iv. [Google Scholar] [CrossRef]
- Canullo, L.; Troiano, G.; Sbricoli, L.; Guazzo, R.; Laino, L.; Caiazzo, A.; Pesce, P. The Use of Antibiotics in Implant Therapy: A Systematic Review and Meta-Analysis with Trial Sequential Analysis on Early Implant Failure. Int. J. Oral Maxillofac. Implant. 2020, 35, 485–494. [Google Scholar] [CrossRef]
- Jain, A.; Rai, A.; Singh, A.; Taneja, S. Efficacy of preoperative antibiotics in prevention of dental implant failure: A Meta-analysis of randomized controlled trials. Oral Maxillofac. Surg. 2020, 24, 469–475. [Google Scholar] [CrossRef]
- Braun, R.S.; Chambrone, L.; Khouly, I. Prophylactic antibiotic regimens in dental implant failure: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2019, 150, e61–e91. [Google Scholar] [CrossRef]


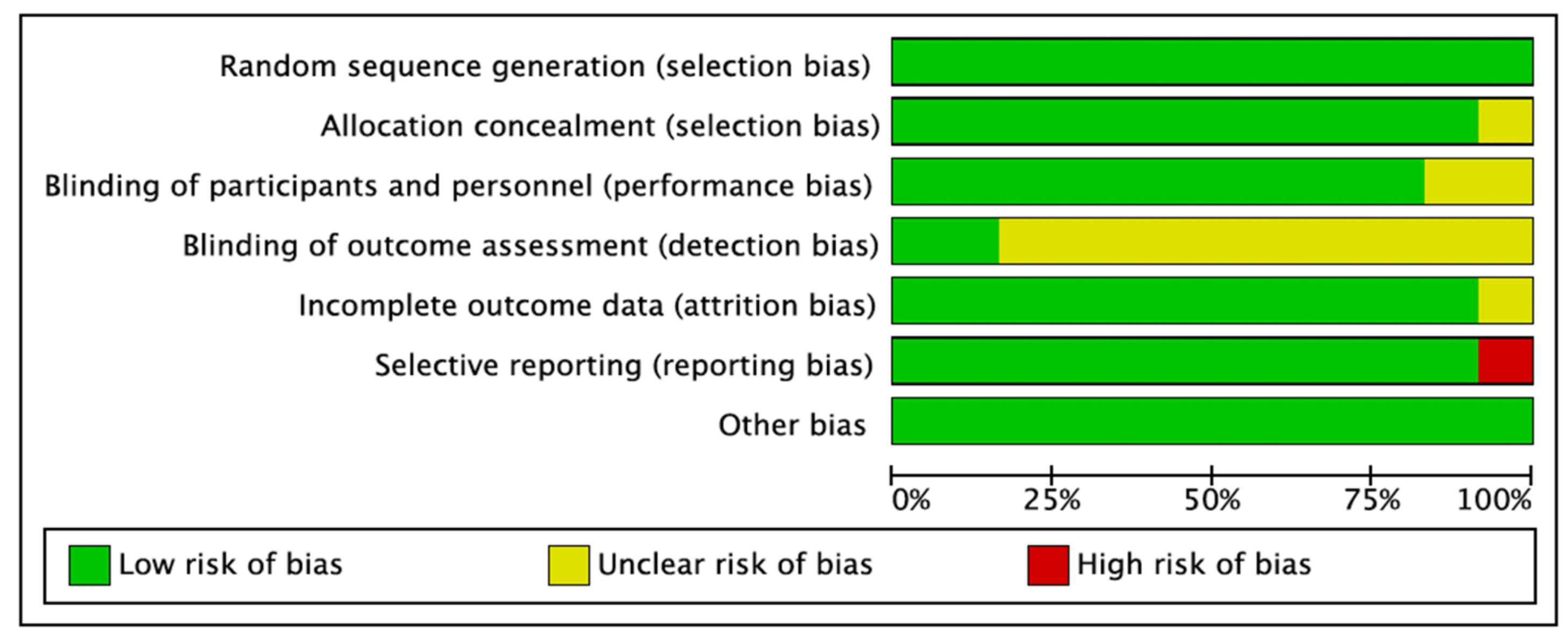
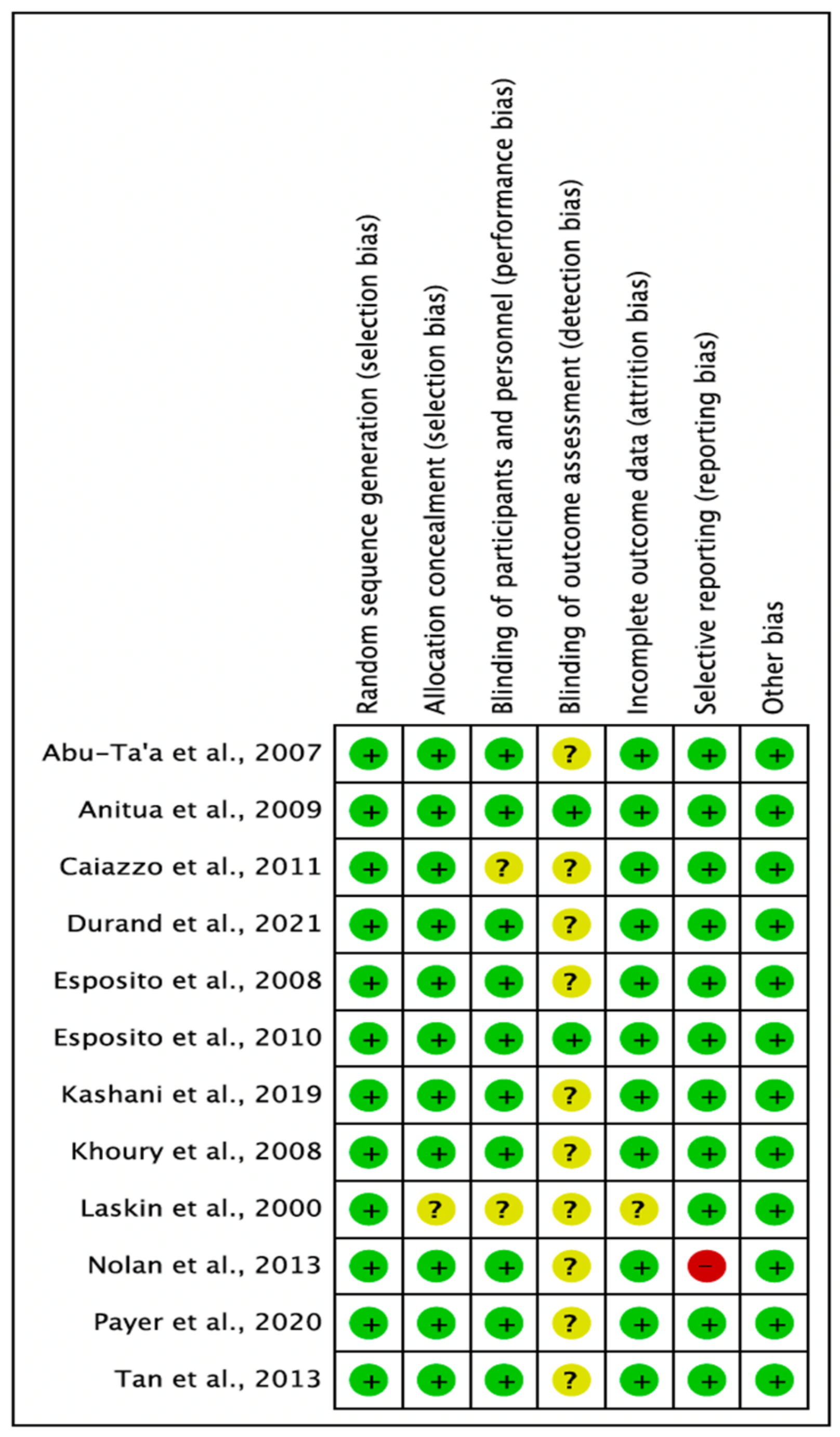
| Citation | Design | Sample Age Gender | Study Aim | Antibiotic | Control | Co-Treatment | Inflammation and Pain Relief | Antibiotic Dosing Time | Follow-Up | Study Period | Settings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Caiazzo et al., 2011 [25] Italy | Prospective, controlled, randomised clinical study Multi-centre | n = 100 ≥18 years F: 58 M: 42 | To evaluate and compare the efficacy of different antibiotic durations in the attempt of founding the minimum effective duration of amoxicillin chemoprophylaxis in dental implant surgery. | SPAB: amoxicillin 2 g 1 h before surgery PPAB: amoxicillin 2 g 1 h before surgery and 1 g twice a day for 7 days following surgery POAB: amoxicillin 1 g twice a day started after surgery and continued for 1 week after surgery. | NOAB | Preop: chlorhexidine gluconate 0.2% solution for 1 min before each procedure Postop: chlorhexidine gluconate 0.2% twice daily for 15 days | Postoperative cold therapy (ice packs) and Nimesulide 100 mg twice daily for 3 days. | 1 h before surgery | 3 months | September 2006–September 2007 | Dental practice |
| Esposito et al., 2010 [31] Italy | Double-blinded randomised controlled trial Multi-centre | n = 506 >18 years F: 270 M: 236 | To compare the effectiveness of 2 g amoxicillin with identical placebo tablets taken orally 1 h prior to implant placement. | 2 g amoxicillin | Placebo | Preop: chlorhexidine mouthwash 0.2% for 1 min prior to implant placement Postop: chlorhexidine Postoperative: chlorhexidine mouthwash 0.2% for 1 min twice a day for at least 1 week | N/R | 1 h before implant placement | 4 months after implant placement | April 2008– November 2009 | Dental clinics |
| Anitua et al., 2009 [26] Spain | Double-blinded placebo-controlled randomised clinical trial Multi-centre | n = 105 ≥18 years F: 70 M: 35 | Efficacy and safety of 2 g amoxicillin orally has been compared with identical placebo tablets 1 h before implant placement of single implants in patients with bone types II and III | 2 g amoxicillin | Placebo | Preop: chlorhexidine mouthwash For 1 min Postoperative: an intravenous or intramuscular administration of 4 mg of dexamethasone, followed by decreasing doses of oral dexamethasone | Paracetamol (maximum 1 g, 8 hourly) both before and after the intervention + metamizol (575 mg, 1 or 2 tablets, 8 hourly) | 1 h before implant surgery | 3 months after implant placement | January 2006–September 2007 | Dental clinic |
| Abu-Ta’a et al., 2007 [32] Belgium | Prospective, double-blinded randomised, controlled clinical trial Single centre | n = 80 ≥26 years F: 37 M: 43 | To compare usefulness of pre- and postop antibiotics while strict asepsis was followed during periodontal surgery, investigate the effect of Preop antibiotics on the perioral skin microbial flora and to detect its impact on S. aureus from the noses of the patients involved. | 1 g amoxicillin, 1 h Preop and 500 mg four times per day, 2 days post-operatively | NOAB | Preop: chlorhexidine di gluconate, PerioAid (0.12 solution without alcohol) for 1 min just before surgery Postop: chlorhexidine di gluconate, PerioAid (0.12 solution without alcohol twice a day for 1 min up to 7–10 days later | N/R | 1 h before surgery | 5 months | N/R | University |
