Clinical Application of Intravitreal Aflibercept Injection for Diabetic Macular Edema Comparing Two Loading Regimens
Abstract
1. Introduction
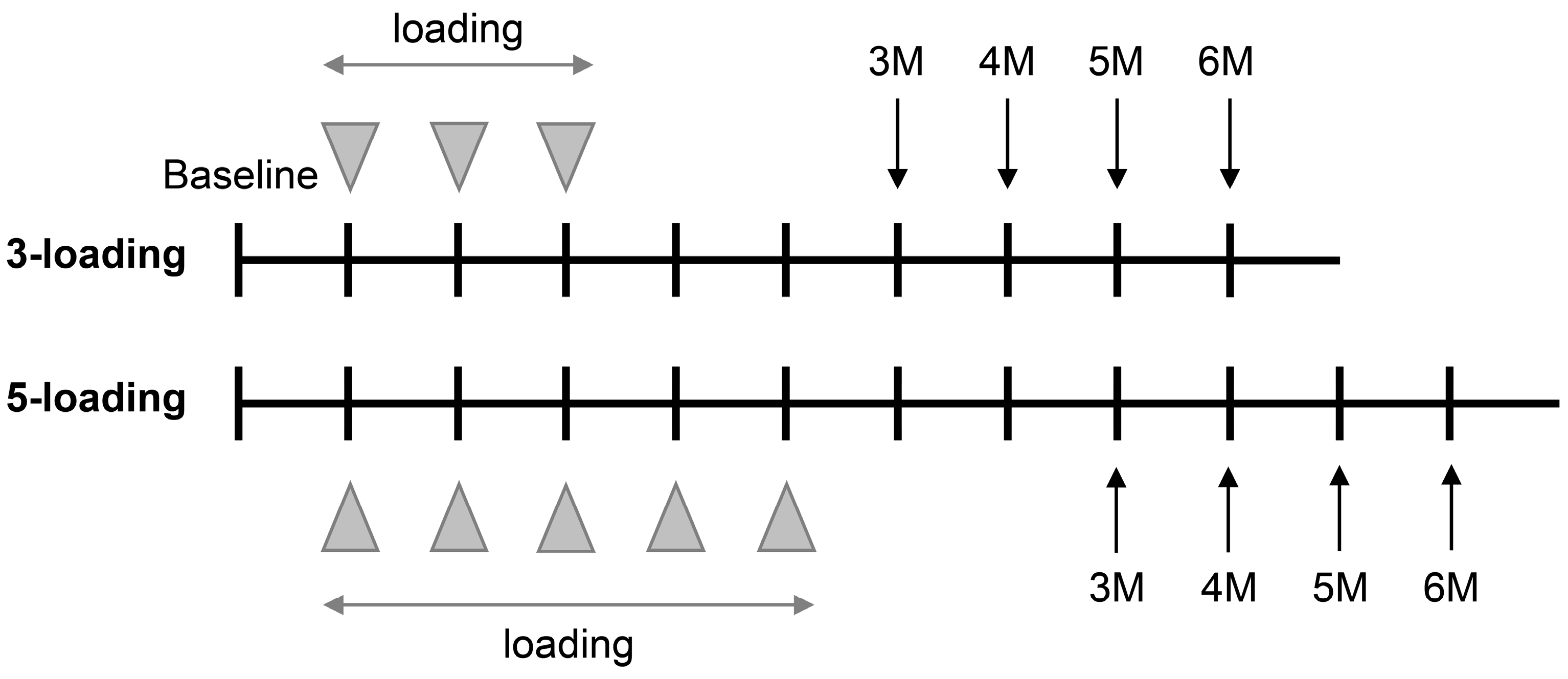
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, Y.R.; Kim, Y.H.; Lee, B.J.; Byeon, H.E.; Lee, K. Proteinuria as a potential biomarker for the efficacy of intravitreal bevacizumab injection in patients with diabetic macular edema. J. Retina 2019, 4, 69–76. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Stewart, M.W.; Browning, D.J.; Landers, M.B. Current management of diabetic tractional retinal detachments. Indian J. Ophthalmol. 2018, 66, 1751–1762. [Google Scholar] [CrossRef]
- Azzolini, C.; Pagani, I.S.; Pirrone, C.; Borroni, D.; Donati, S.; Al Oum, M. Expression of VEGF-A, Otx homeobox and p53 family genes in proliferative vitreoretinopathy. Mediat. Inflamm. 2013, 2013, 857380. [Google Scholar] [CrossRef]
- Virgili, G.; Parravano, M.; Evans, J.R.; Gordon, I.; Lucenteforte, E. Anti-vascular endothelial growth factor for diabetic macular oedema: A network meta-analysis. Cochrane Database Syst. Rev. 2017, 6, Cd007419. [Google Scholar] [CrossRef]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016, 123, 1351–1359. [Google Scholar] [CrossRef]
- Bressler, N.M.; Beaulieu, W.T.; Maguire, M.G.; Glassman, A.R.; Blinder, K.J.; Bressler, S.B. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in Protocol T. Am. J. Ophthalmol. 2018, 195, 93–100. [Google Scholar] [CrossRef]
- Korobelnik, J.F.; Do, D.V.; Schmidt-Erfurth, U.; Boyer, D.S.; Holz, F.G.; Heier, J.S. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014, 121, 2247–2254. [Google Scholar] [CrossRef]
- Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Holz, F.G.; Boyer, D.S.; Midena, E. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015, 122, 2044–2052. [Google Scholar] [CrossRef]
- Heier, J.S.; Korobelnik, J.F.; Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Midena, E. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology 2016, 123, 2376–2385. [Google Scholar] [CrossRef]
- Do, D.V.; Schmidt-Erfurth, U.; Gonzalez, V.H.; Gordon, C.M.; Tolentino, M.; Berliner, A.J. The DA VINCI Study: Phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology 2011, 118, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, T.; Azzolini, C.; Bandello, F.; Boscia, F.; De Falco, S.; Fornasari, D. Aflibercept in the treatment of diabetic macular edema: A review and consensus paper. Eur. J. Ophthalmol. 2017, 27, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Sorour, O.A.; Levine, E.S.; Baumal, C.R.; Elnahry, A.G.; Braun, P.; Girgis, J.; Waheed, N.K. Persistent diabetic macular edema: Definition, incidence, biomarkers, and treatment methods. Surv. Ophthalmol. 2023, 27, 147–174. [Google Scholar] [CrossRef] [PubMed]
