Abstract
Background and Objectives: Opioid analgesics, which are used for cancer-related pain management, cause opioid-induced constipation (OIC). Naldemedine, a peripheral opioid receptor antagonist, is an OIC-modifying agent, but no focused efficacy and safety analysis has been conducted for its use in hepatobiliary pancreatic cancers. We performed a multi-institutional study on the efficacy and safety of naldemedine in patients with hepatobiliary pancreatic cancer using opioids in clinical practice. Materials and Methods: We retrospectively evaluated patients with hepatobiliary pancreatic cancer (including liver, biliary tract, and pancreatic cancers) treated with opioids and naldemedine during hospitalization at ten institutions in Japan from June 2017 to August 2019. We assessed the frequency of bowel movements before and after the initiation of naldemedine therapy. Responders were defined as patients who defecated ≥3 times/week, with an increase from a baseline of ≥1 defecations/week over seven days after the initiation of naldemedine administration. Results: Thirty-four patients were observed for one week before and one week after starting naldemedine. The frequency of bowel movements increased by one over the baseline frequency or to at least thrice per week in 21 patients. The response rate was 61.7% (95% confidence interval: 45.4–78.0%). The median number of weekly bowel movements before and after naldemedine treatment was 2 (range: 0–9) and 6 (range: 1–17), respectively, in the overall population (n = 34); the increase in the number of bowel movements following naldemedine administration was statistically significant (Wilcoxon signed-rank test, p < 0.0001). Diarrhea was the predominant gastrointestinal symptom, and 10 (29.4%) patients experienced grade 1, grade 2, or grade 3 adverse events. The only other adverse event included fatigue in one patient; grade 2–4 adverse events were absent. Conclusions: Naldemedine is effective, and its use may be safe in clinical practice for patients with hepatobiliary pancreatic cancer receiving opioid analgesics.
1. Introduction
Hepatobiliary pancreatic cancer includes liver cancer, gallbladder cancer, biliary tract cancer, and pancreatic cancer [1,2,3]. Globally, the number of patients newly diagnosed with hepatobiliary pancreatic cancer in 2018 was approximately 1.85 million, accounting for 10% of all newly diagnosed cancer patients [4]. Hepatobiliary and pancreatic cancers have been associated with a poor prognosis owing to their high level of invasiveness, potential for distant metastasis, and resistance to conventional treatment modalities such as systemic chemotherapy [5,6,7]. Opioids are widely used as analgesic agents that play a central role in cancer pain management [8,9]. Opioids are effective for cancer pain treatment but often cause adverse events that negatively affect the patients’ quality of life; such adverse events frequently lead to the interruption of opioid therapy [10,11]. Opioid-induced constipation (OIC) is one of the most common side effects in patients receiving opioid treatment and is observed in more than 50% of such patients in the absence of preventive measures [12,13]. OIC is a type of functional constipation defined as altered bowel habits and defecation patterns after opioid administration [14]. The incidence varies depending on the type of opioid analgesic and the route of administration, but in general, opioid analgesics have been reported to have a high potential to cause OIC [15,16]. Unlike that for other adverse effects, such as nausea and vomiting, tolerance towards OIC is not likely to improve with the continued use of opioids [17]. Prolonged constipation has been reported to increase the incidence of delirium, nausea, vomiting, appetite loss, and abdominal pain [18]. These unpleasant adverse effects are major barriers to cancer pain management; they not only deteriorate the quality of life of patients but also lead to a decrease in the use of analgesics and rescue therapies [19]. Thus, addressing OIC in patients receiving opioids for the management of cancer-related pain is important. An earlier study reported that the cumulative incidence of OIC in Japanese patients with cancer was 56% in a week, as per the international Rome IV diagnostic criteria for OIC [20]. Furthermore, OIC and a decline in quality of life can occur rapidly in cancer patients after opioid administration [20]. Opioids have various effects on gastrointestinal function, leading to symptoms of dysphagia, nausea and/or vomiting, bloating, constipation, and abdominal pain. Thus, to effectively treat patients who are on chronic opioid therapy, physicians need to be able to identify these gastrointestinal adverse events and treat them appropriately [21]. In addition, opioids are known to cause the contraction of the sphincter of the Oddi muscle, which interferes with the expulsion of bile and pancreatic juice. Therefore, caution must be exercised when using opioids in patients with gallbladder disorders, cholelithiasis, or pancreatitis [21]. Patients with hepatobiliary and pancreatic cancers are expected to have reduced bile output; the contraction of the sphincter of Oddi induced by opioid administration may further reduce the output and decrease bile acid content, rendering the OIC more severe.