| Nolan et al., 2014 [33] Ireland | Prospective, double-blind, placebo-controlled, randomised clinical trial Single centre | n = 55 >40 years F: 36 M: 19 | To investigate the influence of pre-operative prophylactic antibiotics on post-operative morbidity: swelling, bruising, wound dehiscence and suppuration/ Postop pain and interference with daily activities and successful osseointegration of dental implants | 3 g amoxicillin | Placebo | Preop: chlorhexidine 0.2% mouth rinse for at least 60 s before surgery Postop: chlorhexidine 0.2% mouthwash 4–5 times daily for the first post-operative week | Paracetamol 500 mg as required (maximum of 4 g/day) | 1 h before surgery | 3–4 months post-operatively | N/R | University |
| Tan et al., 2014 [27] Singapore Hong Kong China Australia Spain Taiwan Iceland | Single blinded RCT Multi-centre | n = 329 >19 years F: 147 M: 182 | To determine the effects of various systemic antibiotic prophylaxis regimes on PROMs and prevalence of postsurgical complications in patients undergoing conventional implant installation | G1 (positive control, PC): 2 g amoxycillin Preop G2 (test 1, T1): 2 g Amoxycillin immediately postoperatively; G3 (test 2, T2): 2 g amoxycillin Preop, and 500 mg three times a day (8 hourly) on days 2 and 3 | Placebo | Preop: 0.2 % chlorhexidine for 1 min | Description of analgesics was not provided | 1 h before conventional implant placement | 8 weeks | August 2009–October 2011 | Dental Centre and University |
| Kashani et al., 2019 [34] Sweden | Prospective Double blinded RCT Multi-centre | n = 447 ≥15 years F: 143 M: 175 | Compare the outcome of oral implant treatment due to early failures | AB group: 2 g amoxicillin or if allergic, 600 mg clindamycin 1 h pre-operatively | NOAB | N/R | N/R | 1 h before surgery | 7–14 days | N/R | Dental Centre and University |
| Payer et al., 2020 [28] Austria Singapore China Australia Hong Kong Iceland Shanghai | Double blinded RCT Multi-centre | n = 236 ≥21 years F: 111 M: 125 | To investigate the effect of systemic antibiotics primarily on PROMs and secondarily on post-surgical complications in medically and periodontally healthy patients undergoing oral implant therapy and simultaneous GBR | 2 g amoxicillin 1 h prior to implant placement and GBR + single dose of 500 mg amoxicillin 8 h after surgery and 500 mg thrice daily (8 hourly) on days 1–3 following implant placement and GBR | Placebo | Preop: 0.2% chlorhexidine for 1 min prior to surgery Postoperative: 0.2% chlorhexidine mouth rinse twice daily for 2 weeks | Paracetamol thrice daily for two days after surgery | 1 h before surgery | 12 weeks | August 2014–September 2017 | Dental Centre and University |
| Durand et al., 2021 [29] Canada | Double blinded RCT Single centre | n = 37 Mean age 57.4/±11.3 years F: 21 M: 16 | To evaluate the influence of postoperative antibiotics on peri-implant crestal bone remodelling after 1 year, postoperative pain and morbidity and one-year implant survival rate | 2 g amoxicillin 1 h before implant placement followed by 7 days postoperative regimen (500 mg three times a day) | Placebo | Preop: 0.12% chlorhexidine gluconate for one minute, 1 h prior surgery Postop: 0.12% chlorhexidine twice daily for 1 week | Ibuprofen 600 mg with a maximum of four tablets per day to be taken every four hours for the first 48 h + supplemental dose of analgesic (500 mg paracetamol) taken only if needed for the first 48 h after surgery | 1 h before implant placement | 1 year | N/R | University |
| Khoury et al., 2008 [35] United states of America | Prospective observational trial—case control Single centre | n = 20 >18 years F: 9 M: 11 | To investigate the clinical and biologic markers of early soft tissue healing around dental implants placed in the presence and absence of antibiotic prophylaxis | 2 g amoxicillin, 1 h prior to surgery + 500 mg, three times a day | NOAB | Postop: 0.12% chlorhexidine rinse twice daily for 1 week | Ibuprofen 600 mg + 2 g of amoxicillin | 1 h before surgery | 1 week | September 2005–March 2007 | University |
