Diagnostic Tests in the Prediction of Neonatal Outcome in Early Placental Fetal Growth Restriction
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obstet. 2021, 152 (Suppl. S1), 3–57. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound. Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Selvaratnam, R.J.; Wallace, E.M.; Treleaven, S.; Hooper, S.B.; Davis, P.G.; Davey, M.A. Does detection of fetal growth restriction improve neonatal outcomes? J. Paediatr. Child Health 2021, 57, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Van Wassenaer-Leemhuis, A.G.; Marlow, N.; Lees, N.; Wolf, H.; the TRUFFLE Investigators. The association of neonatal morbidity with long-term neurological outcome in infants who were growth restricted and preterm at birth: Secondary analyses from TRUFFLE (Trial of Randomized Umbilical and Fetal Flow in Europe). BJOG 2017, 124, 1072–1078. [Google Scholar] [CrossRef]
- Thornton, J.G.; Hornbuckle, J.; Vail, A.; Spiegelhalter, D.J.; Levene, M.; GRIT Study Group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): Multicentred randomised controlled trial. Lancet 2004, 364, 513–520. [Google Scholar]
- Griffin, I.J.; Lee, H.C.; Profit, J.; Tancedi, D.J. The smallest of the small: Short-term outcomes of profoundly growth restricted and profoundly low birth weight preterm infants. J. Perinatol. 2015, 35, 503–510. [Google Scholar] [CrossRef]
- Monier, I.; Ancel, P.Y.; Ego, A.; Jarreau, P.H.; Lebeaux, C.; Kaminski, M.; Goffinet, F.; Zeitlina, J.; EPIPAGE 2 Study Group. Fetal and neonatal outcomes of preterm infants born before 32 weeks of gestation according to antenatal vs postnatal assessments of restricted growth. Am. J. Obstet. Gynecol. 2017, 216, 516.e1–516.e10. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333. [Google Scholar] [CrossRef]
- Durutovic-Gligorovic, S. Intrauterine Growth of Newborn Babies; Medical Faculty, University of Belgrade: Belgrade, Serbia, 2000. [Google Scholar]
- Rodrıguez, G.; Samper, M.P.; Olivares, J.L.; Ventura, P.; Moreno, L.A.; Perez-Gonzalez, J.M. Skinfold measurements at birth: Sex and anthropometric influence. Arch Dis. Child Fetal Neonatal Ed. 2005, 90, F273–F275. [Google Scholar] [CrossRef]
- Lees, C.; Parra, M.; Missfelder-Lobos, H. Individualized risk assesment for adverse pregnancy outcome by uterine artery Doppler at 23 weeks. Obstet. Gynecol. 2001, 98, 369. [Google Scholar] [PubMed]
- Manning, F.A.; Hill, L.M.; Platt, L.D. Qualitative amniotic fluid volume determination by ultrasound: Antepartum detection of intrauterin growth retardation. Am. J. Obstet. Gynecol. 1981, 139, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Manning, F.A.; Platt, L.D.; Sipos, L. Antepartum fetal evaluation: Development of fetal biophysical profile. Am. J. Obstet. Gynecol. 1980, 136, 787–791. [Google Scholar] [CrossRef]
