A New Parameter for Calcium Oxalate Stones: Impact of Linear Calculus Density on Non-Contrast Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. NCCT Factors and Stone Analyses
2.3. Statistical Analyses
3. Results
3.1. Demographic Data According to the Mayo Clinic Classification
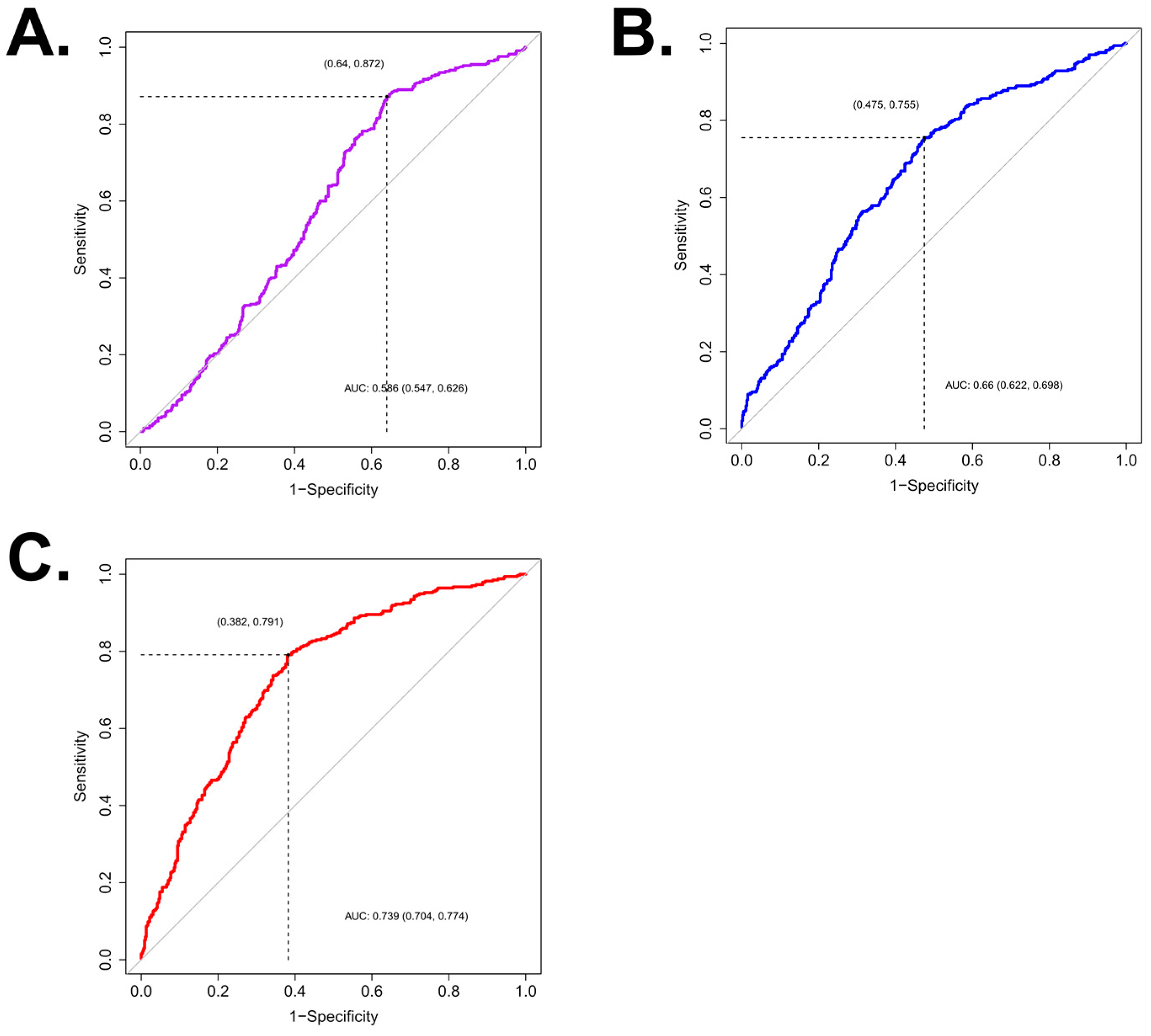
3.2. Predictive Model for CaOx Stone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.Y.; Kang, H.W.; Kim, K.; Ha, Y.S.; Kim, W.T.; Kim, Y.J.; Yun, S.J.; Kim, W.J.; Lee, S.C. Nutritional status assessed by the controlling nutritional status (conut) score as a predictor of recurrence of urolithiasis. Investig. Clin. Urol. 2021, 62, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.D.; Moon, Y.J.; Almujalhem, A.J.; Alqahtani, A.A.; Alkhureeb, M.A.; Lee, J.Y. The first 100 cases of endoscopic combined intrarenal surgery in korea: Matched cohort analyses versus shock-wave lithotripsy. Yonsei Med. J. 2022, 63, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, H.D.; Moon, Y.J.; Han, H.; Cheon, B.; Han, J.; Cho, S.Y.; Lee, J.Y.; Kwon, D.S. In vivo feasibility test of a new flexible ureteroscopic robotic system, easyuretero, for renal stone retrieval in a porcine model. Yonsei Med. J. 2022, 63, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, O.; El-Bendary, M.; Ragab, M.; Rasheed, M. Role of combined use of potassium citrate and tamsulosin in the management of uric acid distal ureteral calculi. Urol. Res. 2012, 40, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.S.; Kwon, H.S.; Yang, W.; Lee, W.; Choi, C.; Kim, J.K.; Lee, S.H.; Rim, D.; Han, J.H. Prediction of the composition of urinary stones using deep learning. Investig. Clin. Urol. 2022, 63, 441–447. [Google Scholar] [CrossRef]
- Al-Ali, B.M.; Patzak, J.; Lutfi, A.; Pummer, K.; Augustin, H. Impact of urinary stone volume on computed tomography stone attenuations measured in hounsfield units in a large group of austrian patients with urolithiasis. Cent. Eur. J. Urol. 2014, 67, 289–295. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.H.; Kang, D.H.; Chung, D.Y.; Lee, D.H.; Do Jung, H.; Kwon, J.K.; Cho, K.S. Stone heterogeneity index as the standard deviation of hounsfield units: A novel predictor for shock-wave lithotripsy outcomes in ureter calculi. Sci. Rep. 2016, 6, 23988. [Google Scholar] [CrossRef]