The primary mechanism by which opioids exert their analgesic effects is the activation of opioid receptors in the central nervous system. Stimulation of the μ-opioid receptors in the enteric tract inhibits intestinal peristalsis [22]; additionally, opioid-induced intestinal dysfunction develops relatively fast following the initiation of opioid administration [23]. Naldemedine is a peripheral-acting μ-opioid receptor antagonist (PAMORA) that reduces OIC by acting on opioid receptors in the gastrointestinal tract [24]. It recovers the gastrointestinal function by suppressing the effects of opioids on the intrinsic nervous system while preserving their analgesic effects. The phase III randomized trials COMPOSE-4 and COMPOSE-5 reported that naldemedine was efficacious and safe in cancer patients with OIC [25,26]. The most common toxicities reported were diarrhea (19.6%), fatigue (4.1%), appetite loss (3.1%), and vomiting (3.1%); 9.3% of patients were reported to discontinue treatment due to adverse events [25]. However, to date, those studies have been conducted on carefully selected patients with good performance status (PS) scores and adequate organ function, and no data have been reported on the efficacy and tolerability of naldemedine in patients with hepatobiliary pancreatic cancer. Although we previously evaluated the effectiveness and safety of naldemedine in combination with opioids in clinical practice [27,28], we did not adequately investigate its effectiveness in cancer patients with hepatobiliary pancreatic cancer. Furthermore, to date, there have been no reports focusing on the digestive system and hepatobiliary pancreatic cancer. Thus, the analysis of the effectiveness and toxicity of naldemedine for treating OIC in patients with hepatobiliary pancreatic cancers is important and warranted. We performed a multi-institutional retrospective analysis on the effectiveness and toxicity of naldemedine treatment in patients with hepatobiliary pancreatic cancer receiving opioids in clinical practice. Our results showed that naldemedine was efficacious in improving defecation frequency and was safe to use in clinical practice in hepatobiliary pancreatic cancer patients receiving opioid treatment.
2. Materials and Methods
2.1. Patients
This multi-institutional, retrospective study was conducted at ten institutions in Japan on patients with hepatobiliary pancreatic cancer treated with a combination of naldemedine and opioids between 7 June 2017, and 31 August 2019. Eligible patients who initiated naldemedine treatment during the target period were selected from pharmacy databases, and their clinical data were extracted from the electronic medical charts. Patients who met the following four criteria were included in the current analysis:
(i) cytologically or pathologically diagnosed with hepatobiliary pancreatic malignancies; (ii) naldemedine administration started during hospitalization; (iii) naldemedine administrated in combination with opioids; and (iv) hospitalized for at least seven days before and seven days after naldemedine treatment initiation. Data on the number of bowel movements were extracted from the medical records maintained by medical staff. First, we discerned 56 patients with hepatobiliary pancreatic cancer who were administered naldemedine for the first time in combination with an opioid during hospitalization. Of the eligible ones, 22 patients could not be followed for at least seven days before and seven days after the initiation of naldemedine and were therefore excluded. Overall, 34 patients were enrolled in this study (Figure S1). The data of the 34 patients included in this study have been previously published [27,28]. Patient charts were reviewed for data on patient characteristics, response to naldemedine, and adverse events. This study was approved by the Institutional Review Board of each participating institution. All procedures complied with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. Due to the retrospective nature of the study, the requirement for obtaining informed consent was waived, but the opportunity to refuse participation was guaranteed to eligible patients using an opt-out method.
2.2. Treatment
Cancer patients who had previously never received naldemedine were included. The treatment comprised an oral intake of 0.2 mg naldemedine once daily with an opioid. The administration of naldemedine was continued until the development of intolerable adverse events, withdrawal of consent of treatment, or until the physician determined that discontinuation was necessary. The decision to initiate and terminate naldemedine administration was made at the discretion of the attending physician.
