Efficacy of Different Fluoride Therapies on Hypersensitive Carious Lesions in Primary Teeth
Abstract
:1. Introduction
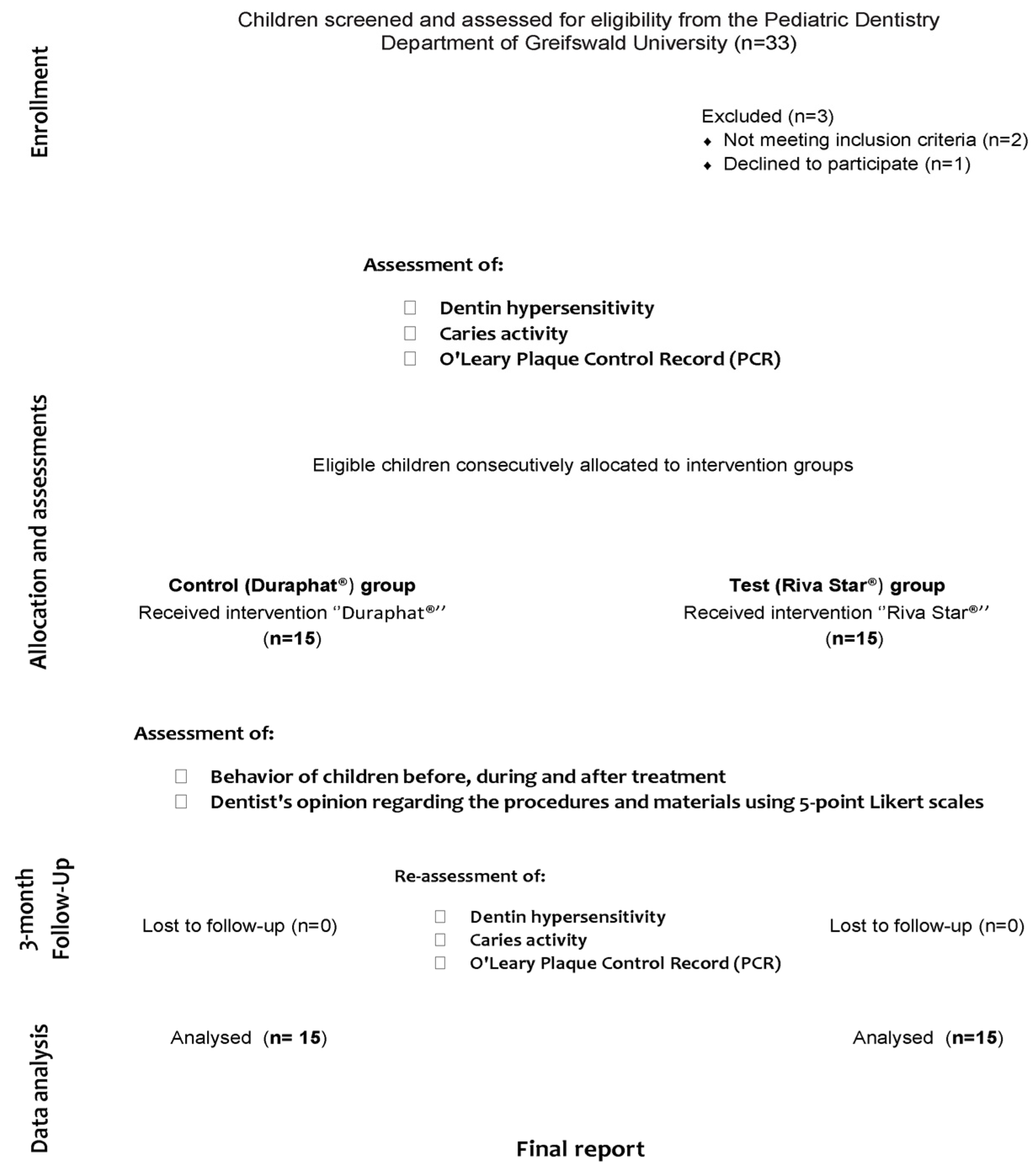
2. Materials and Methods
2.1. Sample
2.2. Treating Dentists
2.3. Participant Screening, Eligibility, and Baseline Assessment
- Hypersensitivity pain;
- Carious lesion activity and pulp status;
- Behavior of children before, during, and after treatment;
- Dentist’s opinion regarding the procedures, materials, procedure duration, and child’s discomfort within the procedure in both groups using 5-point Likert scales;
- O′Leary Plaque Control Record (PCR).