| Esposito et al., 2008 [36] Italy | Double-blinded randomised controlled trial Multi-centre | n = 316 ≥18 years F: 174 M: 142 | To compare the efficacy of 2 g amoxicillin with identical placebo tablets taken 1 h prior to implant placement | 2 g amoxicillin | Placebo | Postop: chlorhexidine mouthwash 0.2% for 1 min twice a day for at least 1 week | N/R | 1 h before implant placement | 4 months | September 2006–March 2007 | Dental clinics |
| Laskin et al., 2000 [37] United states of America New Zealand | Case control Multi-centre | n = 702 30–90 years F: 47 M: 632 | To assess the influence of preop antibiotics on long-term clinical survival of endosseous dental implants of different designs and surfaces | Penicillin 2 g | Placebo | Postop: 0.12% chlorhexidine rinse for 2 weeks | N/R | 1 h before surgery | 36 months | 1997–2000 | University Hospital |
| Citation | Main Results | Appraisal Summary |
|---|---|---|
| Caiazzo et al., 2011 [25] | The RCTs concluded that single preoperative, combined pre- and postoperative antibiotic and postoperative antibiotic coverage versus no antibiotic treatment did not reveal any significant differences regarding implant failure in healthy patients under normal circumstances. | This study included healthy patients. The implant type places were titanium screw-type external hex, but the implant system was not reported. The overall reported success rate was 98.65 with two failures in the NOAB group. Study limitations: The mean age included in the metanalysis was between 42- and 52-years-old, which makes the results not applicable to the global population. There was no clear explanation of the ethics approval, surgical protocol, inclusion criteria or exclusion criteria. The authors were contacted by the reviewer who requested the missing data, and they responded that ‘’the data for clinical diagnosis for the infected surgical wound was not collected’’, which can be considered as reporting bias, especially, insufficient details of the assessors blinding were also prevalent. The planned sample size could not be obtained, and reasons were not explained. |
| Esposito et al., 2010 [31] | Single administration of 2 g amoxicillin initiated one hour prior to implant placement could decrease early implant failure in patients not requiring a bone augmentation procedure. However, there were no significant differences for post-operative complications observed. | Implant procedures were conducted in 10 Italian private dental practices. The logistic regression model was fitted to determine confounding factors and the influence of the site effect on implant failure. No effect was observed between centres, and all had similar results (p = 0.97). The mean age of 49 and age range of 18–85 make the results applicable to the global population. Study limitations: No explanation provided on ethics approval or the preoperative surgical protocol. The planned sample size was 9 and could not be achieved and was insufficient to implicate a statistically significant difference. |
| Anitua et al., 2009 [26] | The finding does not support the use of 2 g of preoperative amoxicillin versus 2 g of placebo one hour before the placement of single dental implants in bone type II and III, and the results failed to show any advantages of preoperative antibiotics in the probability of having postoperative infection, characteristics of the saprophytic flora and incidence of antibiotic adverse events. | Single implants were placed in the maxilla or mandible. All patients received Biotechnology Institute (BTI) dental implants. Implant preparation rich in growth factor (PRGF) was applied in the operative protocol. Both researchers and patients remained blinded to the group receiving treatment. A logistic regression method was applied to determine confounding factors and the influence of different variables, including the centre, antibiotic or placebo, duration of the intervention, age and smoking habits. Study limitations: Participants had bone quality II or III; thus, the inclusion of patients with bone type I and IV could have altered the results. The planned sample size could not be achieved for a statistically significant difference. A larger sample size would be required to rule out the possibility of a difference between groups. |