2.3. Assessment of Therapeutic Efficacy
Information on the weekly frequency of bowel movements (times/week) for the week prior to and the week following the initiation of naldemedine administration was extracted from the medical records. A responder was defined as a patient with ≥3 bowel movements/week in the first seven days following initiation of naldemedine administration and who exhibited an increase of at least one bowel movement from the baseline value. Baseline defecation frequency was defined as the number of bowel movements during the seven days prior to the initiation of naldemedine administration. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events version 5.0.
2.4. Statistical Analysis
Fisher’s exact test was used to analyze categorical variables. After checking for normality and equal variances, we adopted the Wilcoxon signed-rank test to evaluate the correspondence between the two groups. Statistical analysis was performed with multivariate ordered logistic regression analysis to determine factors that predict treatment effectiveness. The results obtained from the multivariate ordered logistic regression analysis were presented as odds ratios and 95% confidence intervals. Statistical significance was defined based on a two-sided p-value ≤ 0.05. All statistical analyses were performed using the JMP for Windows software, version 12.0 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patient Backgrounds
Of the 34 patients enrolled in this study, 28 had died of the underlying disease by the data-cutoff time. The background characteristics of the patients are listed in Table 1. The median age of the patients in the analysis was 72 years (range, 43–87 years), and 13 (38.2%) patients were aged ≥75 years. Regarding sex, 16 patients were men and 18 were women. Based on the Eastern Cooperative Oncology Group Performance Status (ECOG-PS) criteria, the PS scores were as follows: 11 (32.4%) patients, PS 0 or 1; 7 (20.6%) patients, PS 2; and 16 (47.0%) patients, PS 3 or 4 (considered to be a poor PS). The distribution of cancer types was as follows: 23 (67.7%) patients had pancreatic cancer, 8 (23.5%) had liver cancer, and 3 (8.8%) had cholangiocarcinoma of the gallbladder and bile ducts.

Table 1.
Baseline patient characteristics.
Table 2 presents the data on the usage of opioids, laxatives, and antiemetic drugs in the current study population. The median regular opioid dose in oral morphine equivalents was 30 mg/day (range: 7.5–600 mg). With regard to opioid use, the most commonly administered opioid was oxycodone, which was administered to 20 patients (58.8%), followed by fentanyl, which was administered to 11 patients (32.4%). Regarding the use of laxatives, 24 (70.6%) patients received concomitant laxatives, of which 21 (87.5%) received magnesium oxide; sennoside and lubiprostone were administered to 4 (11.8%) patients each. Furthermore, the median value for days from the first opioid treatment to the initiation of naldemedine administration was five days (range, 1–287 days); 15 (44.1%) patients initiated naldemedine treatment within 14 days of the initiation of opioid administration. With regard to antiemetic medications, 15 patients (44.1%) received concomitant antiemetic medications (regular or occasional use), with prochlorperazine being the most commonly used antiemetic agent (8 patients; 53.3%).

Table 2.
Opioids, laxatives, and antiemetic drugs used in this study.
3.2. Treatment Efficacy and Safety
The frequency of bowel movements was examined for at least seven days before and seven days after the initiation of naldemedine treatment in all 34 included patients. The number of responders and non-responders is shown in Figure 1; 21 (61.7%, 95% CI: 45.4–78.0%) patients were responders, and 13 were non-responders. Table 3 shows the patient background information based on the response. The background characteristics of the responders and non-responders were not significantly different as per Fisher’s exact test.
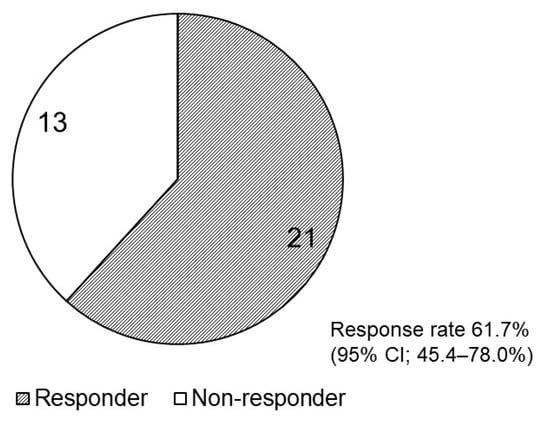
Figure 1.
