Posture-Induced Intraocular Pressure Changes after iStent Inject W Combined with Phacoemulsification in Open Angle Glaucoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. iStent Inject W Device and Surgical Procedures
2.3. Data Collection
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Patient Demographic and Clinical Characteristics
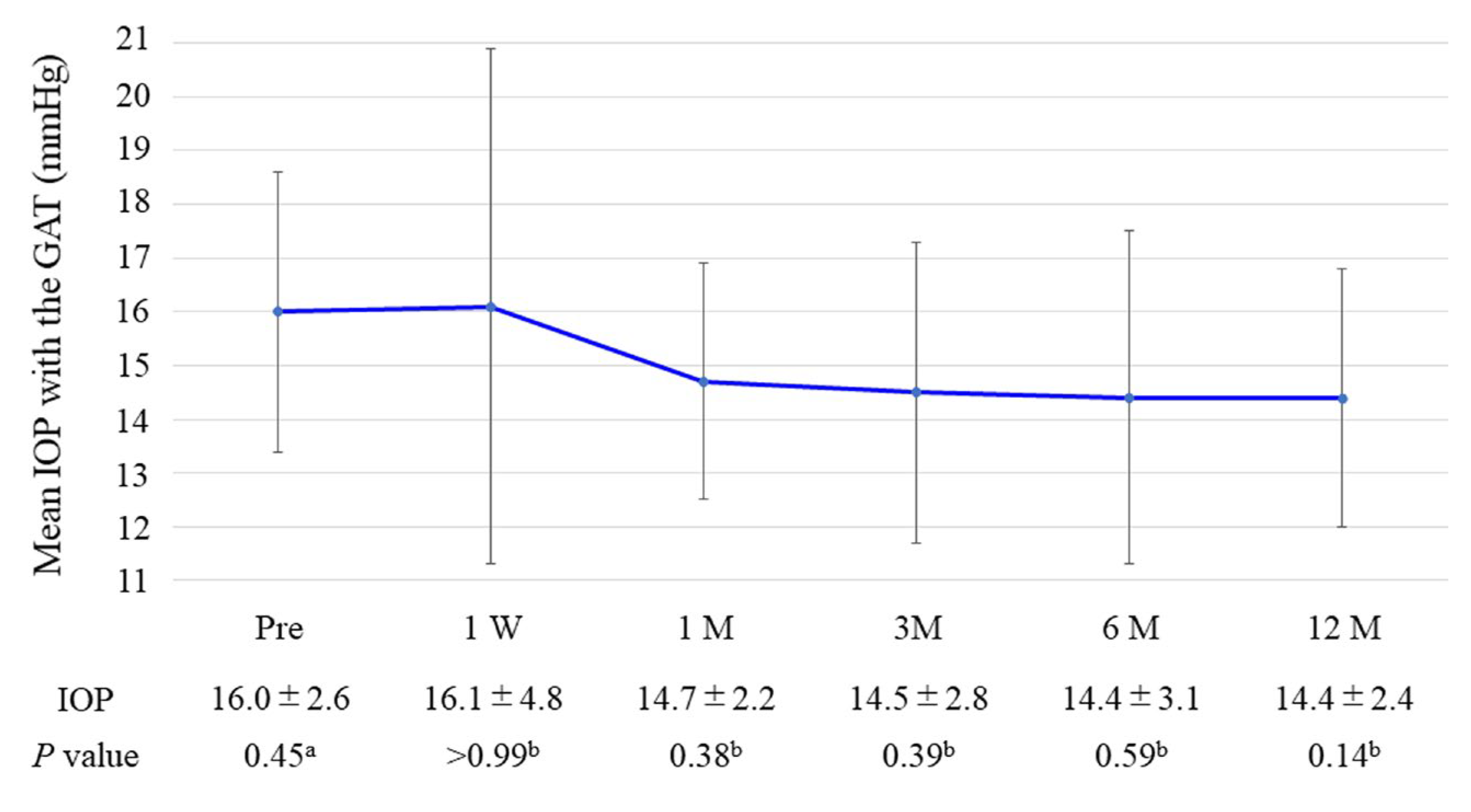
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, D.R.; Grant, W.M. The influence of position on intraocular pressure. Investig. Ophthalmol. 1973, 12, 204–212. Available online: https://pubmed.ncbi.nlm.nih.gov/4692261/ (accessed on 16 November 1972).