| Abu Ta’a et al., 2007 [32] | Single dose of preoperative 1 g amoxicillin and postoperative regimen of 500 mg four times per day for 2 days does not provide any advantages concerning peri-oral aerobic and anaerobic flora on nasal aerobic and anaerobic bacteria and also failed to have a noticeable impact on post-operative infection and implant failure, while strict asepsis applies during periodontal surgery. | High-end policy of asepsis was followed for asepsis prevention from the oral and nasal cavity. Both the surgical team and the patients were blinded to the groups. A team of several peri odontologist surgeons, with different levels of experience, and nurses performed the implant surgery. Postoperative infection and implant failure were assessed at the follow-up visits for up to 5 months after the implant procedures. The implant system was not described in this study. Study limitations: Details of the enrolment period, explanation of how consent was obtained and details of the assessors blinding were missing. There were reports of patient-related variables with different conditions, such as blood-clotting problems, heavy smokers who consumed >40 cigarettes a day and patients with parafunctions included in the study, which may have affected the results. |
| Nolan et al., 2013 [33] | The study favoured the preoperative use of 3 g amoxicillin to improve the survival of dental implants, to effectively reduce postoperative pain, and improve interference with daily activity, considering that the duration of surgery and the number of implants had an effect on implant survival. | Only patients with the presence of a partial edentulous or edentulous alveolar ridge and presence of a tooth or several teeth regarded as non-restorable with the intention of immediate implant placement were included in the study. The number of implants in each of the study groups was not described. Multiple implant systems were used; however, 65% of implants placed were Biomet 3i. A higher level of postoperative pain following implant surgery was reported in patients with implant failure after 2 days and after 7 days. Study limitations: There was some proportion of smoker patients in antibiotic and placebo groups. The sample size was low, with only patients over the age of 40 years; therefore, the results may not be generalisable to the global population. In this study, factors related to osseointegration of the implants, such as the site of the implant and previous bone augmentation, were not described. |
| Tan et al., 2014 [27] | The study demonstrated that the administration of pre-, peri- or postsurgical prophylactic antibiotics did not provide beneficial effects on patient-reported outcome measures (PROMs), such as pain, swelling, bruising and bleeding, and also did not influence clinical parameters of postsurgical complications, such as flap closures, suppuration, swelling, implant stability and pain. | The study examined three antibiotic regimens and one control group using randomisation tables allocating the patient a number with a corresponding envelope. In addition, all patients were treated periodontally for one minute prior to the implant installation. The pre-operative dosage of amoxicillin applied was based on the antibiotic dosage recommendation by the AHA for the prevention of infective endocarditis. The patients were not blinded to the allocated treatment, with panned single-tooth edentulous space in the maxilla or mandible placement with adequate pristine bone for a standard oral implant placement