Pie chart presenting the number of responders and non-responders after naldemedine initiation. CI, confidence interval (CI).

Table 3.
Patient backgrounds based on response.
In addition, changes in the weekly frequency of bowel movements in the week prior to and the week after the start of naldemedine treatment were examined for the following patient cohorts: all 34 patients and those who had less than three bowel movements in the seven days prior to naldemedine administration (Figure 2). In all the eligible patients (n = 34), the median number of bowel movements that occurred in the seven days before and seven days following naldemedine treatment was 2 (range: 0–9) and 6 (range: 1–17), respectively; hence, the number of bowel movements increased significantly following naldemedine initiation (p <0.0001; Figure 2a). Furthermore, we compared the weekly number of bowel movements during the week prior to and the week following naldemedine initiation in patients who had less than three bowel movements in the week prior to naldemedine treatment (n = 19). The median number of bowel movements that occurred during the seven days before and seven days following naldemedine initiation was 1 (range, 0–2) and 3 (range, 0–7), respectively; hence, the number of bowel movements increased significantly following naldemedine initiation (p = 0.0001; Figure 2b).
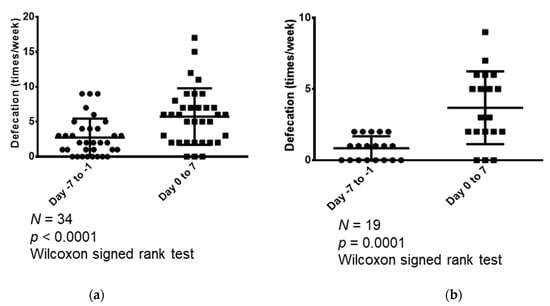
Figure 2.
Comparison of the weekly number of bowel movements in the week (seven days) prior to and the week following naldemedine initiation. (a) Comparison of the number of bowel movements that occurred during the seven days before and seven days following naldemedine initiation in all patients (n = 34); (b) Comparison of the number of bowel movements that occurred during the seven days before and seven days following naldemedine initiation in patients who had less than three bowel movements in the seven days prior to naldemedine administration (n = 19).
As listed in Table 4, diarrhea was the most common adverse event likely associated with naldemedine, which was reported (regardless of grade) in 10 patients (29.4%); of these, nine (90.0%) patients reported grade 1 or 2 diarrhea. None of the patients experienced adverse events of grade 4 or higher.

Table 4.
Toxicities during naldemedine treatment.
3.3. Clinical Factors Influencing Treatment Response
Finally, multivariate logistic regression analysis was performed to assess the relationship between naldemedine efficacy and various clinical factors, as shown in Table 5. There were no statistically significant differences in the efficacy of naldemedine with respect to age, PS, or daily opioid dose in oral morphine equivalents.

Table 5.
Multivariate logistic regression analysis of the clinical factors indicative of response in patients treated with naldemedine.
4. Discussion
In the current analysis, we assessed changes in the number of bowel movements and toxicities after naldemedine initiation in patients with hepatobiliary pancreatic cancer who were treated with opioids and hospitalized for at least seven days before and seven days following initiation of naldemedine administration. Additionally, we examined the effects of various associated factors that may affect treatment response.
In the current study, the response rate was 61.7%, which is comparable to those reported by the COMPOSE-4 study (71%) and another clinical trial on naloxegol (73%), a PAMORA drug similar to naldemedine [25,29]. However, patient characteristics differed between the previous studies and the present study, and thus the results of the studies cannot be compared. Furthermore, in the present study, a significant increase in the number of bowel movements was found following naldemedine administration in the entire study population. In addition, a statistically significant increase in the number of bowel movements occurred in the patients who had fewer than three bowel movements in the week prior to naldemedine treatment. Thus, the present results suggest that naldemedine is effective for hepatobiliary pancreatic cancer patients with OIC. As per our multivariate logistic regression analysis, none of the clinical factors we examined (age, PS, or daily opioid dose in oral morphine equivalents) were significant with reference to the effectiveness of naldemedine. These results are similar to those of previous studies that have reported that the efficacy of naldemedine in patients with OIC is independent of the baseline characteristics of patients [30,31].