- Prata, T.S.; De Moraes, C.G.V.; Kanadani, F.N.; Ritch, R.; Paranhos, A. Posture-induced intraocular pressure changes: Considerations regarding body position in glaucoma patients. Surv. Ophthalmol. 2010, 55, 445–453. [Google Scholar] [CrossRef]
- Sawada, A.; Yamamoto, T. Posture-induced intraocular pressure changes in eyes with open-angle glaucoma, primary angle closure with or without glaucoma medications, and control eyes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7631–7635. [Google Scholar] [CrossRef]
- Hirooka, K.; Shiraga, F. Relationship between postural change of the intraocular pressure and visual field loss in primary open-angle glaucoma. J. Glaucoma 2003, 12, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, T.; Motoyama, Y.; Oshika, T. Relationship of progression of visual field damage to postural changes in intraocular pressure in patients with normal-tension glaucoma. Ophthalmology 2006, 113, 2150–2155. [Google Scholar] [CrossRef] [PubMed]
- Mizokami, J.; Yamada, Y.; Negi, A.; Nakamura, M. Postural changes in intraocular pressure are associated with asymmetrical retinal nerve fiber thinning in treated patients with primary open-angle glaucoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2011, 249, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Sawada, A.; Yamamoto, T. Localization in glaucomatous visual field loss vulnerable to posture-induced intraocular pressure changes in open-angle glaucoma. Am. J. Ophthalmol. 2020, 213, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, T.; Motoyama, Y.; Oshika, T. Influence of ocular hypotensive eyedrops on intraocular pressure fluctuation with postural change in eyes with normal-tension glaucoma. Am. J. Ophthalmol. 2007, 143, 693–695. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, B. Postural behaviour of intraocular pressure following trabeculoplasty. Int. Ophthalmol. 1992, 16, 163–166. [Google Scholar] [CrossRef]
- Cairns, J.E. Trabeculectomy: Preliminary report of a new method. Am. J. Ophthalmol. 1968, 66, 673–679. [Google Scholar] [CrossRef]
- Hirooka, K.; Takenaka, H.; Baba, T.; Takagishi, M.; Mizote, M.; Shiraga, F. Effect of trabeculectomy on intraocular pressure fluctuation with postural change in eyes with open-angle glaucoma. J. Glaucoma 2009, 18, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Weizer, J.S.; Goyal, A.; Ple-Plakon, P.; Trzcinka, A.; Strong, B.D.; Bruno, C.A.; Junn, J.; Tseng, I.; Niziol, L.M.; Musch, D.C.; et al. Bleb morphology characteristics and effect on positional intraocular pressure variation. Ophthalmic Surg. Lasers Imaging 2010, 41, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Yamamoto, T. Effects of trabeculectomy on posture-induced intraocular pressure changes over time. Graefes. Arch. Clin. Exp. Ophthalmol. 2012, 250, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Arimura, S.; Takamura, Y.; Inatani, M. Clinical practice preferences for glaucoma surgery in Japan: A survey of Japan Glaucoma Society specialists. Jpn. J. Ophthalmol. 2020, 64, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.V.; Corcoran, K.J.; Lee, A.Y. Changes in performance of glaucoma surgeries 1994 through 2017 based on claims and payment data for United States medicare beneficiaries. Ophthalmol. Glaucoma 2021, 4, 463–471. [Google Scholar] [CrossRef]
