The Effect of Postinduction Blood Glucose on Intraoperative Hypothermia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and Data Collection
2.3. Anesthetic Methods
2.4. Temperature Collection and Management
2.5. Definitions
2.6. Outcomes
2.7. Statistical Analyses
3. Results
3.1. Study Population and Patient Characteristics
3.2. Hypothermic Patients Presented with a Lower Temperature
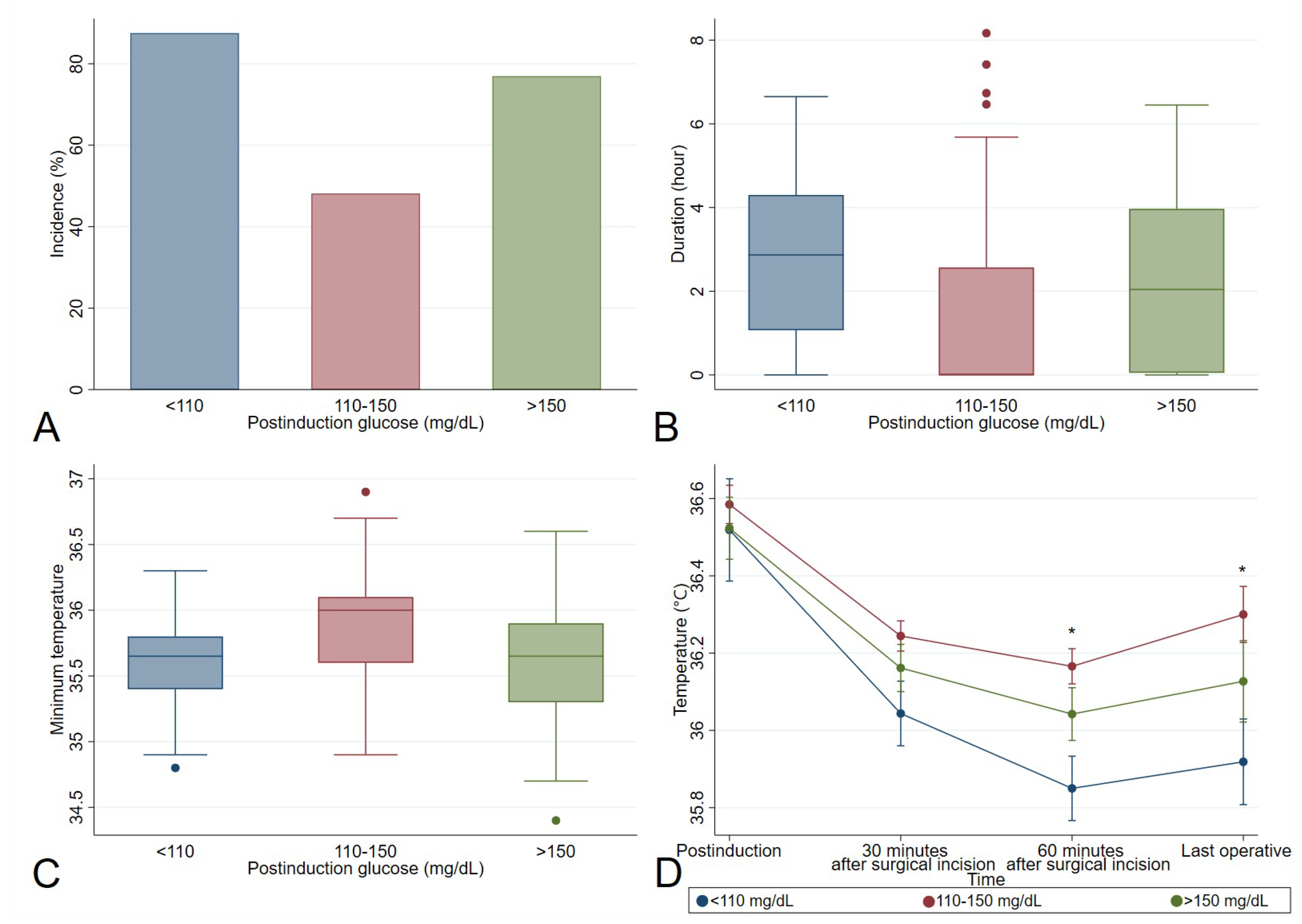
3.3. Perioperative Glucose Variables Associated with Hypothermia
3.4. Out-of-Range Postinduction Glucose in Diabetics Is at a Higher Risk for Hypothermia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurz, A.; Sessler, D.I.; Lenhardt, R. Perioperative Normothermia to Reduce the Incidence of Surgical-Wound Infection and Shorten Hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996, 334, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.M.; Fleisher, L.A.; Breslow, M.J.; Higgins, M.S.; Olson, K.F.; Kelly, S.; Beattie, C. Perioperative Maintenance of Normothermia Reduces the Incidence of Morbid Cardiac Events. A Randomized Clinical Trial. JAMA 1997, 277, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Mascha, E.; Na, J.; Sessler, D.I. The Effects of Mild Perioperative Hypothermia on Blood Loss and Transfusion Requirement. Anesthesiology 2008, 108, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lenhardt, R.; Marker, E.; Goll, V.; Tschernich, H.; Kurz, A.; Sessler, D.I.; Narzt, E.; Lackner, F. Mild Intraoperative Hypothermia Prolongs Postanesthetic Recovery. Anesthesiology 1997, 87, 1318–1323. [Google Scholar] [CrossRef]
- Rauch, S.; Miller, C.; Bräuer, A.; Wallner, B.; Bock, M.; Paal, P. Perioperative Hypothermia-A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 8749. [Google Scholar] [CrossRef]
- Okoué, R.; Calabrese, D.; Nzé, P.; Msika, S.; Keita, H. Efficacy of Forced-Air Warming to Prevent Perioperative Hypothermia in Morbidly-Obese Versus Non-Obese Patients. Obes. Surg. 2018, 28, 1955–1959. [Google Scholar] [CrossRef]
- Xu, H.; Xu, G.; Ren, C.; Liu, L.; Wei, L. Effect of Forced-Air Warming System in Prevention of Postoperative Hypothermia in Elderly Patients: A Prospective Controlled Trial. Medicine 2019, 98, e15895. [Google Scholar] [CrossRef]