- Yamashita, S.; Kohjimoto, Y.; Iguchi, T.; Nishizawa, S.; Iba, A.; Kikkawa, K.; Hara, I. Variation coefficient of stone density: A novel predictor of the outcome of extracorporeal shockwave lithotripsy. J. Endourol. 2017, 31, 384–390. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, K.S.; Lee, S.H.; Yoon, Y.E.; Kang, D.H.; Jeong, W.S.; Jung, H.D.; Kwon, J.K.; Lee, J.Y. Stone heterogeneity index on single-energy noncontrast computed tomography can be a positive predictor of urinary stone composition. PLoS ONE 2018, 13, e0193945. [Google Scholar] [CrossRef]
- Kim, J.C.; Cho, K.S.; Kim, D.K.; Chung, D.Y.; Jung, H.D.; Lee, J.Y. Predictors of uric acid stones: Mean stone density, stone heterogeneity index, and variation coefficient of stone density by single-energy non-contrast computed tomography and urinary ph. J. Clin. Med. 2019, 8, 243. [Google Scholar] [CrossRef]
- Yamashita, S.; Kohjimoto, Y.; Iwahashi, Y.; Iguchi, T.; Nishizawa, S.; Kikkawa, K.; Hara, I. Noncontrast computed tomography parameters for predicting shock wave lithotripsy outcome in upper urinary tract stone cases. BioMed Res. Int. 2018, 2018, 9253952. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Rule, A.D.; Krambeck, A.E.; Williams, J.C.; Bergstralh, E.J.; Mehta, R.A.; Moyer, T.P. Stone composition as a function of age and sex. Clin. J. Am. Soc. Nephrol. 2014, 9, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Coursey, C.A.; Casalino, D.D.; Remer, E.M.; Arellano, R.S.; Bishoff, J.T.; Dighe, M.; Fulgham, P.; Goldfarb, S.; Israel, G.M.; Lazarus, E.; et al. Acr appropriateness criteria® acute onset flank pain--suspicion of stone disease. Ultrasound Q 2012, 28, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Chan, M.; Brown, V.; Huo, Y.R.; Chan, L.; Ridley, L. Systematic review and meta-analysis of the diagnostic accuracy of low-dose computed tomography of the kidneys, ureters and bladder for urolithiasis. J. Med. Imaging Radiat. Oncol. 2017, 61, 582–590. [Google Scholar] [CrossRef]
- Federle, M.P.; McAninch, J.W.; Kaiser, J.A.; Goodman, P.C.; Roberts, J.; Mall, J.C. Computed tomography of urinary calculi. AJR Am. J. Roentgenol. 1981, 136, 255–258. [Google Scholar] [CrossRef]
- Yang, S.Y.; Jung, H.D.; Kwon, S.H.; Lee, E.K.; Lee, J.Y.; Lee, S.H. Does early retrograde intrarenal surgery improve the cost-effectiveness of renal stone management? Yonsei Med. J. 2020, 61, 515–523. [Google Scholar] [CrossRef]