- Staurenghi, G.; Sadda, S.; Chakravarthy, U.; Spaide, R.F. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: The IN•OCT consensus. Ophthalmology 2014, 121, 1572–1578. [Google Scholar] [CrossRef]
- Kocur, I.; Resnikoff, S. Visual impairment and blindness in Europe and their prevention. Br. J. Ophthalmol. 2002, 86, 716–722. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Coscas, G. Diagnosis of macular edema. Ophthalmologica 2010, 224 (Suppl. S1), 2–7. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic macular edema pathophysiology: Vasogenic versus inflammatory. J. Diabetes Res. 2016, 2016, 2156273. [Google Scholar] [CrossRef]
- Starr, M.R.; Salabati, M.; Mahmoudzadeh, R.; Patel, L.G.; Ammar, M.J.; Hsu, J. Fluctuations in central subfield thickness associated with worse visual outcomes in patients with diabetic macular edema in clinical trial setting. Am. J. Ophthalmol. 2021, 232, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.Y.; Kuo, B.L.; Chen, A.X.; Wang, K.; Greenlee, T.E.; Conti, T.F.; Singh, R.P. Fluctuations in macular thickness in patients with diabetic macular oedema treated with anti-vascular endothelial growth factor agents. Eye 2022, 36, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Beaulieu, W.T.; Glassman, A.R.; Blinder, K.J.; Bressler, S.B.; Jampol, L.M. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: A secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018, 136, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Li, F.Z.; Liang, S.Z.; Wang, J.; Qian, C.; Wan, G.M. Efficacy of conversion to aflibercept for diabetic macular edema previously refractory to bevacizumab or ranibizumab: A meta-analysis of high-quality nonrandomized studies. Ann. Pharmacother. 2020, 54, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, C.D.; Glassman, A.R.; Ferris, F.L., III; Liu, D.; Maguire, M.G.; Allen, J.B. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N. Engl. J. Med. 2022, 387, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.S.; Sobol, E.K.; Lema, G.M.; Lee, J.G.; Rosen, R.B.; Deobhakta, A. Intravitreal dexamethasone insert in diabetic macular edema super-refractory to anti-vascular endothelial growth factor therapy. Eur. J. Ophthalmol. 2022, 32, NP31–NP47. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Shin, J.P.; Pak, K.Y.; Kim, H.W.; Sagong, M.; Lee, S.J. Two-year outcomes of the treat-and-extend regimen using aflibercept for treating diabetic macular oedema. Sci. Rep. 2020, 10, 22030. [Google Scholar] [CrossRef]
- Garweg, J.G.; Štefanickova, J.; Hoyng, C.; Niesen, T.; Schmelter, T.; Leal, S.; Sivaprasad, S. Dosing regimens of intravitreal aflibercept for diabetic macular edema beyond the first rear: VIOLET, a prospective randomized trial. Adv. Ther. 2022, 39, 2701–2716. [Google Scholar] [CrossRef]