- Torossian, A.; Van Gerven, E.; Geertsen, K.; Horn, B.; Van de Velde, M.; Raeder, J. Active Perioperative Patient Warming Using a Self-Warming Blanket (BARRIER EasyWarm) Is Superior to Passive Thermal Insulation: A Multinational, Multicenter, Randomized Trial. J. Clin. Anesth. 2016, 34, 547–554. [Google Scholar] [CrossRef]
- Lauronen, S.-L.; Mäkinen, M.-T.; Annila, P.; Huhtala, H.; Yli-Hankala, A.; Kalliomäki, M.-L. Thermal Suit Connected to a Forced-Air Warming Unit for Preventing Intraoperative Hypothermia: A Randomised Controlled Trial. Acta Anaesthesiol. Scand. 2021, 65, 176–181. [Google Scholar] [CrossRef]
- Abdelmalak, B.B.; Knittel, J.; Abdelmalak, J.B.; Dalton, J.E.; Christiansen, E.; Foss, J.; Argalious, M.; Zimmerman, R.; Van den Berghe, G. Preoperative Blood Glucose Concentrations and Postoperative Outcomes after Elective Non-Cardiac Surgery: An Observational Study. Br. J. Anaesth. 2014, 112, 79–88. [Google Scholar] [CrossRef]
- Dronge, A.S.; Perkal, M.F.; Kancir, S.; Concato, J.; Aslan, M.; Rosenthal, R.A. Long-Term Glycemic Control and Postoperative Infectious Complications. Arch. Surg. 2006, 141, 375–380, discussion 380. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, N.A.; Alodhayani, A.A.; Joy, S.S.; Isnani, A.; Mujammami, M.; Alfadda, A.A.; Siddiqui, K. Evaluation of Risk Factors for Diabetic Peripheral Neuropathy Among Saudi Type 2 Diabetic Patients with Longer Duration of Diabetes. Diabetes Metab. Syndr. Obes. 2022, 15, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Mallet, M.-L.; Hadjivassiliou, M.; Sarrigiannis, P.G.; Zis, P. The Role of Oxidative Stress in Peripheral Neuropathy. J. Mol. Neurosci. 2020, 70, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Karahmet, E.; Prnjavorac, B.; Bego, T.; Softić, A.; Begić, L.; Begić, E.; Karahmet, E.; Prnjavorac, L.; Prnjavorac, I. Clinical Use of an Analysis of Oxidative Stress and IL-6 as the Promoters of Diabetic Polyneuropathy. Med. Glas. 2021, 18, 12–17. [Google Scholar] [CrossRef]
- Karahmet, E.; Prnjavorac, B.; Bego, T.; Meseldžić, N.; Imamović, S.; Karahmet, E.; Sher, F.; Lekić, L.; Begić, E. IL-1β in Correlation to the Common Diabetic Complications. Acta Sci. Med. Sci. 2021, 5, 25–29. [Google Scholar] [CrossRef]