- Joseph, P.; Mandal, A.K.; Singh, S.K.; Mandal, P.; Sankhwar, S.N.; Sharma, S.K. Computerized tomography attenuation value of renal calculus: Can it predict successful fragmentation of the calculus by extracorporeal shock wave lithotripsy? A preliminary study. J. Urol. 2002, 167, 1968–1971. [Google Scholar] [CrossRef]
- Zarse, C.A.; Hameed, T.A.; Jackson, M.E.; Pishchalnikov, Y.A.; Lingeman, J.E.; McAteer, J.A.; Williams, J.C., Jr. Ct visible internal stone structure, but not hounsfield unit value, of calcium oxalate monohydrate (com) calculi predicts lithotripsy fragility in vitro. Urol. Res. 2007, 35, 201–206. [Google Scholar] [CrossRef]
- Siener, R.; Herwig, H.; Rüdy, J.; Schaefer, R.M.; Lossin, P.; Hesse, A. Urinary stone composition in germany: Results from 45,783 stone analyses. World J. Urol. 2022, 40, 1813–1820. [Google Scholar] [CrossRef]
- Jung, H.D.; Seo, I.Y.; Lee, J.Y. Large database study of urinary stone composition in south korea: Korean society of endourology and robotics (kser) research series. Investig. Clin. Urol. 2021, 62, 462–469. [Google Scholar] [CrossRef]
- Türk, C.; Petřík, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Knoll, T. Eau guidelines on interventional treatment for urolithiasis. Eur. Urol. 2016, 69, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Seo, S.P.; Lee, H.Y.; Kim, K.; Ha, Y.S.; Kim, W.T.; Kim, Y.J.; Yun, S.J.; Kim, W.J.; Lee, S.C. A high basal metabolic rate is an independent predictor of stone recurrence in obese patients. Investig. Clin. Urol. 2021, 62, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.M.; Friedlander, J.I.; Hartman, C.; Elsamra, S.E.; Smith, A.D.; Okeke, Z. Using 24-h urinalysis to predict stone type. J. Urol. 2013, 190, 2106–2111. [Google Scholar] [CrossRef]
- Ahn, S.H.; Oh, T.H.; Seo, I.Y. Can a dual-energy computed tomography predict unsuitable stone components for extracorporeal shock wave lithotripsy? Korean J. Urol. 2015, 56, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Motley, G.; Dalrymple, N.; Keesling, C.; Fischer, J.; Harmon, W. Hounsfield unit density in the determination of urinary stone composition. Urology 2001, 58, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Teichman, J.M.; Long, R.D.; Hulbert, J.C. Long-term renal fate and prognosis after staghorn calculus management. J. Urol. 1995, 153, 1403–1407. [Google Scholar] [CrossRef]