- Hirano, T.; Toriyama, Y.; Takamura, Y.; Sugimoto, M.; Nagaoka, T.; Sugiura, Y. Treat-and-extend therapy with aflibercept for diabetic macular edema: A prospective clinical trial. Jpn. J. Ophthalmol. 2021, 65, 354–362. [Google Scholar] [CrossRef]
- Dascalu, A.M.; Rizzo, M.; Rizvi, A.A.; Stoian, A.P.; Iancu, R.C.; Stana, D. Safety and outcomes of intravitreal aflibercept in diabetic macular edema—A systematic review. Curr. Pharm. Des. 2022, 28, 1758–1768. [Google Scholar]
- Salimi, A.; Vila, N.; Modabber, M.; Kapusta, M. One-year outcomes of Aflibercept for refractory diabetic macular edema in Bevacizumab nonresponders. Indian J. Ophthalmol. 2021, 69, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Handa, C.; Hirano, K.; Sunaya, T.; Kondo, M. Intravitreal aflibercept for diabetic macular edema in real-world clinical practice in Japan: 24-month outcomes. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.; Schiefelbein, J.; Fu, D.J.; Schworm, B.; Sim, D.; Herold, T. Two year visual acuity and structural outcomes in patients with diabetic macular oedema treated with intravitreal aflibercept—A retrospective cohort study. Clin. Ophthalmol. 2020, 14, 533–541. [Google Scholar] [CrossRef]
- Rahimy, E.; Baker, K.; Thompson, D.; Saroj, N. Impact of systemic dipeptidyl peptidase-4 inhibitor use in diabetic macular edema. Ophthalmic Surg. Lasers Imaging Retina 2020, 51, 226–234. [Google Scholar] [CrossRef] [PubMed]

| Variables | 3 Injections | 5 Injections | p Value |
|---|---|---|---|
| No. of eyes | 30 | 14 | |
| Age (years) | 57.8 ± 12.0 | 57.6 ± 11.7 | 0.960 † |
| Sex, male | 18 (60.0%) | 7 (50.0%) | 0.533 * |
| Treatment—naïve | 9 (30.0%) | 2 (14.3%) | 0.262 * |
| Hypertension | 14 (46.7%) | 8 (57.1%) | 0.517 * |
| Systolic BP (mmHg) | 138.7 ± 19.4 | 134.9 ± 16.3 | 0.525 † |
| Diastolic BP (mmHg) | 75.5 ± 16.3 | 79.4 ± 12.8 | 0.330 † |
| Antidiabetic medications | |||
| Biguanides | 22 (73.3%) | 9 (64.3%) | 0.428 * |
| Sulfonylurea | 20 (66.7%) | 9 (64.3%) | 0.759 * |
| SGLT-2 inhibitor | 1 (3.3%) | 3 (21.4%) | 0.094 * |
| DPP-4 inhibitor | 20 (66.7%) | 5 (35.7%) | 0.038 * |
| Thiazolidinedione | 2 (6.7%) | 4 (28.6%) | 0.055 * |
| Insulin | 7 (23.3%) | 4 (28.6%) | 0.755 * |
| DM duration (years) | 14.4 ± 9.8 | 12.1 ± 7.5 | 0.449 † |
| NPDR | 14 (46.7%) | 9 (64.3%) | 0.495 * |
| Chronic kidney disease | 7 (23.3%) | 0 | 0.078 * |
| HbA1c (%) | 7.3 ± 1.3 | 7.7 ± 1.5 | 0.422 † |
| Variables | 3 Injections | 5 Injections | p Value |
| No. of eyes | 30 | 14 | |
| Baseline CRT (μm) | 490.8 ± 123.5 | 461.4 ± 95.1 | 0.437 * |
| CRT at 1 month after loading (μm) | 308.2 ± 89.1 | 320.9 ± 65.2 | 0.635 * |
| p value within group | <0.001 † | <0.001 † | 0.350 ‡ |
| CRT change from baseline (μm) | −182.6 ± 151.4 | −140.5 ± 99.6 | 0.350 * |
| CRT change from baseline (%) | −34.0 ± 21.6 | −28.7 ± 15.4 | 0.832 * |
| Refractory DME | 4 (13.3%) | 0 | 0.152 § |
| No. of focal laser | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.674 * |