- Silverman, H.A.; Tynan, A.; Hepler, T.D.; Chang, E.H.; Gunasekaran, M.; Li, J.H.; Huerta, T.S.; Tsaava, T.; Chang, Q.; Addorisio, M.E.; et al. Transient Receptor Potential Ankyrin-1-Expressing Vagus Nerve Fibers Mediate IL-1β Induced Hypothermia and Reflex Anti-Inflammatory Responses. Mol. Med. 2023, 29, 4. [Google Scholar] [CrossRef]
- Kitamura, A.; Hoshino, T.; Kon, T.; Ogawa, R. Patients with Diabetic Neuropathy Are at Risk of a Greater Intraoperative Reduction in Core Temperature. Anesthesiology 2000, 92, 1311–1318. [Google Scholar] [CrossRef]
- Stansberry, K.B.; Hill, M.A.; Shapiro, S.A.; McNitt, P.M.; Bhatt, B.A.; Vinik, A.I. Impairment of Peripheral Blood Flow Responses in Diabetes Resembles an Enhanced Aging Effect. Diabetes Care 1997, 20, 1711–1716. [Google Scholar] [CrossRef]
- Kenny, G.P.; Sigal, R.J.; McGinn, R. Body Temperature Regulation in Diabetes. Temperature 2016, 3, 119–145. [Google Scholar] [CrossRef]
- Neil, H.A.; Dawson, J.A.; Baker, J.E. Risk of Hypothermia in Elderly Patients with Diabetes. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 416–418. [Google Scholar] [CrossRef]
- Zhang, X.; Gregg, E.W.; Williamson, D.F.; Barker, L.E.; Thomas, W.; Bullard, K.M.; Imperatore, G.; Williams, D.E.; Albright, A.L. A1C Level and Future Risk of Diabetes: A Systematic Review. Diabetes Care 2010, 33, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.F.; Dellinger, E.P.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Kongsayreepong, S.; Chaibundit, C.; Chadpaibool, J.; Komoltri, C.; Suraseranivongse, S.; Suwannanonda, P.; Raksamanee, E.-O.; Noocharoen, P.; Silapadech, A.; Parakkamodom, S.; et al. Predictor of Core Hypothermia and the Surgical Intensive Care Unit. Anesth. Analg. 2003, 96, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Stryker, L.S.; Abdel, M.P.; Morrey, M.E.; Morrow, M.M.; Kor, D.J.; Morrey, B.F. Elevated Postoperative Blood Glucose and Preoperative Hemoglobin A1C Are Associated with Increased Wound Complications Following Total Joint Arthroplasty. J. Bone Jt. Surg. Am. 2013, 95, 808–814. [Google Scholar] [CrossRef]