included in this study. The reason for the implant was a single-tooth edentulous space in the maxilla or mandible with adequate pristine bone for a standard oral implant placement without the need of simultaneous bone augmentation. The implant surgery was performed by oral and maxillofacial surgeons. Only one oral implant system (Straumann SLA) was used. The oral implants were either Standard Plus or Bone Level implants and placed into pristine bone, without any simultaneous bone augmentation. Moreover, a one-stage implant installation protocol was employed. Study limitations: Light smokers who consumed fewer than 20 cigarette a day were included in the study. The lack of patient blinding, as well as descriptions of the results for postoperative complications and missing the number of implant failures, resulted in detecting reporting bias. |
| Kashani et al., 2019 [34] | The administration of single dose of prophylactic antibiotics in conjunction with implant placement surgery had a statistically significant lower early implant failure rate based on the early implant rate on both implant and patient levels in healthy individuals. | No preoperative oral hygiene protocol was applied in this study. Both patients and the surgeon remained blinded in the present trial. Patients were healthy and were divided into subgroups to identify potential confounding variables. Regarding the implant system, four implant brands were used: 611 implants from Nobel Biocare, 236 implants from Astra Tech, 112 implants from Straumann and implants from Ankylos. The jaw type, bone graft and number of implants placed per patient were sufficiently described. Study limitations: Details of the enrolment period and surgical protocol were not described. |
| Payer et al., 2020 [28] | Systemic antibiotics in comparison with a placebo group were seen to provide no improvement in patient-reported outcome measures (PROMs) and no significant reduction in post-surgical complications in healthy patients undergoing advanced oral implant surgery and simultaneous GBR. | Only periodontally and medically healthy patients with a score of 1 or 2 according to the physical status classification of the American Society of Anaesthesiologists (ASA) with defects allowing for simultaneous fixture implant placement and GBR were included. The examiners and patients remained blinded. One implant system of the Straumann Standard Plus or Straumann Bone level was applied. The number of implants inserted in each group was not described. Up to four surgeons were included to perform the clinical procedure in each centre. Payer et al. (2020) were contacted to supply the missing information through e-mail inquiries; however, the corresponding author did not respond. Study limitations: The planned sample size could not be achieved, and it was supported by a grant from the international team for implantology (ITI Foundation). |
| Durand et al., 2021 [29] | A single preoperative dose of 2 gr of amoxicillin one hour prior to implant placement was seen to be more beneficial than an additional postoperative antibiotic regimen for the prevention of implant complications after placing straightforward platform-switched implants for uncomplicated implant surgery in healthy patients, and the addition of amoxicillin 500 mg (three times daily) 7-day postoperatively was of no advantage or benefit on peri-implant crestal bone remodelling, post-operative morbidities and implant survival after placing straightforward platform-switched implants in healthy patients after a 1-year follow-up. | Only periodontally healthy patients or those presenting with gingivitis with adequate oral hygiene undergoing straightforward platform-switched implant placement from multiple ethnic backgrounds were included in the study. The groups were homogenous in age, sex, ethnicity, education and, smoking status. In addition, the groups were similar in surgical parameters (insertion torque, incision length, bone quality, implant location [maxilla vs. mandible]) and implant system, except two parameters: unequal distribution of implant insertion in each group, despite using random allocation, and the mean surgery duration was significantly longer in the control group. All implant surgeries were conducted in one treatment centre. The surgeons, participants and examiners were all unaware of subject allocation throughout the study. The measured outcomes were mesial and distal peri-implant crestal bone levels, postoperative pain severity and postoperative morbidity. The author was contacted to provide the missing information through e-mail inquiries; however, the corresponding authors did not respond. Study limitations: Since the sample size was low and study population was healthy, the results of this study prevent any generalisation for global populations, with comorbidities, smokers, more complex surgeries involving additional bone grafting procedures and when implant surgeries were executed by inexperienced surgeons. The duration of the enrolment period was not described. There was no clear explanation of ethical approval in this study. The decision whether to prescribe perioperative antibiotics was left to the surgeon, and this lack of randomisation could contribute to selection bias. In addition, adverse events were self-reported at the follow-up appointment and not collected on a daily basis, possibly introducing a recall bias. The sample size could not achieve statistical power. |
| Khoury et al., 2008 [35] | Systemic amoxicillin may have a modest effect on clinical parameters during the first postoperative week and may have a limited effect on biomarkers. | Only systemically and periodontally healthy individuals or those presenting with mild gingivitis for a single tooth implant were included in this study. The two implant systems from “Astra Tech” and “Zimmer Dental Implant” were used in this study. These two implant systems have different thread pattern designs and require different osteotomy procedures, and both systems require implant placement flush with the alveolar crest. All implant surgeries were conducted in one treatment centre. Screw-type, root-form, two-piece dental implants were inserted. The majority of implants (81%) were located in the posterior sextant, and the distribution between the maxilla and mandible was similar. The short-term evaluation of one week was chosen to determine early events occurring at the soft tissue level within the time limitation of antibiotic prescription. Study limitations: This study lacked relevant outcomes measured in this review. No specification given to who performed the surgery. |
| Esposito et al., 2008 [36] | 2 g of preoperative amoxicillin versus 2 g of placebo one hour prior to the placement of dental implants resulted in no statistically significant differences in implant failures and postoperative complications in patients not requiring bone augmentation procedures. | Randomisation was performed using sequentially numbered, identical, opaque, sealed envelopes for concealment of allocation through the application of computer-generated restricted randomisation lists. Patients were grouped into three groups, non-smokers, light smokers (up to 10 cigarettes per day) and heavy smokers (more than 10 cigarettes per day). Dentists with extensive experience in implant treatment performed the implant surgery in each centre. Various implant systems were used. The majority of implants were manufactured by “Zimmer”. There was no apparent relevant baseline imbalance between the two groups in terms of patient characteristics (gender, age, non-smoker, duration of intervention in minutes, total number of inserted implants, patients who took postoperative antibiotics and incidence of intraoperative complications). Patients and investigators remained blinded for the entire duration of the trial. Study limitations: There was no clear explanation of ethical approval. The sample size was underpowered to detect a statistically significant difference. This study was not sponsored; however, the placebo and antibiotic used in this study were donated by a drug company manufacturing generic drugs. |