- Akagashi, K.; Tanda, H.; Kato, S.; Ohnishi, S.; Nakajima, H.; Nanbu, A.; Nitta, T.; Koroku, M.; Sato, Y.; Hanzawa, T. Characteristics of patients with staghorn calculi in our experience. Int. J. Urol. 2004, 11, 276–281. [Google Scholar] [CrossRef]
- Viprakasit, D.P.; Sawyer, M.D.; Herrell, S.D.; Miller, N.L. Changing composition of staghorn calculi. J. Urol. 2011, 186, 2285–2290. [Google Scholar] [CrossRef]
- Haden, T.; Kuhlmann, P.; Ross, J.; Kalkhoff, S.; Johans, C.; Jones, A.; Weinstein, S.; Wakefield, M.; Hoyt, D.; Cummings, J.; et al. Is there a shift from infectious stones in staghorn calculi?: Mp01-13. J. Urol. 2017, 197, e5–e6. [Google Scholar] [CrossRef]
- Terry, R.S.; Preminger, G.M. Metabolic evaluation and medical management of staghorn calculi. Asian J. Urol. 2020, 7, 122–129. [Google Scholar] [CrossRef]
- Rahman, N.U.; Meng, M.V.; Stoller, M.L. Infections and urinary stone disease. Curr. Pharm. Des. 2003, 9, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.A.; Ogan, K. Pathophysiology and management of infectious staghorn calculi. Urol. Clin. N. Am. 2007, 34, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Yasui, T.; Okada, A.; Hamamoto, S.; Shimizu, H.; Itoh, Y.; Tozawa, K.; Kohri, K. Renal tubular epithelial cell injury and oxidative stress induce calcium oxalate crystal formation in mouse kidney. Int. J. Urol. 2010, 17, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Tozawa, K.; Okada, A.; Hamamoto, S.; Higashibata, Y.; Gao, B.; Hayashi, Y.; Shimizu, H.; Kubota, Y.; Yasui, T.; et al. Role of osteopontin in early phase of renal crystal formation: Immunohistochemical and microstructural comparisons with osteopontin knock-out mice. Urol. Res. 2012, 40, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: Evidence from clinical and experimental investigations. J. Urol. 2013, 189, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Okada, A.; Hamamoto, S.; Ando, R.; Taguchi, K.; Tozawa, K.; Kohri, K. Pathophysiology-based treatment of urolithiasis. Int. J. Urol. 2017, 24, 32–38. [Google Scholar] [CrossRef]
- Saw, K.C.; McAteer, J.A.; Monga, A.G.; Chua, G.T.; Lingeman, J.E.; Williams, J.C., Jr. Helical ct of urinary calculi: Effect of stone composition, stone size, and scan collimation. AJR Am. J. Roentgenol. 2000, 175, 329–332. [Google Scholar] [CrossRef]