- Park, J.; Oh, A.R.; Lee, S.-H.; Lee, J.-H.; Min, J.J.; Kwon, J.-H.; Kim, J.; Yang, K.; Choi, J.-H.; Lee, S.-C.; et al. Associations Between Preoperative Glucose and Hemoglobin A1c Level and Myocardial Injury After Noncardiac Surgery. J. Am. Heart Assoc. 2021, 10, e019216. [Google Scholar] [CrossRef]
- Lim, S.; Yeh, H.-H.; Macki, M.; Mansour, T.; Schultz, L.; Telemi, E.; Haider, S.; Nerenz, D.R.; Schwalb, J.M.; Abdulhak, M.; et al. Preoperative HbA1c > 8% Is Associated with Poor Outcomes in Lumbar Spine Surgery: A Michigan Spine Surgery Improvement Collaborative Study. Neurosurgery 2021, 89, 819–826. [Google Scholar] [CrossRef]
- Halkos, M.E.; Lattouf, O.M.; Puskas, J.D.; Kilgo, P.; Cooper, W.A.; Morris, C.D.; Guyton, R.A.; Thourani, V.H. Elevated Preoperative Hemoglobin A1c Level Is Associated with Reduced Long-Term Survival after Coronary Artery Bypass Surgery. Ann. Thorac. Surg. 2008, 86, 1431–1437. [Google Scholar] [CrossRef]
- Cheisson, G.; Jacqueminet, S.; Cosson, E.; Ichai, C.; Leguerrier, A.-M.; Nicolescu-Catargi, B.; Ouattara, A.; Tauveron, I.; Valensi, P.; Benhamou, D.; et al. Perioperative Management of Adult Diabetic Patients. Intraoperative Period. Anaesth. Crit. Care Pain Med. 2018, 37 (Suppl. S1), S21–S25. [Google Scholar] [CrossRef]
- Jiang, J.; Li, S.; Zhao, Y.; Zhou, Z.; Zhang, J.; Sun, R.; Luo, A. Intensive Glucose Control during the Perioperative Period for Diabetic Patients Undergoing Surgery: An Updated Systematic Review and Meta-Analysis. J. Clin. Anesth. 2021, 75, 110504. [Google Scholar] [CrossRef]
- Lai, J.; Li, Q.; He, Y.; Zou, S.; Bai, X.; Rastogi, S. Glycemic Control Regimens in the Prevention of Surgical Site Infections: A Meta-Analysis of Randomized Clinical Trials. Front. Surg. 2022, 9, 855409. [Google Scholar] [CrossRef]
- Antoniou, S.; Naka, K.K.; Bechlioulis, A.; Papadakis, M.; Tsatsoulis, A.; Michalis, L.K.; Tigas, S. Vascular Effects Following Intensification of Glycemic Control in Poorly Controlled Patients with Long-Standing Type 2 Diabetes Mellitus. Hormones 2021, 20, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Yoon, H. Influence of Pre-Operative Fasting Time on Blood Glucose in Older Patients. J. Korean Acad. Nurs. 2011, 41, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Molnar, G.W.; Read, R.C. Hypoglycemia and Body Temperature. JAMA 1974, 227, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.A.; Bennett, T.; Green, J.H.; MacDonald, I.A. Hypoglycaemia, Hypothermia and Shivering in Man. Clin. Sci. 1981, 61, 463–469. [Google Scholar] [CrossRef]