- Acharyia, G. Reference ranges for serial measurements of umbilical artery Doppler indices in the second part of pregnancy. Am J. Obstet. Gynecol. 2005, 192, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.; Wright, A.; Syngelaki, A.; Wright, D.; Akolekar, R.; Nicolaides, K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019, 53, 465–472. [Google Scholar] [CrossRef]
- Ebbing, C.; Rasmussen, S.; Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: Longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet. Gynecol. 2007, 30, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Rasmussen, S.; Hanson, M.; Kiserud, T. Longitudinal reference ranges for ductus venosus flow velocities and waveform indices. Ultrasound Obstet. Gynecol. 2006, 28, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A. Planning management and delivery of the growth-restricted fetus. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 53–56. [Google Scholar] [CrossRef]
- Jensen, E.A.; Foglia, E.E.; Dysart, K.C.; Simmons, R.A.; Aghai, Z.H.; Cook, A.; Greenspan, J.S.; DeMauro, S.B. Adverse effects of small for gestational age differ by gestational week among very preterm infants. Arch Dis. Child Fetal Neonatal Ed. 2019, 104, F192–F198. [Google Scholar] [CrossRef]
- Visser, G.H.A.; Bilardo, C.M.; Derks, J.B.; Ferrazzi, E.; Fratelli, N.; Frusca, T.; Ganzevoort, W.; Lees, C.C.; Napolitano, R.; Todros, T.; et al. Fetal monitoring indications for delivery and 2-year outcome in 310 infants with fetal growth restriction delivered before 32 weeks’ gestation in the TRUFFLE study. Ultrasound Obstet. Gynecol. 2017, 50, 347–352. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Taylor, M.; Shields, D.; Parker, D.; Scardo, J.A.; Magann, E.F. Intrauterine growth restriction and oligohydramnios among high-risk patients. Am. J. Perinatol. 2007, 24, 215–221. [Google Scholar] [CrossRef]
- Magann, E.F.; Haas, D.M.; Hill, J.B.; Chauhan, S.P.; Watson, E.M.; Learman, L.A. Oligohydramnios, small for gestational age and pregnancy outcomes: An analysis using precise measures. Gynecol. Obstet. Invest. 2011, 72, 239–244. [Google Scholar] [CrossRef]
- Odibo, A.O.; Quinones, J.N.; Lawrence-Cleary, K.; Stamilio, D.M.; Macones, G.A. What antepartum fetal test should guide the timing of delivery of the preterm growth-restricted fetus? A decision-analysis. Am. J. Obstet. Gynecol. 2004, 191, 1477–1482. [Google Scholar] [CrossRef]
- Kaur, S.; Picconi, J.L.; Chadha, R.; Kruger, M.; Mari, G. Biophysical profile in the treatment of intrauterine growth-restricted fetuses who weigh <1000 g. Am. J. Obstet. Gynecol. 2008, 199, 264.e1–264.e4. [Google Scholar]
- Crimmins, S.; Desai, A.; Block-Abraham, D.; Berg, C.; Gembruch, U.; Baschat, A.A. A comparison of Doppler and biophysical findings between liveborn and stillborn growth-restricted fetuses. Am. J. Obstet. Gynecol. 2014, 211, 669.e1–669.e10. [Google Scholar] [CrossRef]