- Fujita, A.; Hashimoto, Y.; Matsui, H.; Yasunaga, H.; Aihara, M. Recent trends in glaucoma surgery: A nationwide database study in Japan, 2011–2019. Jpn. J. Ophthalmol. 2022, 66, 183–192. [Google Scholar] [CrossRef]
- Tanihara, H.; Negi, A.; Akimoto, M.; Terauchi, H.; Okudaira, A.; Kozaki, J.; Takeuchi, A.; Nagata, M. Surgical effects of trabeculotomy ab externo on adult eyes with primary open angle glaucoma and pseudoexfoliation syndrome. Arch. Ophthalmol. 1993, 111, 1653–1661. [Google Scholar] [CrossRef]
- Honjo, M.; Tanihara, H.; Negi, A.; Hangai, M.; Taniguchi, T.; Honda, Y.; Mizoguchi, T.; Matsumura, M.; Nagata, M. Trabeculotomy ab externo, cataract extraction, and intraocular lens implantation: Preliminary report: Preliminary report. J. Cataract. Refract. Surg. 1996, 22, 601–606. [Google Scholar] [CrossRef]
- Tanito, M.; Ohira, A.; Chihara, E. Surgical outcome of combined trabeculotomy and cataract surgery. J. Glaucoma 2001, 10, 302–308. [Google Scholar] [CrossRef]
- Bahler, C.K.; Hann, C.R.; Fjield, T.; Haffner, D.; Heitzmann, H.; Fautsch, M.P. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am. J. Ophthalmol. 2012, 153, 1206–1213. [Google Scholar] [CrossRef]
- Pereira, I.C.F.; van de Wijdeven, R.; Wyss, H.M.; Beckers, H.J.M.; den Toonder, J.M.J. Conventional glaucoma implants and the new MIGS devices: A comprehensive review of current options and future directions. Eye 2021, 35, 3202–3221. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, T.W.; Sarkisian, S.R.; Lubeck, D.M.; Stiles, M.C.; Duh, Y.J.; Romo, E.A.; Giamporcaro, J.E.; Hornbeak, D.M.; Katz, L.J.; iStent inject Study Group. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: Two-year results. Ophthalmology 2019, 126, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Gillmann, K.; Mansouri, K.; Ambresin, A.; Bravetti, G.E.; Mermoud, A. A prospective analysis of iStent inject microstent implantation: Surgical outcomes, endothelial cell density, and device position at 12 months. J. Glaucoma 2020, 29, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Lapointe, J.; Harasymowycz, P. One-year outcomes of second-generation trabecular micro-bypass stents (iStent inject) implantation with cataract surgery in different glaucoma subtypes and severities. Ophthalmol. Ther. 2019, 8, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Hengerer, F.H.; Auffarth, G.U.; Conrad-Hengerer, I. iStent inject Trabecular Micro-Bypass with or without Cataract Surgery Yields Sustained 5-year Glaucoma Control. Adv. Ther. 2022, 39, 1417–1431. [Google Scholar] [CrossRef]
- Guedes, R.A.P.; Gravina, D.M.; Lake, J.C.; Guedes, V.M.P.; Chaoubah, A. One-year Comparative Evaluation of iStent or iStent inject Implantation Combined with Cataract Surgery in a Single Center. Adv. Ther. 2019, 36, 2797–2810. [Google Scholar] [CrossRef]
- Manning, D. Real-world case series of iStent or iStent inject trabecular micro-bypass stents combined with cataract surgery. Ophthalmol. Ther. 2019, 8, 549–561. [Google Scholar] [CrossRef]
- Clement, C.I.; Howes, F.; Ioannidis, A.S.; Shiu, M.; Manning, D. One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: Multicenter, multi-surgeon study. Clin. Ophthalmol. 2019, 13, 491–499. [Google Scholar] [CrossRef]
- Sachdeva, R.; Iordanous, Y.; Lin, T. Comparison of intraocular pressure measured by iCare tonometers and Goldmann applanation tonometer. Can. J. Ophthalmol. 2022; in press. [Google Scholar] [CrossRef]