- O’Brien, C.; Young, A.J.; Sawka, M.N. Hypohydration and Thermoregulation in Cold Air. J. Appl. Physiol. 1998, 84, 185–189. [Google Scholar] [CrossRef]
- Chiasson, J.-L.; Aris-Jilwan, N.; Bélanger, R.; Bertrand, S.; Beauregard, H.; Ekoé, J.-M.; Fournier, H.; Havrankova, J. Diagnosis and Treatment of Diabetic Ketoacidosis and the Hyperglycemic Hyperosmolar State. CMAJ 2003, 168, 859–866. [Google Scholar]
- Zeng, S.; Chen, Q.; Wang, X.-W.; Hong, K.; Li, J.-X.; Li, P.; Cheng, X.-S.; Su, H. Longer Rewarming Time in Finger Cooling Test in Association with HbA1c Level in Diabetics. Microvasc. Res. 2016, 107, 72–75. [Google Scholar] [CrossRef]
- Yan, L.; Tan, J.; Chen, H.; Xiao, H.; Zhang, Y.; Yao, Q.; Li, Y. A Nomogram for Predicting Unplanned Intraoperative Hypothermia in Patients with Colorectal Cancer Undergoing Laparoscopic Colorectal Procedures. AORN J. 2023, 117, e1–e12. [Google Scholar] [CrossRef]
- Scott, A.R.; MacDonald, I.A.; Bennett, T.; Tattersall, R.B. Abnormal Thermoregulation in Diabetic Autonomic Neuropathy. Diabetes 1988, 37, 961–968. [Google Scholar] [CrossRef]
- Wick, D.E.; Roberts, S.K.; Basu, A.; Sandroni, P.; Fealey, R.D.; Sletten, D.; Charkoudian, N. Delayed Threshold for Active Cutaneous Vasodilation in Patients with Type 2 Diabetes Mellitus. J. Appl. Physiol. 2006, 100, 637–641. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Su, L.-J.; Wu, H.-Z.; Zou, H.; Yang, R.; Zhu, Y.-X. Risk Factors for Inadvertent Intraoperative Hypothermia in Patients Undergoing Laparoscopic Surgery: A Prospective Cohort Study. PLoS ONE 2021, 16, e0257816. [Google Scholar] [CrossRef]
- Mehta, O.H.; Barclay, K.L. Perioperative Hypothermia in Patients Undergoing Major Colorectal Surgery. ANZ J. Surg. 2014, 84, 550–555. [Google Scholar] [CrossRef]