| Laskin et al., 2000 [37] | The study showed that the use of preoperative antibiotics significantly increased dental implant survival according to the incision type, mobility at implant placement and implant type. | The study did not compromise patients with mild and severe systematic disease. One implant system of the Spectra system was used in this study. Oral hygiene was prescribed for two weeks post-operatively. Patients were followed up from the time of placement to 36 months. Study limitations: The study did not define the inclusion and exclusion criteria and has been funded by USA Government-supported research. It was not clear who performed the implant surgery. The number of participants for whom implant failure was measured in each intervention group was missing and the method was unclear, with respect to the description of the randomisation procedure, method of sequence generation and method of allocation concealment, with inadequate reports of outcomes, causing performance bias, reporting bias and attrition bias. |
| Citation | Selection Bias | Study Design | Confounders | Blinding | Data Collection | Withdrawals & Dropouts | Global Rating |
|---|---|---|---|---|---|---|---|
| Caiazzo et al., 2011 [25] | 1 | 1 | 0 | 2 | 2 | 1 | 1.17 |
| Esposito et al., 2010 [31] | 2 | 1 | 1 | 1 | 2 | 1 | 1.33 |
| Anitua et al., 2009 [26] | 1 | 1 | 1 | 1 | 2 | 1 | 1.17 |
| Abu’-Ta’a et al., 2008 [32] | 1 | 1 | 0 | 1 | 2 | 1 | 1.00 |
| Nolan et al., 2014 [33] | 2 | 1 | 0 | 1 | 2 | 1 | 1.17 |
| Tan et al., 2014 [27] | 1 | 1 | 2 | 2 | 2 | 1 | 1.50 |
| Kashani et al., 2019 [34] | 1 | 1 | 0 | 1 | 2 | 1 | 1.00 |
| Payer et al., 2020 [28] | 1 | 1 | 2 | 1 | 2 | 1 | 1.33 |
| Durand et al., 2021 [29] | 2 | 1 | 1 | 1 | 1 | 1 | 1.17 |
| Khoury et al., 2008 [35] | 2 | 2 | 0 | 2 | 2 | 1 | 1.50 |
| Esposito et al., 2008 [36] | 1 | 1 | 2 | 1 | 2 | 1 | 1.33 |
| Laskin et al., 2000 [37] | 1 | 2 | 1 | 3 | 3 | 1 | 1.83 |
| Citation | Comparison |
|---|---|
| Canullo et al., 2020 [43] | This study found a statistically significant benefit of antibiotics in the reduction of early implant failure at both the implant level and patient level (RR = 0.32 [0.20, 0.51], p < 0.001 and RR = 0.31 [0.20, 0.49], p < 0.001, respectively). The reported finding was based on the fixed effect model. It revealed low risk of bias for only three of the studies included. The current meta-analysis found that the majority of studies included had a low risk of bias, except one (moderate). NNT was not analysed by Canullo et al. (2020). A strength of the current metanalysis is the inclusion of a recently published study and calculation of the NNT. |
| Ata-Ali, Ata-Ali and Ata-Ali, 2014 [40] | The study by Ata-Ali et al., (2014) included only four, large-sample size double-blinded placebo-controlled trails with a total of 2063 implants (including 1077 in the antibiotic treatment group and 986 in the control group). The results affirmed that antibiotic treatment provides a statistically significant beneficial reduction in implant failure (p = 0.003) with an NNT of 48, and it was concluded that antibiotic administration lowers the odds of implant failure by 66.9%. In contrast, failing to reach statistical significance was a reduction in the incidence of postoperative infection (p = 0.754). The current meta-analysis is consistent with previously published meta-analyses but included a larger number of studies regardless the sample size of studies (eight double blinded placebo-controlled trails, including a combined total of 2086 implants, including 1930 implants in the antibiotic versus 1566 in the placebo group) and revealed a statistically significant reduction in implant failure (67%). This meta-analysis used risk ratio as the measure of effect, while other meta-analyses in the literature have used the odds ratio (OR) as the primary measure. To add to the limitations, the study included RCTs assessing the effect of amoxicillin as the antibiotic treatment, whereas this meta-analysis included any type of antibiotic. |