- Tasian, G.E.; Kabarriti, A.E.; Kalmus, A.; Furth, S.L. Kidney stone recurrence among children and adolescents. J. Urol. 2017, 197, 246–252. [Google Scholar] [CrossRef]
- Tublin, M.E.; Murphy, M.E.; Delong, D.M.; Tessler, F.N.; Kliewer, M.A. Conspicuity of renal calculi at unenhanced ct: Effects of calculus composition and size and ct technique. Radiology 2002, 225, 91–96. [Google Scholar] [CrossRef]
- Bellin, M.F.; Renard-Penna, R.; Conort, P.; Bissery, A.; Meric, J.B.; Daudon, M.; Mallet, A.; Richard, F.; Grenier, P. Helical ct evaluation of the chemical composition of urinary tract calculi with a discriminant analysis of ct-attenuation values and density. Eur. Radiol. 2004, 14, 2134–2140. [Google Scholar] [CrossRef]
- Zarse, C.A.; McAteer, J.A.; Tann, M.; Sommer, A.J.; Kim, S.C.; Paterson, R.F.; Hatt, E.K.; Lingeman, J.E.; Evan, A.P.; Williams, J.C., Jr. Helical computed tomography accurately reports urinary stone composition using attenuation values: In vitro verification using high-resolution micro-computed tomography calibrated to fourier transform infrared microspectroscopy. Urology 2004, 63, 828–833. [Google Scholar] [CrossRef] [PubMed]
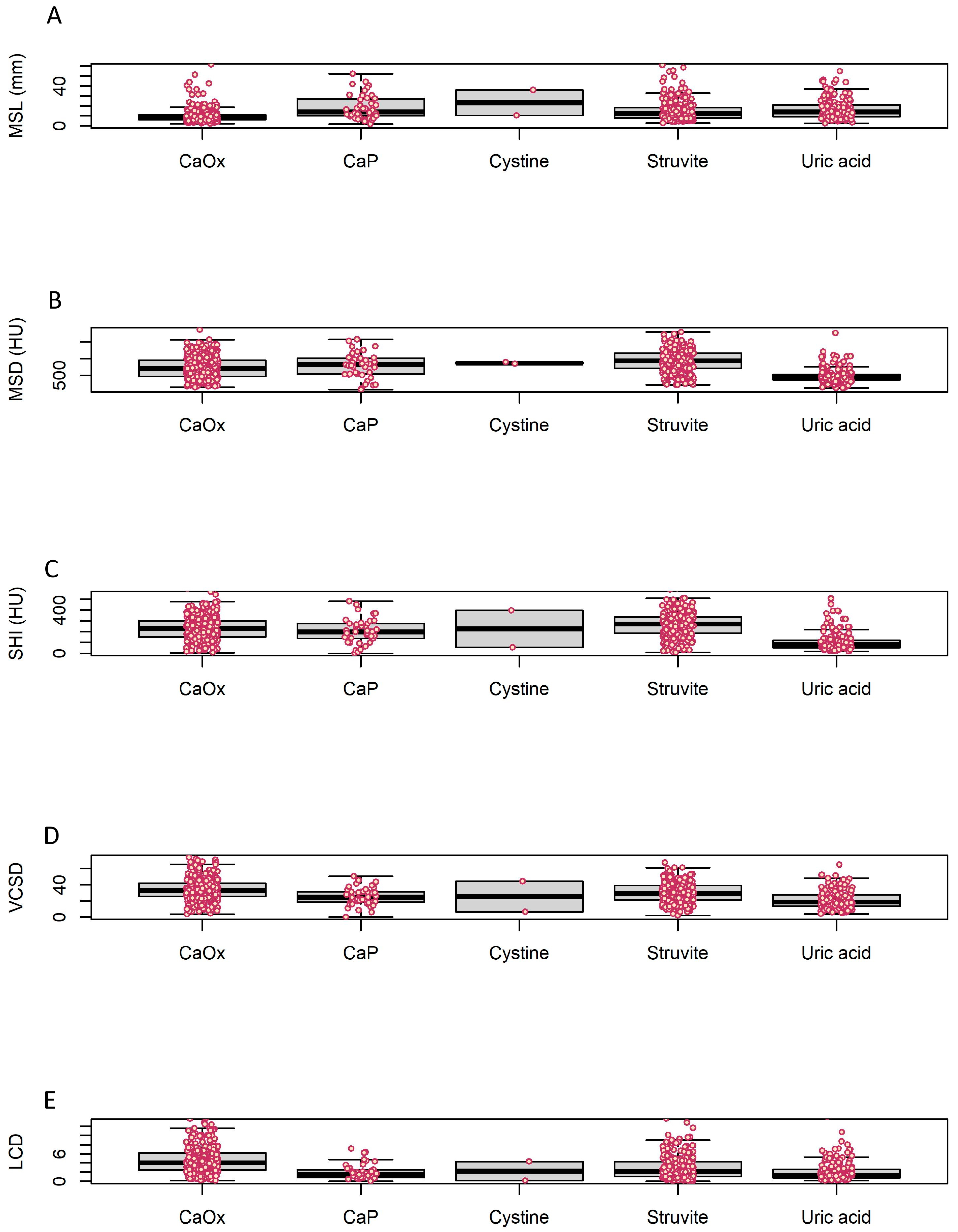
| Total (n = 790) | Struvite (n = 239) | Cystine (n = 2) | Uric Acid (n = 172) | CaOx (n = 335) | CaP (n = 42) | p-Value | |
|---|---|---|---|---|---|---|---|
| Age | 56.4 ± 15.6 | 55.7 ± 15.0 | 42.5 ± 30.4 | 62.2 ± 13.9 | 54.5 ± 15.5 | 52.2 ± 20.8 | <0.001 a |
| Sex | |||||||
| Male | 494 (62.5%) | 129 (54.0%) | 1 (50.0%) | 130 (75.6%) | 215 (64.2%) | 19 (45.2%) | <0.001 b |
| Female | 296 (37.5%) | 110 (46.0%) | 1 (50.0%) | 42 (24.4%) | 120 (35.8%) | 23 (54.8%) | |
| MSL | 15.4 ± 47.4 | 21.8 ± 84.7 | 23.1 ± 18.0 | 16.5 ± 10.1 | 9.8 ± 7.0 | 18.6 ± 12.3 | 0.052 a |
| MSD | 735.5 ± 347.4 | 919.9 ± 339.0 | 864.8 ± 38.3 | 488.8 ± 224.3 | 722.6 ± 324.1 | 792.3 ± 362.0 | <0.001 a |
| SHI | 213.0 ± 123.6 | 263.0 ± 116.5 | 225.1 ± 242.9 | 109.1 ± 95.6 | 230.9 ± 107.5 | 210.8 ± 137.3 | <0.001 a |
| VCSD | 29.5 ± 13.6 | 30.0 ± 12.8 | 25.4 ± 27.0 | 21.5 ± 11.2 | 33.8 ± 13.5 | 25.5 ± 11.2 | <0.001 a |
| LCD | 3.5 ± 2.9 | 3.0 ± 2.5 | 2.2 ± 2.9 | 2.1 ± 2.1 | 4.7 ± 3.1 | 2.0 ± 1.7 | <0.001 a |
| Urine pH | 6.0 ± 0.9 | 6.3 ± 0.9 | 5.8 ± 1.1 | 5.4 ± 0.6 | 6.0 ± 0.8 | 6.8 ± 1.0 | <0.001 a |
| Total (N = 790) | CaOx (N = 335) | Non-CaOx (N = 455) | p-Value a | |
|---|---|---|---|---|
| Age | 56.4 ± 15.6 | 54.5 ± 15.5 | 57.8 ± 15.6 | 0.004 |