- Yi, J.; Lei, Y.; Xu, S.; Si, Y.; Li, S.; Xia, Z.; Shi, Y.; Gu, X.; Yu, J.; Xu, G.; et al. Intraoperative Hypothermia and Its Clinical Outcomes in Patients Undergoing General Anesthesia: National Study in China. PLoS ONE 2017, 12, e0177221. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Mendonça, F.T.; Ferreira, J.D.S.; Guilardi, V.H.F.; Guimarães, G.M.N. Prevalence of Inadvertent Perioperative Hypothermia and Associated Factors: A Cross-Sectional Study. Ther. Hypothermia Temp. Manag. 2021, 11, 208–215. [Google Scholar] [CrossRef]
- Sari, S.; Aksoy, S.M.; But, A. The Incidence of Inadvertent Perioperative Hypothermia in Patients Undergoing General Anesthesia and an Examination of Risk Factors. Int. J. Clin. Pract. 2021, 75, e14103. [Google Scholar] [CrossRef]
- Horn, E.-P.; Bein, B.; Böhm, R.; Steinfath, M.; Sahili, N.; Höcker, J. The Effect of Short Time Periods of Pre-Operative Warming in the Prevention of Peri-Operative Hypothermia. Anaesthesia 2012, 67, 612–617. [Google Scholar] [CrossRef]
- Lau, A.; Lowlaavar, N.; Cooke, E.M.; West, N.; German, A.; Morse, D.J.; Görges, M.; Merchant, R.N. Effect of Preoperative Warming on Intraoperative Hypothermia: A Randomized-Controlled Trial. Can. J. Anaesth. 2018, 65, 1029–1040. [Google Scholar] [CrossRef]
- Jeyadoss, J.; Thiruvenkatarajan, V.; Watts, R.W.; Sullivan, T.; van Wijk, R.M.A.W. Intraoperative Hypothermia Is Associated with an Increased Intensive Care Unit Length-of-Stay in Patients Undergoing Elective Open Abdominal Aortic Aneurysm Surgery: A Retrospective Cohort Study. Anaesth. Intensive Care 2013, 41, 759–764. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kim, D.-K.; Kim, J.-K.; Lee, E.-J.; Kim, J.-Y. A Retrospective Analysis on the Relationship between Intraoperative Hypothermia and Postoperative Ileus after Laparoscopic Colorectal Surgery. PLoS ONE 2018, 13, e0190711. [Google Scholar] [CrossRef]
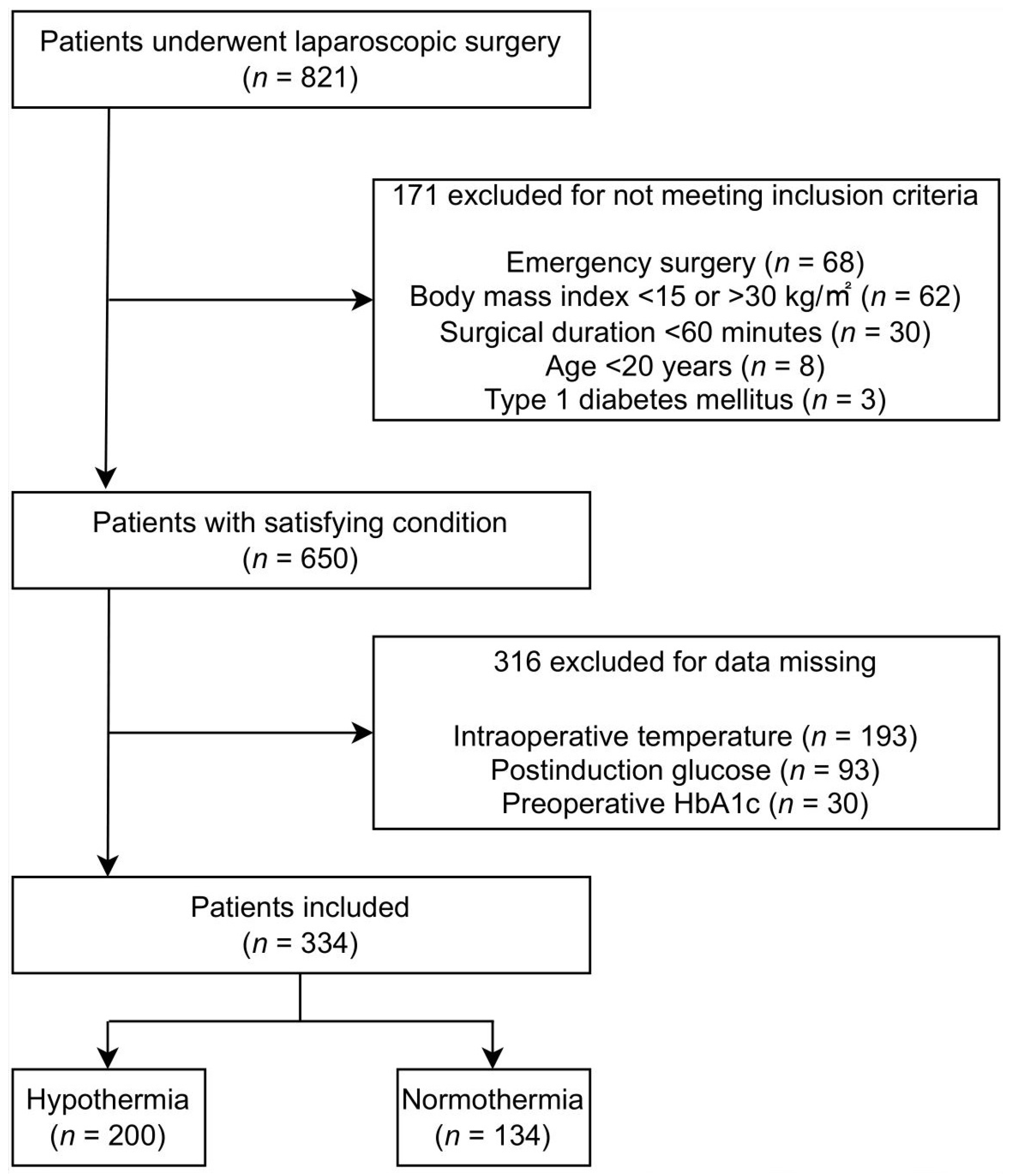
| Hypothermic (n = 200) | Normothermic (n = 134) | p Value | |
|---|---|---|---|
| Age (years) | 70 [65; 77] | 68 [56; 74] | 0.007 |
| Age ≥65 years n (%) | 151 (65.9) | 78 (34.1) | 0.001 |
| Male n (%) | 122 (61.0) | 85 (63.4) | 0.653 |
| BMI (kg/m2) | 22.3 [20.1; 24.5] | 23.0 [21.5; 25.3] | 0.003 |
| ASA-Score n (%) | 0.070 | ||
| I | 35 (17.5) | 35 (26.1) | |
| II | 129 (64.5) | 84 (62.7) | |
| >II | 36 (18.0) | 15 (11.2) | |
| Diabetes n (%) | 72 (36.0) | 49 (36.6) | 0.916 |
| Intraoperative parameters | |||
| Total intravenous/volatile anesthesia n | 62/138 | 34/100 | 0.265 |