| Jain, Rai, Singh and Taneja, 2020 [44] | The metanalysis implemented by Jain et al. (2020) included five RCTs rated as having a low risk of bias assessing the use of preoperative amoxicillin 1 h prior to surgery with a total of 1032 patients (514 in antibiotic and 518 in placebo groups) and 1919 implants (952 in the antibiotic group and 967 in the placebo group) for the prevention of both the number of implant failures and the number of patients with implant failures after a minimum of 3 months, from implant placement in healthy individuals, and concluded that the number of implants failed was statistically fewer in the antibiotic group, p = 0.006, with 0% heterogeneity. The number of patients that suffered from implant failure was significantly lower in the antibiotic group, p = 0.006 with 0% heterogeneity. This study is consistent with the current finding; however, our study included a larger number of randomised controlled trials including any antibiotic regardless of the timing of administration and revealed statistically significant results. |
| Braun, Chambrone and Khouly, 2019 [45] | The systematic review and metanalysis performed by Braun et al., (2019) included six studies with a combined total of 1162 participants and a total combined number of implants placed of 2250, assessing the efficacy of antibiotic use in the prevention of both the number of implant failures and the number of patients with implant failures after a minimum of 3 months after dental implant placement in otherwise healthy individuals receiving dental implants and suggested that the number of implants performed with the use of antibiotic had a significant effect on the survival rate (p = 0.005; RR, 0.35; 95% CI, 0.16 to 0.72), with an NNT of 43 for the number of implants, and the number of patients with implant failure was statistically lower in the antibiotic groups (p = 0.002; RR, 0.33; 95% CI, 0.16 to 0.67), with an NNT of 24 for the number of patients. The current meta-analysis is consistent but included a higher number of total participants and found a smaller NNT for both implant failure analysis by the number of implants and by patient numbers: NNT, 32 and NNT, 14. The research conducted by Braun et al. (2019) assessed antibiotic-associated adverse events and prosthetic failure as a secondary outcome. The results showed no statistically significant difference for any of the other outcome adverse events (RR, 1.00, 95% CI, 0.06 to 15.85) and prosthetic failure (p = 0.08; RR, 0.43; 95% CI, 0.17 to 1.11). The conclusion for the antibiotic-associated adverse events was based on the inclusion of three RCTs, while the current study included five RCTs with three studies reporting no adverse events in either group. Braun included two studies on prosthetic failure in the meta-analysis, while the current meta-analysis combined the total number of prostheses failed due to implant loss and total number of implant failures together. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torof, E.; Morrissey, H.; Ball, P.A. Antibiotic Use in Dental Implant Procedures: A Systematic Review and Meta-Analysis. Medicina 2023, 59, 713. https://doi.org/10.3390/medicina59040713
Torof E, Morrissey H, Ball PA. Antibiotic Use in Dental Implant Procedures: A Systematic Review and Meta-Analysis. Medicina. 2023; 59(4):713. https://doi.org/10.3390/medicina59040713
Chicago/Turabian StyleTorof, Elham, Hana Morrissey, and Patrick A. Ball. 2023. "Antibiotic Use in Dental Implant Procedures: A Systematic Review and Meta-Analysis" Medicina 59, no. 4: 713. https://doi.org/10.3390/medicina59040713
APA StyleTorof, E., Morrissey, H., & Ball, P. A. (2023). Antibiotic Use in Dental Implant Procedures: A Systematic Review and Meta-Analysis. Medicina, 59(4), 713. https://doi.org/10.3390/medicina59040713