In our study, 47.0% of patients exhibited PS of ≥3, whereas the COMPOSE-4 and COMPOSE-5 prospective studies of naldemedine in patients with OIC included cancer patients with PS 0–2 and excluded patients with PS ≥ 3 [25]. Thus, the effectiveness and tolerability of naldemedine in clinical practice have not been assessed in prospective studies. Additionally, there are no prospective clinical trials focused specifically on patients with hepatobiliary pancreatic cancer. Furthermore, although naldemedine is prescribed to many outpatients in real-world clinical settings, the accurate assessment of the number of bowel movements in such patients in practice is difficult; thus, the data in the current study were limited to inpatients. Inpatient data are thought to be more reliable because they are recorded and assessed by healthcare professionals. Hospitalization for at least seven days before and seven days following the initiation of naldemedine treatment was required to gather sufficient data for the comparative analysis. However, the results should be interpreted with caution because the patients may have been hospitalized due to complications, such as poor PS, or because they required concomitant therapy.
In terms of tolerability, diarrhea and abdominal pain were the most frequently occurring toxicities, with incidence rates ranging from 19.6% to 39.7% and 1.7%, respectively. This is in agreement with the results from other prospective clinical studies in cancer patients with OIC [25,32]. The occurrence of diarrhea and abdominal pain in the current analysis population was 29.4% and 0%, respectively, almost equal to that in the randomized phase III study [25,32]. Despite the fact that the study population comprised patients with PS 3–4 and those aged 75 years and over, only one serious adverse event (2.9%, grade 3 diarrhea) was observed, suggesting that naldemedine treatment was well tolerated in hepatobiliary pancreatic cancer patients in clinical practice.
A study on a variety of malignancies, including gastrointestinal cancers and other abdominal organs, has shown results comparable to those of the COMPOSE-4 and -5 phase III studies with regard to the effectiveness and toxicities of naldemedine [25]. However, patient selection bias was inherent in the cited study as only naldemedine-eligible patients without gastrointestinal obstruction who were able to receive naldemedine orally were enrolled. This study included patients with hepatobiliary pancreatic cancer in clinical practice; however, unlike the patients enrolled in the clinical trial, not all patients had a good general condition or were complication-free, but naldemedine administration was possible because they were able to take it orally. In terms of age, almost one-third of cancers are diagnosed in patients aged 70 years or over; this poses additional challenges with respect to the optimal therapeutic approach and prognosis in this patient population [33]. In general, older cancer patients exhibit a higher number of comorbidities and reduced organ function compared to younger cancer patients; therefore, treatment-related adverse events in geriatric patients is a noteworthy concern. While geriatric patients are usually excluded from randomized phase III studies, COMPOSE-4 and -5 included patients aged 20 years and older, and no upper age limit was stipulated [25]. In addition, a subgroup analysis of a phase III study in patients with chronic non-cancer-related pain demonstrated that naldemedine treatment was generally effective and tolerable, even in patients aged 65 years and older [34]. Similar to this analysis, the current study found no statistically significant difference in the effectiveness of naldemedine in patient groups older and younger than 75 years, showing that naldemedine may be effective in treating elderly patients with hepatobiliary pancreatic cancer. Opioids affect the flow of bile in the bile ducts, and the current evidence suggests that increasing the frequency of contraction of the sphincter of Oddi reduces the filling of the peristaltic segments of the bile ducts and decreases the emptying of the bile ducts [35]. The mechanism appears to involve the suppression of inhibitory motoneurons controlling the sphincter muscle. Opioids also decrease the contractility of the gallbladder. In a previous animal study, opioid agonists inhibited excitatory neurotransmission at the presynaptic and neuromuscular terminals [36]; moreover, a human study reported a decrease in biliary ejection fraction in 76% of patients who received opioids followed by cholecystokinin [37], potentially predisposing the patients to gallbladder stasis. The latter study also reported an increased flow of hepatic bile toward the gallbladder rather than the small intestine (such as that in a fasting state), which may be due to the effect of opioid administration on the sphincter of Oddi [37]. In patients with hepatobiliary pancreatic cancer, the ability to expel bile is expected to be reduced, and the contraction of the sphincter of Oddi induced by opioid administration may further reduce bile acid expulsion [21]; this may increase the severity of OIC. It is therefore important to manage OIC adequately in these patients. Although there have been no reports on the effectiveness and tolerability of naldemedine in gastrointestinal cancers, we previously reported on the effectiveness and tolerability of naldemedine in thoracic cancers [38]. The results showed that 65.0% of patients were responders, and the safety profile was comparable to that described in the present study in hepatobiliary pancreatic cancer; diarrhea occurred in 27.5% of the patients. The above-mentioned results imply that no special considerations need to be taken into account for patients with hepatobiliary pancreatic cancer with reference to the efficacy and adverse events of OIC treatment; such treatment can be applied in the same manner as in other malignancies.