- Kato, Y.; Nakakura, S.; Matsuo, N.; Yoshitomi, K.; Handa, M.; Tabuchi, H.; Kiuchi, Y. Agreement among Goldmann applanation tonometer, iCare, and Icare PRO rebound tonometers; non-contact tonometer; and Tonopen XL in healthy elderly subjects. Int. Ophthalmol. 2018, 38, 687–696. [Google Scholar] [CrossRef]
- Güler, M.; Bilak, Ş.; Bilgin, B.; Şimşek, A.; Çapkin, M.; Hakim Reyhan, A. Comparison of intraocular pressure measurements obtained by Icare PRO rebound tonometer, Tomey FT-1000 noncontact tonometer, and Goldmann applanation tonometer in healthy subjects. J. Glaucoma 2015, 24, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, S.; Asaoka, R.; Terao, E.; Nagata, Y.; Fukuma, Y.; Oogi, S.; Shiraishi, M.; Kiuchi, Y. Evaluation of rebound tonometer iCare IC200 as compared with IcarePRO and Goldmann applanation tonometer in patients with glaucoma. Eye Vis. 2021, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Martinez-De-La-Casa, J.M.; Garcia-Feijoo, J.; Castillo, A.; Garcia-Sanchez, J. Reproducibility and clinical evaluation of rebound tonometry. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4578–4580. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.N.; Bartlett, H.; Mallen, E.A.H.; Wolffsohn, J.S. Clinical evaluation of rebound tonometer. Acta Ophthalmol. Scand. 2006, 84, 206–209. [Google Scholar] [CrossRef]
- Munkwitz, S.; Elkarmouty, A.; Hoffmann, E.M.; Pfeiffer, N.; Thieme, H. Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer over a wide IOP range. Graefes. Arch. Clin. Exp. Ophthalmol. 2008, 246, 875–879. [Google Scholar] [CrossRef]
- Jóhannesson, G.; Hallberg, P.; Eklund, A.; Lindén, C.; Pascal, I. Pascal, ICare and Goldmann applanation tonometry—A comparative study. Acta Ophthalmol. 2008, 86, 614–621. [Google Scholar] [CrossRef]
- Schweier, C.; Hanson, J.V.M.; Funk, J.; Töteberg-Harms, M. Repeatability of intraocular pressure measurements with Icare PRO rebound, Tono-Pen AVIA, and Goldmann tonometers in sitting and reclining positions. BMC Ophthalmol. 2013, 13, 44. [Google Scholar] [CrossRef]
- Badakere, S.V.; Chary, R.; Choudhari, N.S.; Rao, H.L.; Garudadri, C.; Senthil, S. Agreement of intraocular pressure measurement of Icare ic200 with Goldmann applanation tonometer in adult eyes with normal cornea. Ophthalmol. Glaucoma 2021, 4, 89–94. [Google Scholar] [CrossRef]
- Dielemans, I.; Vingerling, J.R.; Hofman, A.; Grobbee, D.E.; de Jong, P.T.V.M. Reliability of intraocular pressure measurement with the Goldmann applanation tonometer in epidemiological studies. Graefes. Arch. Clin. Exp. Ophthalmol. 1994, 232, 141–144. [Google Scholar] [CrossRef]
- Baek, S.U.; Ha, A.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Effect of manual eyelid manipulation on intraocular pressure measurement by rebound tonometry. Br. J. Ophthalmol. 2018, 102, 1515–1519. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, J.Y.; Moon, J.I.; Park, M.H. Effectiveness of the Icare rebound tonometer in patients with overestimated intraocular pressure due to tight orbit syndrome. Jpn. J. Ophthalmol. 2014, 58, 496–502. [Google Scholar] [CrossRef]
- Gurnani, B.; Tripathy, K. Minimally invasive glaucoma surgery. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/35881761/ (accessed on 13 December 2022).