- Baschat, A.A.; Galan, H.L.; Lee, W.; DeVore, G.R.; Mari, G.; Hobbins, J.; Vintzileos, A.; Platt, L.D.; Manning, F.A. The role of the fetal biophysical profile in the management of fetal growth restriction. Am. J. Obstet. Gynecol. 2022, 226, 475–486. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, K.G.; Wang, S.M.; Chen, C.P. Two-year neurological outcome of very-low-birth-weight children with prenatal absent or reversed end-diastolic flow velocity in the umbilical artery. Taiwan J. Obstet. Gynecol. 2013, 52, 323–328. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, V.; Burke, G.; Unterscheider, J.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; O’Donoghue, K.; Hunter, A.; Morrison, J.J.; et al. Defining the residual risk of adverse perinatal outcome in growth-restricted fetuses with normal umbilical artery blood flow. Am. J. Obstet. Gynecol. 2014, 211, e1–e420. [Google Scholar] [CrossRef] [PubMed]
- Gerber, S.; Hohlfeld, P.; Viquerat, F.; Tolsa, J.F.; Vial, Y. Intrauterine growth restriction and absent or reverse end-diastolic blood flow in umbilical artery (Doppler class II or III): A retrospective study of short- and long-term fetal morbidity and mortality. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 126, 20–26. [Google Scholar] [CrossRef]
- Regan, J.; Masters, H.; Warshak, C.R. Estimation of the growth rate in fetuses with an abnormal cerebroplacental ratio compared to those with suspected growth restriction without evidence of centralization of blood flow. J. Ultrasound Med. 2015, 34, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Kutuk, M.S.; Sahin, M.; Gorkem, S.B.; Doganay, S.; Ozturk, A. Relationship between Doppler findings and fetal brain apparent diffusion coefficient in early-onset intra-uterine growth restriction. J. Matern. Fetal Neonatal Med. 2018, 31, 3201–3208. [Google Scholar] [CrossRef]
- Vollgraff Heidweiller-Schreurs, C.A.; De Boer, M.A.; Heymans, M.W.; Schoonmade, L.J.; Bossuyt, P.M.M.; Mol, B.W.J.; De Groot, C.J.M.; Bax, C.J. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 313–322. [Google Scholar] [CrossRef]
- Vollgraff Heidweiller-Schreurs, C.A.; van Osch, I.R.; Heymans, M.W.; Ganzevoort, W.; Schoonmade, L.J.; Bax, C.J.; Mol, B.; de Groot, C.; Bossuyt, P.; de Boer, M.A.; et al. Cerebroplacental ratio in predicting adverse perinatal outcome: A meta-analysis of individual participant data. BJOG 2021, 128, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, N.; Amighetti, S.; Bhide, A.; Fichera, A.; Khalil, A.; Papageorghiou, A.T.; Prefumo, F.; Thilaganathan, B. Ductus venosus Doppler waveform pattern in fetuses with early growth restriction. Acta Obstet. Gynecol. Scand. 2020, 99, 608–614. [Google Scholar] [CrossRef]