Several limitations exist in the current study. First, the population analyzed in this study is small. However, the study has clinical significance because the inclusion criteria specified hospitalized hepatobiliary pancreatic cancer patients whose number of bowel movements was closely followed and evaluated by healthcare professionals for at least seven days before and seven days following naldemedine administration. Second, owing to the retrospective nature of the study, objective evaluations, such as the Bristol stool form scale [39], bowel function index [40], and defecation diary, were not applicable. This study limitation may affect the validity of our results. Third, the decision to start, skip, or discontinue naldemedine administration and the use of various laxatives was at the discretion of each attending physician, which may have introduced discrepancies due to subjectivity. Fourth, owing to the nature of this retrospective analysis, standardizing the effects of other concomitant medications was not possible.
5. Conclusions
In summary, the results of the current study indicate that naldemedine treatment for hepatobiliary pancreatic cancer patients is effective and well tolerated in clinical practice; this drug can be administered to elderly patients and patients with poor PS. Overall, naldemedine is effective and well tolerated for OIC in most hepatobiliary pancreatic cancer patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina59030492/s1, Figure S1: Flow chart showing patient selection.
Author Contributions
Conceptualization and methodology, H.I. and T.K.; formal analysis and data curation, H.I. and K.K.; project administration, visualization, and writing—original draft preparation, H.I. and T.K.; supervision, S.K., T.S., K.O., K.K. and K.M.; investigation and resources, T.K., Y.F., H.I., E.H., T.M., S.Y., H.T., M.S., S.T., K.A., H.N. and J.M.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of GUNMA PREFECTURAL CANCER CENTER (No. 405-31046; approval date: 27 September 2019).
Informed Consent Statement
The requirement for written informed consent was waived due to the retrospective nature of this study. However, the opportunity to refuse to participate through an opt-out method was guaranteed.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available.
Acknowledgments
We thank Hiromi Sakamoto, Tomoko Nakajima, and Mie Kotake for their assistance in preparing this manuscript.
Conflicts of Interest
The authors declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; in the decision to publish the results.
References
- Cazacu, I.M.; Singh, B.S.; Saftoiu, A.; Bhutani, M.S. Recent developments in hepatopancreatobiliary EUS. Endosc. Ultrasound 2019, 8, 146–150. [Google Scholar] [CrossRef]
- Kovacevic, B.; Karstensen, J.G.; Havre, R.F.; Pham, K.D.; Giovannini, M.; Dabizzi, E.; Arcidiacono, P.; Santo, E.; Sequeiros, E.V.; Klausen, P.; et al. Initial experience with EUS-guided microbiopsy forceps in diagnosing pancreatic cystic lesions: A multicenter feasibility study (with video). Endosc. Ultrasound 2018, 7, 383–388. [Google Scholar] [CrossRef]
- Saftoiu, A.; Bhutani, M.S.; Itoi, T.; Arcidiacono, P.G.; Bories, E.; Cazacu, I.M.; Constantin, A.; Coronel, E.; Dietrich, C.F.; Duda, D.G.; et al. Changes in tumor vascularity depicted by contrast-enhanced EUS as a predictor of prognosis and treatment efficacy in patients with unresectable pancreatic cancer (PEACE): A study protocol. Endosc. Ultrasound 2019, 8, 235–240. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Llovet, J.M.; Zucman-Rossi, J.; Pirkarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gorges, G. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Bianki, A.V.; Neale, R.E.; Temperao, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef]