- Iwasaki, K.; Takamura, Y.; Orii, Y.; Arimura, S.; Inatani, M. Performances of glaucoma operations with Kahook dual Blade or iStent combined with phacoemulsification in Japanese open angle glaucoma patients. Int. J. Ophthalmol. 2020, 13, 941–945. [Google Scholar] [CrossRef] [PubMed]
- ElMallah, M.K.; Seibold, L.K.; Kahook, M.Y.; Williamson, B.K.; Singh, I.P.; Dorairaj, S.K.; KDB Goniotomy Study Group. 12-month retrospective comparison of Kahook dual blade excisional goniotomy with istent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv. Ther. 2019, 36, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Arnljots, T.S.; Economou, M.A. Kahook dual Blade goniotomy vs iStent inject: Long-Term Results in Patients with Open-Angle Glaucoma. Clin. Ophthalmol. 2021, 15, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Slabaugh, M.A.; Chen, P.P. The effect of cataract extraction on intraocular pressure. Curr. Opin. Ophthalmol. 2014, 25, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Young, C.E.C.; Seibold, L.K.; Kahook, M.Y. Cataract surgery and intraocular pressure in glaucoma. Curr. Opin. Ophthalmol. 2020, 31, 15–22. [Google Scholar] [CrossRef]
- Inatani, M.; Kohama, I.; Chu, A. iStent trabecular micro-bypass stent implantation combined with phacoemulsification for open-angle glaucoma: A 2-year post-marketing surveillance study in Japan. Adv. Ther. 2022, 39, 4076–4093. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Yamada, Y.; Morokado, S.; Sugiyama, K. iStent trabecular micro-bypass stent implantation with cataract surgery in a Japanese glaucoma population. Clin. Ophthalmol. 2020, 14, 3381–3391. [Google Scholar] [CrossRef]
- Storr-Paulsen, A.; Norregaard, J.C.; Ahmed, S.; Storr-Paulsen, T.; Pedersen, T.H. Endothelial cell damage after cataract surgery: Divide-and-conquer versus phaco-chop technique. J. Cataract. Refract. Surg. 2008, 34, 996–1000. [Google Scholar] [CrossRef]
- Lee, T.E.; Yoo, C.; Kim, Y.Y. Effects of different sleeping postures on intraocular pressure and ocular perfusion pressure in healthy young subjects. Ophthalmology 2013, 120, 1565–1570. [Google Scholar] [CrossRef]
- Jonas, J.B.; Ritch, R.; Panda-Jonas, S. Cerebrospinal fluid pressure in the pathogenesis of glaucoma. Prog. Brain Res. 2015, 221, 33–47. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total (n = 15) |
|---|---|
| Age (years) | 73.1 ± 6.3 |
| Sex, n (%) | |
| Male | 4 (27) |
| Female | 11 (73) |
| Glaucoma type, n (%) | |
| Primary open-angle glaucoma | 12 (80) |
| Exfoliation glaucoma | 3 (20) |
| IOP with GAT (mmHg) | 16.0 ± 2.6 |
| Number of glaucoma medications, n | 2.5 ± 1.2 |
| BCVA (logMAR) | 0.45 ± 0.3 |
| Central corneal ECD (cell/mm2) | 2525 ± 321 |
| Visual field MD (dB) | −9.8 ± 4.6 |
| Complication | n (%) |
|---|---|
| Hyphema | 0 (0) |
| IOP spikes | 0 (0) |
| One iStent occlusion by iris | 2 (13) |
| Additional glaucoma surgery | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, K.; Arimura, S.; Orii, Y.; Inatani, M. Posture-Induced Intraocular Pressure Changes after iStent Inject W Combined with Phacoemulsification in Open Angle Glaucoma Patients. Medicina 2023, 59, 423. https://doi.org/10.3390/medicina59030423
Iwasaki K, Arimura S, Orii Y, Inatani M. Posture-Induced Intraocular Pressure Changes after iStent Inject W Combined with Phacoemulsification in Open Angle Glaucoma Patients. Medicina. 2023; 59(3):423. https://doi.org/10.3390/medicina59030423
Chicago/Turabian StyleIwasaki, Kentaro, Shogo Arimura, Yusuke Orii, and Masaru Inatani. 2023. "Posture-Induced Intraocular Pressure Changes after iStent Inject W Combined with Phacoemulsification in Open Angle Glaucoma Patients" Medicina 59, no. 3: 423. https://doi.org/10.3390/medicina59030423
APA StyleIwasaki, K., Arimura, S., Orii, Y., & Inatani, M. (2023). Posture-Induced Intraocular Pressure Changes after iStent Inject W Combined with Phacoemulsification in Open Angle Glaucoma Patients. Medicina, 59(3), 423. https://doi.org/10.3390/medicina59030423