| Epidural-general anesthesia/general anesthesia n | 131/69 | 98/36 | 0.141 |
| Duration of surgery (hour) | 4.8 [3.7; 6.5] | 4.8 [3.6; 7.3] | 0.963 |
| Intravenous fluid administration (mL) | 2516 [1906; 3161] | 2398 [1728; 3335] | 0.605 |
| Estimated blood loss and urine output (mL) | 453 [266; 775] | 415 [238; 753] | 0.557 |
| Intraoperative insulin dose (IU) (n = 8) | (n = 4) 6.4 [1.6; 9.6] | (n = 4) 6.0 [2.2; 9.1] | 0.999 |
| Intraoperative glucose dose (g) | 13.3 [10.0; 15.0] | 14.0 [10.0; 15.0] | 0.906 |
| Outcome parameters | |||
| Postoperative hospital length of stay (days) | 13 [10; 19] | 12 [10; 22] | 0.971 |
| ICU length of stay (days) | (n = 49) 4 [2; 6] | (n = 36) 5 [2; 5] | 0.757 |
| Hypothermic (n = 200) | Normothermic (n = 134) | p Value | |
|---|---|---|---|
| Perioperative glucose variables | |||
| HbA1c (%) | 5.9 [5.5; 6.4] | 5.8 [5.4; 6.2] | 0.182 |
| HbA1c >6% n (%) | 77 (38.5) | 44 (32.8) | 0.291 |
| Postinduction glucose (mg/dL) | 119 [106; 138] | 125 [112; 137] | 0.063 |
| Postinduction glucose levels n (%) | 0.009 | ||
| Postinduction glucose <110 mg/dL | 60 (30.0) | 24 (17.9) | |
| Postinduction glucose 110–150 mg/dL | 112 (56.0) | 97 (72.4) | |
| Postinduction glucose >150 mg/dL | 28 (14.0) | 13 (9.7) | |
| Perioperative body temperature variables | |||
| Basal body temperature (°C) | 36.4 [36.2; 36.6] | 36.4 [36.2; 36.5] | 0.949 |
| Basal body temperature <36 °C n (%) | 23 (11.5) | 13 (9.7) | 0.603 |
| Postinduction temperature (°C) | 36.4 [36.1; 36.7] | 36.7 [36.6; 37.0] | <0.001 |
| 30 min after surgical incision temperature (°C) | 36.0 [35.8; 36.2] | 36.5 [36.3; 36.7] | <0.001 |
| 60 min after surgical incision temperature (°C) | 35.9 [35.7; 36.1] | 36.5 [36.3; 36.7] | <0.001 |
| Last operative temperature (°C) | 35.9 [35.6; 36.2] | 36.6 [36.4; 37.0] | <0.001 |
| OR (95% CI) | p Value | |
|---|---|---|
| Age ≥65 years | 2.03 (1.22, 3.39) | 0.007 |
| BMI | 0.87 (0.80, 0.95) | 0.001 |
| ASA-Score | 1.36 (0.90, 2.06) | 0.148 |
| Postinduction glucose levels | ||
| Postinduction glucose 110–150 mg/dL * | ||
| Postinduction glucose <110 mg/dL | 2.02 (1.15, 3.55) | 0.015 |
| Postinduction glucose >150 mg/dL | 2.17 (1.02, 4.61) | 0.045 |