- Fallon, M.; Guisti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I.; ESMO Guidelines Committee. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv166–iv191. [Google Scholar] [CrossRef]
- Jara, C.; Del Barco, S.; Gravalos, C.; Hoyos, S.; Hernandez, B.; Munoz, M.; Quintanar, T.; Meana, J.A.; Rodriguez, C.; de Las Penas, R. SEOM clinical guideline for treatment of cancer pain (2017). Clin. Transl. Oncol. 2018, 20, 97–107. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Wee, B.; Moore, R.A. Oral morphine for cancer pain. Cochrane Database Syst. Rev. 2016, 4, CD003868. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Wee, B.; Derry, S.; Bell, R.F.; Moore, A.R. Opioids for cancer pain—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2017, 7, CD012592. [Google Scholar] [CrossRef]
- Bell, T.J.; Panchal, S.J.; Miaskowski, C.; Bolge, S.C.; Milanova, T.; Williamson, R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med. 2009, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Ikesue, H.; Matsunaga, H.; Suemaru, K.; Kitaichi, K.; Suetsugu, K.; Oishi, R.; Sendo, T.; Araki, H.; Itoh, Y.; et al. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin. J. Pain 2012, 28, 373–381. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Derry, S.; Moore, R.A. Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain. Cochrane Database Syst. Rev. 2014, 5, CD011056. [Google Scholar] [CrossRef]
- Laugsand, E.A.; Kaasa, S.; Klepstad, P. Management of opioid-induced nausea and vomiting in cancer patients: Systematic review and evidence-based recommendations. Palliat. Med. 2011, 25, 442–453. [Google Scholar] [CrossRef]
- Coyne, K.S.; Margolis, M.K.; Yeomans, K.; King, F.R.; Chavoshi, S.; Payne, K.A.; LoCasale, R.J. Opioid-induced constipation among patients with chronic noncancer pain in the United States, Canada, Germany, and the United Kingdom: Laxative use, response, and symptom burden over time. Pain Med. 2015, 16, 1551–1565. [Google Scholar] [CrossRef]
- Smonig, R.; Wallenhorst, T.; Bouju, P.; Letheulle, J.; Le Tulzo, Y.; Tadié, J.M.; Gacouin, A. Constipation is independently associated with delirium in critically ill ventilated patients. Intensive Care Med. 2016, 42, 126–127. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, H.; Scopel, J.; Mody, R.R. Impact of constipation on opioid therapy management among long-term opioid users, based on a patient survey. J. Opioid Manag. 2015, 11, 325–338. [Google Scholar] [CrossRef]
- Tokoro, A.; Imai, H.; Fumita, S.; Harada, T.; Noriyuki, T.; Gamoh, M.; Akashi, Y.; Sat, H.; Kizawa, Y. Incidence of opioid-induced constipation in Japanese patients with cancer pain: A prospective observational cohort study. Cancer Med. 2019, 8, 4883–4891. [Google Scholar] [CrossRef]
- Nee, J.; Rangan, V.; Lembo, A. Reduction in pain: Is it worth the gain? The effect of opioids on the GI tract. Neurogastroenterol. Motil. 2018, 30, e13367. [Google Scholar] [CrossRef]
- Poulsen, J.L.; Brock, C.; Olesen, A.E.; Nilsson, M.; Drewes, A.M. Clinical potential of naloxegol in the management of opioid-induced bowel dysfunction. Clin. Exp. Gastroenterol. 2014, 7, 345–358. [Google Scholar] [CrossRef]
- Nilsson, M.; Poulsen, J.L.; Brock, C.; Sandberg, T.H.; Gram, M.; Frøkjær, J.B.; Krogh, K.; Drewes, A.M. Opioid-induced bowel dysfunction in healthy volunteers assessed with questionnaires and MRI. Eur. J. Gastroenterol. Hepatol. 2016, 28, 514–524. [Google Scholar] [CrossRef]