- Lees, C.; Marlow, N.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Derks, J.B.; Duvekot, J.; Frusca, T.; Diemert, A.; Ferrazzi, E.; et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: Cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet. Gynecol. 2013, 42, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Frusca, T.; Todros, T.; Lees, C.; Bilardo, C.M. TRUFFLE Investigators. Outcome in early-onset fetal growth restriction is best combining computerized fetal heart rate analysis with ductus venosus Doppler: Insights from the Trial of Umbilical and Fetal Flow in Europe. Am. J. Obstet. Gynecol. 2018, 218, S783–S789. [Google Scholar] [CrossRef]
- Lees, C.C.; Marlow, N.; van Wassenaer-Leemhuis, A.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Calvert, S.; Derks, J.B.; Diemert, A.; Duvekot, J.J.; et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): A randomised trial. Lancet 2015, 385, 2162–2172. [Google Scholar] [CrossRef]
- Najafzadeh, A.; Dickinson, J.E. Umbilical Venous Blood Flow and Its Measurement in the Human Fetus. J. Clin. Ultrasound 2012, 40, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Ganzevoort, W.; Thornton, J.G.; Marlow, N.; Thilaganathan, B.; Arabin, B.; Prefumo, F.; Lees, C.; Wolf, H.; GRIT Study Group; TRUFFLE Study Group. Comparative analysis of 2-year outcomes in GRIT and TRUFFLE trials. Ultrasound Obstet. Gynecol. 2020, 55, 68–74. [Google Scholar] [CrossRef]
- Pels, A.; Mensing van Charante, N.A.; Vollgraff Heidweiller-Schreurs, C.A.; Limpens, J.; Wolf, H.; de Boer, M.A.; Ganzevoort, W. The prognostic accuracy of short-term variation of fetal heart rate in early-onset fetal growth restriction: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 179–184. [Google Scholar] [CrossRef]
- Tanaka, H.; Furuhashi, F.H.; Toriyabe, K.; Matsumoto, T.; Magawa, S.; Nii, M.; Watanabe, J.; Tanaka, K.; Umekawa, T.; Kamimoto, Y.; et al. Management of fetal growth restriction using the contraction stress test: A case-control study. J. Matern Fetal Neonatal Med. 2019, 32, 3221–3225. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Gratacos, E. Stage-based approach to the management of fetal growth restriction. Prenatal Diagnosis 2014, 34, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, Z.; Stampalija, T.; Dowswell, T. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst. Rev. 2017, 6, CD007529. [Google Scholar] [CrossRef] [PubMed]
- Odibo, A.O.; Goetzinger, K.R.; Cahill, A.G.; Odibo, L.; Macones, G.A. Combined Sonographic testing index and prediction of adverse outcome in preterm Fetal growth restriction. Am. J. Perinatol. 2014, 31, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.; Jackson, R.; Cornforth, C.; Harrold, J.; Turner, M.A.; Kenny, L.; Baker, P.N.; Johnstone, E.D.; Khalil, A.; von Dadelszen, P.; et al. A prediction model for short-term neonatal outcomes in severe early-onset fetal growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 241, 109–118. [Google Scholar] [CrossRef] [PubMed]