| Diabetic (n = 121) | Non-Diabetic (n = 213) | |||||
|---|---|---|---|---|---|---|
| Hypothermic (n = 72) | Normothermic (n = 49) | p Value | Hypothermic (n = 128) | Normothermic (n = 85) | p Value | |
| Age ≥65 years n (%) | 61 (84.7) | 36 (73.5) | 0.128 | 90 (70.3) | 42 (49.4) | 0.002 |
| BMI (kg/m2) | 22.6 [20.5; 24.5] | 23.2 [21.2; 25.2] | 0.183 | 21.9 [19.9; 24.5] | 22.9 [21.5; 25.3] | 0.007 |
| ASA-Score n (%) | 0.162 | 0.125 | ||||
| I | 10 (13.9) | 8 (16.3) | 25 (19.5) | 27 (31.8) | ||
| II | 41 (56.9) | 34 (69.4) | 88 (68.8) | 50 (58.8) | ||
| >II | 21 (29.2) | 7 (14.3) | 15 (11.7) | 8 (9.4) | ||
| Basal body temperature (°C) | 36.4 [36.1; 36.6] | 36.4 [36.1; 36.5] | 0.475 | 36.4 [36.2; 36.5] | 36.4 [36.2; 36.6] | 0.619 |
| Preoperative HbA1c >6% n (%) | 49 (68.1) | 28 (57.1) | 0.221 | 28 (21.9) | 16 (18.8) | 0.590 |
| Postinduction glucose levels n (%) | 0.002 | 0.294 | ||||
| Postinduction glucose <110 mg/dL | 14 (19.4) | 2 (4.1) | 46 (35.9) | 22 (25.9) | ||
| Postinduction glucose 110–150 mg/dL | 38 (52.8) | 41 (83.7) | 74 (57.8) | 56 (65.9) | ||
| Postinduction glucose >150 mg/dL | 20 (27.8) | 6 (12.2) | 8 (6.3) | 7 (8.2) | ||
| Intravenous fluid administration (mL) | 2683 [1959; 3429] | 2450 [1702; 3216] | 0.156 | 2458 [1894; 3098] | 2394 [1772; 3690] | 0.740 |
| Estimated blood loss and urine output (mL) | 493 [275; 949] | 305 [228; 715] | 0.028 | 438 [223; 708] | 465 [273; 775] | 0.326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Kuroda, K.; Morimatsu, H. The Effect of Postinduction Blood Glucose on Intraoperative Hypothermia. Medicina 2023, 59, 395. https://doi.org/10.3390/medicina59020395
Shen Z, Kuroda K, Morimatsu H. The Effect of Postinduction Blood Glucose on Intraoperative Hypothermia. Medicina. 2023; 59(2):395. https://doi.org/10.3390/medicina59020395
Chicago/Turabian StyleShen, Zhangtian, Kosuke Kuroda, and Hiroshi Morimatsu. 2023. "The Effect of Postinduction Blood Glucose on Intraoperative Hypothermia" Medicina 59, no. 2: 395. https://doi.org/10.3390/medicina59020395
APA StyleShen, Z., Kuroda, K., & Morimatsu, H. (2023). The Effect of Postinduction Blood Glucose on Intraoperative Hypothermia. Medicina, 59(2), 395. https://doi.org/10.3390/medicina59020395