- Blair, H.A. Naldemedine: A review in opioid-induced constipation. Drugs 2019, 79, 1241–1247. [Google Scholar] [CrossRef]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized phase III and extension studies of naldemedine in patients with opioid-induced constipation and cancer. J. Clin. Oncol. 2017, 35, 3859–3866. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Harada, T.; Murata, T.; Shinozaki, K.; Tsutsumi, M.; Yokota, T.; Arai, M.; Tada, Y.; Narabayashi, M.; Boku, N. Randomized phase III and extension studies: Efficacy and impacts on quality of life of naldemedine in subjects with opioid-induced constipation and cancer. Ann. Oncol. 2018, 29, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, E.; Fujita, Y.; Imai, H.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Ito, M.; Takei, S.; Matsuura, M.; et al. Real-world patient characteristics and treatment patterns of naldemedine for the treatment of opioid-induced constipation in patients with cancer: A multicenter retrospective chart review study. Medicina 2021, 57, 1233. [Google Scholar] [CrossRef] [PubMed]
- Nishiba, H.; Imai, H.; Fujita, Y.; Hiruta, E.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Ito, M.; Takei, S.; et al. Efficacy and safety of naldemedine for patients with cancer with opioid-induced constipation in clinical practice: A real-world retrospective study. J. Clin. Med. 2022, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, A.; Pointreau, Y.; Narciso, B.; Piloquet, F.-X.; Braniste, V.; Sabaté, J.M. Effectiveness of naloxegol in patients with cancer pain suffering from opioid-induced constipation. Support. Care Cancer 2021, 29, 7577–7586. [Google Scholar] [CrossRef]
- Kubota, R.; Fukumura, K.; Wajima, T. Population pharmacokinetics and exposure-response relationships of naldemedine. Pharm. Res. 2018, 35, 225. [Google Scholar] [CrossRef]
- Osaka, I.; Ishiki, H.; Yokota, T.; Tada, Y.; Sato, H.; Okamoto, M.; Satomi, E. Safety and efficacy of naldemedine in cancer patients with opioid-induced constipation: A pooled, subgroup analysis of two randomised controlled studies. ESMO Open 2019, 4, e000527. [Google Scholar] [CrossRef]
- Katakami, N.; Oda, K.; Tauchi, K.; Nakata, K.; Shinozaki, K.; Yokota, T.; Suzuki, Y.; Narabayashi, M.; Boku, N. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J. Clin. Oncol. 2017, 35, 1921–1928. [Google Scholar] [CrossRef]
- Venuta, F.; Diso, D.; Onorati, I.; Anile, M.; Mantovani, S.; Rendina, E.A. Lung cancer in elderly patients. J. Thorac. Dis. 2016, 8 (Suppl. S11), S908–S914. [Google Scholar] [CrossRef]
- Wild, J.; Webster, L.; Yamada, T.; Hale, M. Safety and efficacy of naldemedine for the treatment of opioid-induced constipation in patients with chronic non-cancer pain receiving opioid therapy: A subgroup analysis of patients >/= 65 years of age. Drugs Aging 2020, 37, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.R. Narcotic analgesic effects on the sphincter of Oddi: A review of the data and therapeutic implications in treating pancreatitis. Am. J. Gastroenterol. 2001, 96, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Guarraci, F.A.; Pozo, M.J.; Palomares, S.M.; Firth, T.A.; Mawe, G.M. Opioid agonists inhibit excitatory neurotransmission in ganglia and at the neuromus3cular junction in Guinea pig gallbladder. Gastroenterology 2002, 122, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Krishnamurthy, G.T. Effect of sequential administration of an opioid and cholecystokinin on gallbladder ejection fraction: Brief communication. J. Nucl. Med. 2006, 47, 1463–1466. [Google Scholar]
- Imai, H.; Fujita, Y.; Hiruta, E.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Kamiya, T.; Sandoh, M.; Takei, S.; Arai, K.; et al. A retrospective study of the efficacy and safety of naldemedine for opioid-induced constipation in thoracic cancer patients. Thorac. Cancer 2022, 13, 2301–2308. [Google Scholar] [CrossRef]
- Müller-Lissner, S.; Bassotti, G.; Coffin, B.; Drewes, A.M.; Breivik, H.; Eisenberg, E.L.; Emmanuel, A.; Laroche, F.; Meissner, W.; Morlion, B. Opioid-induced constipation and bowel dysfunction: A clinical guideline. Pain Med. 2017, 18, 1837–1863. [Google Scholar] [CrossRef]
- Rentz, A.M.; Müller-Lissner, S.; Leyendecker, P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J. Med. Econ. 2009, 12, 371–383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).