| NMM N = 40 | NAA N = 40 | NWMAA N = 40 | p | Overall N = 120 | |
|---|---|---|---|---|---|
| Maternal age | 31.3 ± 6.4 | 32.6 ± 5.7 | 30.9 ± 6.6 | 0.456 b | 31.6 ± 6.2 |
| Gestational age (weeks) | 28.94 ± 1.87 | 30.46 ± 1.08 | 30.91 ± 1.0 | <0.001 b | 30.1 ± 1.6 |
| Parity, n (%) 1 | 30 (75) | 26 (65) | 25 (62.5) | 0.548 a | 81 (67.5) |
| 2 | 5 (12.5) | 10 (25) | 11 (25) | 26 (21.7) | |
| 3+ | 5 (12.5) | 4 (10) | 4 (10) | 13 (10.8) | |
| Pre-eclampsia, n (%) | 28 (70) | 29 (72.5) | 31 (77.5) | 0.742 a | 88 (73.3) |
| Corticosteroid, n (%) | 32 (80) | 38 (95) | 40 (100) | 0.005 a | 110 (91.7) |
| Neuroprotection—MgSO4, n (%) | 19 (47.5) | 25 (62.5) | 25 (62.5) | 0.100 a | 69 (57.5) |
| Fetal indication for delivery, n (%) | 31 (77.5) | 20 (50) | 14 (35) | 0.001 a | 65 (53.7) |
| Cesarean Section, n (%) | 40 (100) | 40 (100) | 39 (97.5) | 0.365 a | 119 (99.7) |
| N gender, n (%) Male | 22 (55) | 18 (45) | 23 (57.5) | 0.496 a | 63 (52,5) |
| Female | 18 (45) | 22 (55) | 17 (42.5) | 57 (47.5) | |
| Neonatal body weight (g) | 907.3 ± 146.7 | 1284.3 ± 166.9 | 1448.5 ± 166.2 | <0.001 b | 1213.3 ± 277.5 |
| 5’Apgar score | 5.53 ± 1.77 | 7.15 ± 0.97 | 8.1 ± 0.81 | <0.001 b | 6.93 ± 1.64 |
| pH | 7.13 ± 0.13 | 7.17 ± 0.44 | 7.29 ± 0.04 | <0.001 b | 7.19 ± 0.11 |
| Base excess | 8.15 ± 5.14 | 7.63 ±3.09 | 2.52 ±1.11 | 0.007 b | 6.10 ±4.33 |
| Hospital stay (days) | 66.78 ± 42.4 | 58.2 ± 17.7 | 55.9 ± 18.9 | 0.208 b | 60.3 ± 28.8 |
| NMM N = 40 | NAA N = 40 | NWMAA N = 40 | Overall N = 120 | |
|---|---|---|---|---|
| Fetal indication for delivery, n (%) | 31 (77.5) | 20 (50) | 14 (35) | 65 (54.2) |
| FHRM (Decelerations/positive CST) | 14 | 9 | 7 | 30 |
| BPS changes/Olygohydramnio | 7 | 5 | 3 | 15 |
| Doppler changes | 10 | 6 | 4 | 20 |
| Maternal indication for delivery, n (%) | 20 (50) | 16 (65) | 55 (45.8) | |
| HELLP | 3 | 4 | 8 | 15 |
| Severe pre-eclampsia | 4 | 11 | 13 | 28 |
| Superimposed pre-eclampsia | 2 | 5 | 5 | 12 |
| NMM N = 40 | NAA N = 40 | NWMAA N = 40 | p | Overall N = 120 | |
|---|---|---|---|---|---|
| AFI | 74.48 ± 30.26 | 100.63 ± 25.65 | 110.38 ± 26.42 | <0.001 b | 95.16 ± 31.24 |
| BPS | 5.45 ± 1.87 | 7.30 ± 1.40 | 8.18 ± 1.43 | <0.001 b | 6.98 ± 1.94 |
| UA PI | 1.78 ± 0.22 | 1.52 ± 0.26 | 1.32 ± 0.28 | <0.001 b | 1.54 ± 0.32 |
| UA blood flow, n (%) | <0.001 a | ||||
| PI ≤ 95th percentile | 2 (5) | 8 (20) | 12 (30) | 22 (18.3) | |
| PI > 95th percentile | 7 (17.5) | 14 (35) | 24 (60) | 45 (37.5) | |
| AREDV | 31 (77.5) | 18 (45) | 4 (10) | 53 (44.2) | |
| MCA Pi | 1.24 ± 0.16 | 1.32 ± 0.14 | 1.54 ± 0.2 | <0.001 b | 1.37 ± 0.21 |
| CPR | 0.72 ± 0.19 | 0.92 ± 0.24 | 1.25 ± 0.41 | <0.001 b | 0.96 ± 0.36 |
| ΔCPR, n (%) | <0.001 a | ||||
| ≥5th percentile | 3 (7.5) | 12 (30) | 29 (72.5) | 44 (36.7) | |