| Sex | 0.455 | |||
| Male | 494 (62.5%) | 215 (64.2%) | 279 (61.3%) | |
| Female | 296 (37.5%) | 120 (35.8%) | 176 (38.7%) | |
| MSL | 15.4 ± 47.4 | 9.8 ± 7.0 | 19.5 ± 61.8 | 0.001 |
| MSD | 735.5 ± 347.4 | 722.6 ± 324.1 | 744.9 ± 363.7 | 0.365 |
| SHI | 213.0 ± 123.6 | 230.9 ± 107.5 | 199.8 ± 132.8 | <0.001 |
| VCSD | 29.5 ± 13.6 | 33.8 ± 13.5 | 26.3 ± 12.7 | <0.001 |
| LCD | 3.5 ± 2.9 | 4.7 ± 3.1 | 2.6 ± 2.3 | <0.001 |
| Urine pH | 6.0 ± 0.9 | 6.0 ± 0.8 | 6.0 ± 0.9 | 0.382 |
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Univariate | |||
| Age | 0.987 | 0.978–0.996 | 0.004 |
| Sex | 1.130 | 0.844–1.516 | 0.412 |
| MSL | 0.906 | 0.884–0.927 | <0.001 |
| MSD | 0.999 | 0.999–1.000 | 0.373 |
| SHI | 1.002 | 1.001–1.003 | <0.001 |
| VCSD | 1.044 | 1.032–1.056 | <0.001 |
| LCD | 1.359 | 1.277–1.450 | <0.001 |
| Urine pH | 1.072 | 0.914–1.258 | 0.392 |
| Multivariate (with MSL & SHI) | |||
| Age | 0.989 | 0.979–0.999 | 0.028 |
| MSL | 0.904 | 0.881–0.926 | <0.001 |
| SHI | 1.002 | 1.001–1.004 | <0.001 |
| Multivariate (with MSL & VCSD) | |||
| Age | 0.991 | 0.981–1.001 | 0.080 |
| MSL | 0.923 | 0.900–0.944 | <0.001 |
| VCSD | 1.028 | 1.016–1.041 | <0.001 |
| Multivariate (with LCD) | |||
| Age | 0.995 | 0.985–1.005 | 0.296 |
| LCD | 1.352 | 1.270–1.444 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.Y.; Cho, K.S.; Kim, D.H.; Jun, D.Y.; Moon, Y.J.; Lee, J.Y. A New Parameter for Calcium Oxalate Stones: Impact of Linear Calculus Density on Non-Contrast Computed Tomography. Medicina 2023, 59, 267. https://doi.org/10.3390/medicina59020267
Jeong JY, Cho KS, Kim DH, Jun DY, Moon YJ, Lee JY. A New Parameter for Calcium Oxalate Stones: Impact of Linear Calculus Density on Non-Contrast Computed Tomography. Medicina. 2023; 59(2):267. https://doi.org/10.3390/medicina59020267
Chicago/Turabian StyleJeong, Jae Yong, Kang Su Cho, Dae Ho Kim, Dae Young Jun, Young Joon Moon, and Joo Yong Lee. 2023. "A New Parameter for Calcium Oxalate Stones: Impact of Linear Calculus Density on Non-Contrast Computed Tomography" Medicina 59, no. 2: 267. https://doi.org/10.3390/medicina59020267
APA StyleJeong, J. Y., Cho, K. S., Kim, D. H., Jun, D. Y., Moon, Y. J., & Lee, J. Y. (2023). A New Parameter for Calcium Oxalate Stones: Impact of Linear Calculus Density on Non-Contrast Computed Tomography. Medicina, 59(2), 267. https://doi.org/10.3390/medicina59020267