| Stable for 3 months | 22 (73.3%) | 14 (100.0%) | 0.033 § |
| Stable for 4 months | 19 (63.3%) | 11 (78.6%) | 0.312 § |
| Stable for 5 months | 17 (56.7%) | 9 (64.3%) | 0.632 § |
| Stable for 6 months | 12 (40.0%) | 5 (35.7%) | 0.786 § |
| Time to recurrence (days) | 116.6 ± 64.6 | 152.2 ± 62.6 | 0.153 * |
| No. of IVA (including loading) | 4.2 ± 1.5 | 6.1 ± 0.9 | <0.001 * |
| No. of additional IVA after initial loading | 1.2 ± 1.5 | 1.1 ± 0.9 | 0.761 * |
| For 3 Months after Loading | For 6 Months after Loading | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.043 | 0.966–1.125 | 0.282 | 0.972 | 0.919–1.027 | 0.304 |
| Sex, male | 1.400 | 0.301–6.505 | 0.668 | 1.702 | 0.488–5.934 | 0.404 |
| Loading, 5 times | 0.999 | 0.833 | 0.224–3.103 | 0.786 | ||
| Treatment—naïve | 1.000 | 0.170–5.866 | 1.000 | 2.400 | 0.598–9.637 | 0.217 |
| Baseline CRT | 0.998 | 0.992–1.004 | 0.539 | 0.994 | 0.987–1.000 | 0.062 |
| Change (%) of CRT after loading | 1.067 | 1.010–1.128 | 0.021 * | 1.015 | 0.983–1.047 | 0.370 |
| Hypertension | 3.750 | 0.665–21.154 | 0.134 | 1.786 | 0.523–6.100 | 0.355 |
| Chronic kidney disease | 1.400 | 0.144–13.568 | 0.772 | 2.476 | 0.476–12.716 | 0.282 |
| Biguanides | 1.040 | 0.173–6.258 | 0.966 | 0.844 | 0.228–3.432 | 0.884 |
| Sulfonylurea | 1.705 | 0.325–8.933 | 0.528 | 0.815 | 0.223–2.982 | 0.757 |
| SGLT-2 inhibitor | 0.999 | 5.357 | 0.508–56.502 | 0.163 | ||
| DPP-4 inhibitor | 0.500 | 0.085–2.926 | 0.442 | 0.471 | 0.135–1.641 | 0.237 |
| Thiazolidinedione | 0.999 | 0.733 | 0.119–4.525 | 0.738 | ||
| Insulin | 0.381 | 0.070–2.066 | 0.263 | 0.835 | 0.203–3.444 | 0.803 |
| DM duration | 1.039 | 0.950–1.137 | 0.402 | 1.004 | 0.938–1.074 | 0.906 |
| PDR | 0.714 | 0.154–3.319 | 0.668 | 1.912 | 0.558–6.554 | 0.302 |
| HbA1c (%) | 1.835 | 0.809–4.162 | 0.146 | 1.121 | 0.682–1.840 | 0.653 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.-R.; Lee, K.H.; Lee, K. Clinical Application of Intravitreal Aflibercept Injection for Diabetic Macular Edema Comparing Two Loading Regimens. Medicina 2023, 59, 558. https://doi.org/10.3390/medicina59030558
Chung Y-R, Lee KH, Lee K. Clinical Application of Intravitreal Aflibercept Injection for Diabetic Macular Edema Comparing Two Loading Regimens. Medicina. 2023; 59(3):558. https://doi.org/10.3390/medicina59030558
Chicago/Turabian StyleChung, Yoo-Ri, Kyung Ho Lee, and Kihwang Lee. 2023. "Clinical Application of Intravitreal Aflibercept Injection for Diabetic Macular Edema Comparing Two Loading Regimens" Medicina 59, no. 3: 558. https://doi.org/10.3390/medicina59030558
APA StyleChung, Y.-R., Lee, K. H., & Lee, K. (2023). Clinical Application of Intravitreal Aflibercept Injection for Diabetic Macular Edema Comparing Two Loading Regimens. Medicina, 59(3), 558. https://doi.org/10.3390/medicina59030558