| <5th percentile | 37 (92.5) | 28 (70) | 11 (27.5) | 76 (63.3) | |
| DV PI | 1.10 ± 0.42 | 0.64 ± 0.3 | 0.55 ± 0.22 | <0.001 b | 0.76 ± 0.4 |
| DV blood flow, n (%) | <0.001 a | ||||
| PI ≤ 95th percentile | 4 (10 | 23 (57.5) | 31 (77.5) | 58 (48.3) | |
| PI > 95th percentile | 16 (40) | 16 (40) | 9 (22.5) | 41 (34.2) | |
| ARA wave | 20 (50) | 1 (2.5) | 0 (0) | 21 (17.5) | |
| UV blood flow, n (%) | <0.001 a | ||||
| Laminar | 15 (37.5) | 36 (90) | 40 (100) | 91 (75.8) | |
| Pulsatile | 25 (62.5) | 4 (10) | 0 (0) | 29 (24.2) | |
| FHRM, n (%) | 0.130 a | ||||
| Normal/Silent, n (%) | 17 (42.5) | 21 (52.5) | 26 (65) | 64 (53.3) | |
| Spontaneous decelerations/Positive CST, n (%) | 23 (57.5) | 19 (47.5) | 14 (35) | 56 (46.7) |
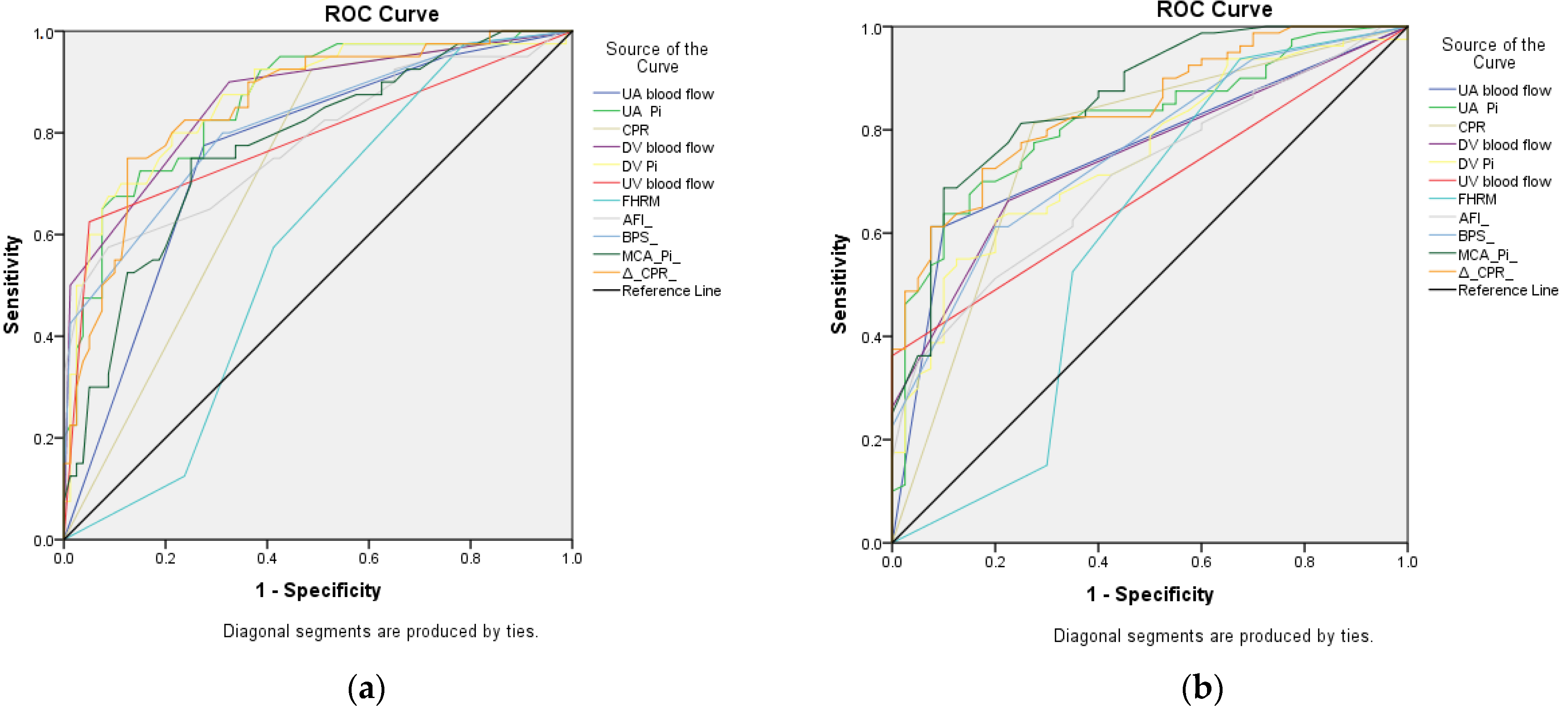
| Test Result Variable(s) | I | II | ||
|---|---|---|---|---|
| AUC (Asymptotic 95% CI) | p | AUC (Asymptotic 95% CI) | p | |
| AFI | 0.783 (0.688–0.877) | <0.001 | 0.711 (0.619–0.803) | <0.001 |
| BPS | 0.816 (0.732–0.900) | <0.001 | 0.758 (0.671–0.845) | <0.001 |
| UA PI | 0.863 (0.793–0.932) | <0.001 | 0.814 (0.736–0.891) | <0.001 |
| UA blood flow | 0.760 (0.669–0.851) | <0.001 | 0.758 (0.670–0.846) | <0.001 |
| MCA PI | 0.774 (0.686–0.863) | <0.001 | 0.859 (0.789–0.928) | <0.001 |
| CPR | 0.719 (0.627–0.810) | <0.001 | 0.769 (0.674–0.864) | <0.001 |
| ΔCPR | 0.855 (0.783–0.927) | <0.001 | 0.840 (0.770–0.910) | <0.001 |
| DV PI | 0.869 (0.798–0/940) | <0.001 | 0.750 (0.662/0.839) | <0.001 |
| DV blood flow | 0.863 (0.790–0.936) | <0.001 | 0.748 (0.662–0.835) | <0.001 |
| UV blood flow | 0.788 (0.690–0.885) | <0.001 | 0.681 (0.588–0.774) | <0.001 |
| FHRM | 0.577 (0.475–0.678) | 0.173 | 0.592 (0.468–0.716) | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandić-Marković, V.; Bogavac, M.; Miković, Ž.; Panić, M.; Pavlović, D.M.; Mitrović, J.; Mandić, M. Diagnostic Tests in the Prediction of Neonatal Outcome in Early Placental Fetal Growth Restriction. Medicina 2023, 59, 406. https://doi.org/10.3390/medicina59020406
Mandić-Marković V, Bogavac M, Miković Ž, Panić M, Pavlović DM, Mitrović J, Mandić M. Diagnostic Tests in the Prediction of Neonatal Outcome in Early Placental Fetal Growth Restriction. Medicina. 2023; 59(2):406. https://doi.org/10.3390/medicina59020406
Chicago/Turabian StyleMandić-Marković, Vesna, Mirjana Bogavac, Željko Miković, Milan Panić, Dejan M. Pavlović, Jelena Mitrović, and Milica Mandić. 2023. "Diagnostic Tests in the Prediction of Neonatal Outcome in Early Placental Fetal Growth Restriction" Medicina 59, no. 2: 406. https://doi.org/10.3390/medicina59020406
APA StyleMandić-Marković, V., Bogavac, M., Miković, Ž., Panić, M., Pavlović, D. M., Mitrović, J., & Mandić, M. (2023). Diagnostic Tests in the Prediction of Neonatal Outcome in Early Placental Fetal Growth Restriction. Medicina, 59(2), 406. https://doi.org/10.3390/medicina59020406