2.4. Treatment Procedures and Assessment
2.5. Statistical Analysis
3. Results
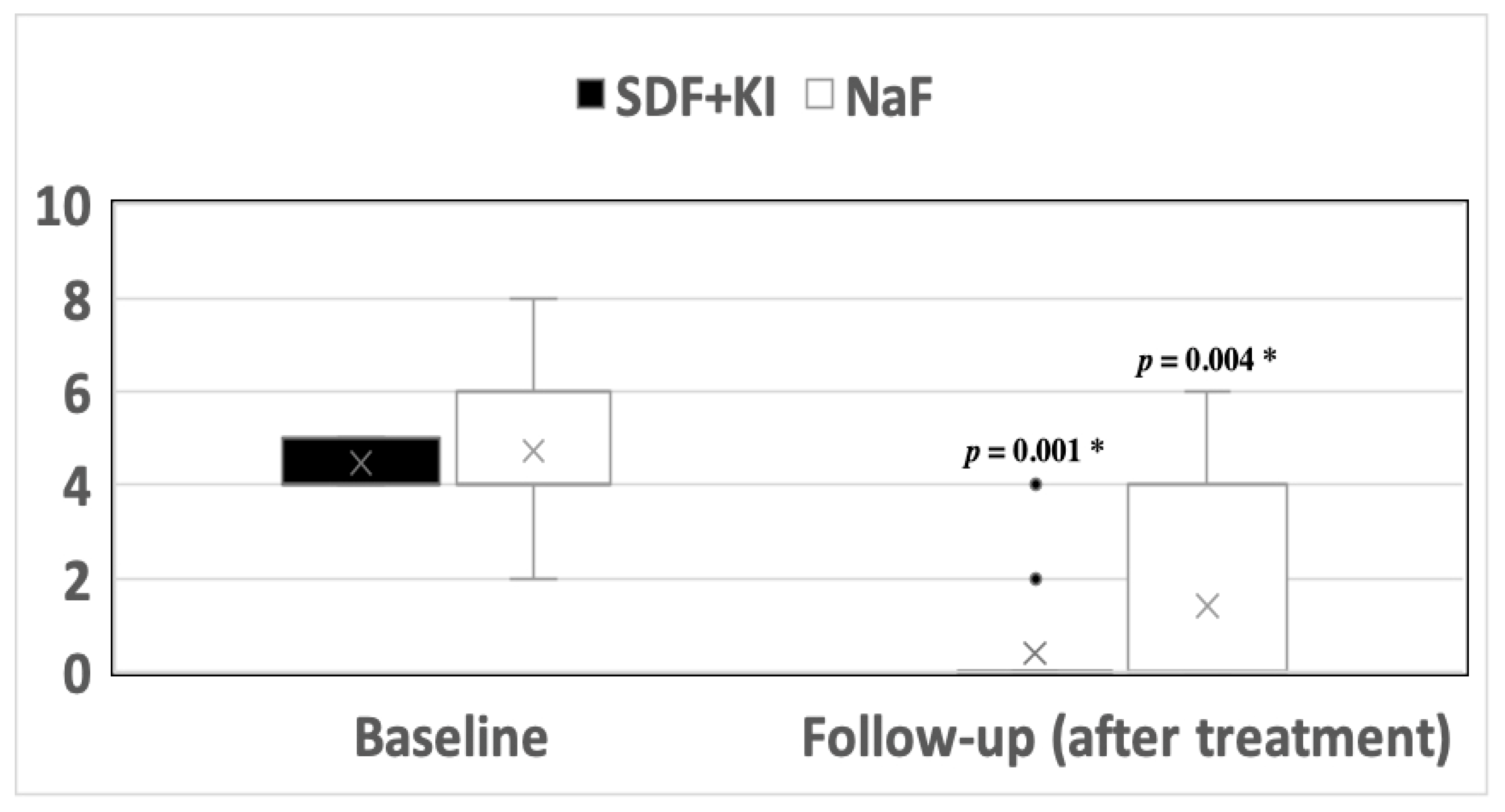
3.1. Hypersensitivity
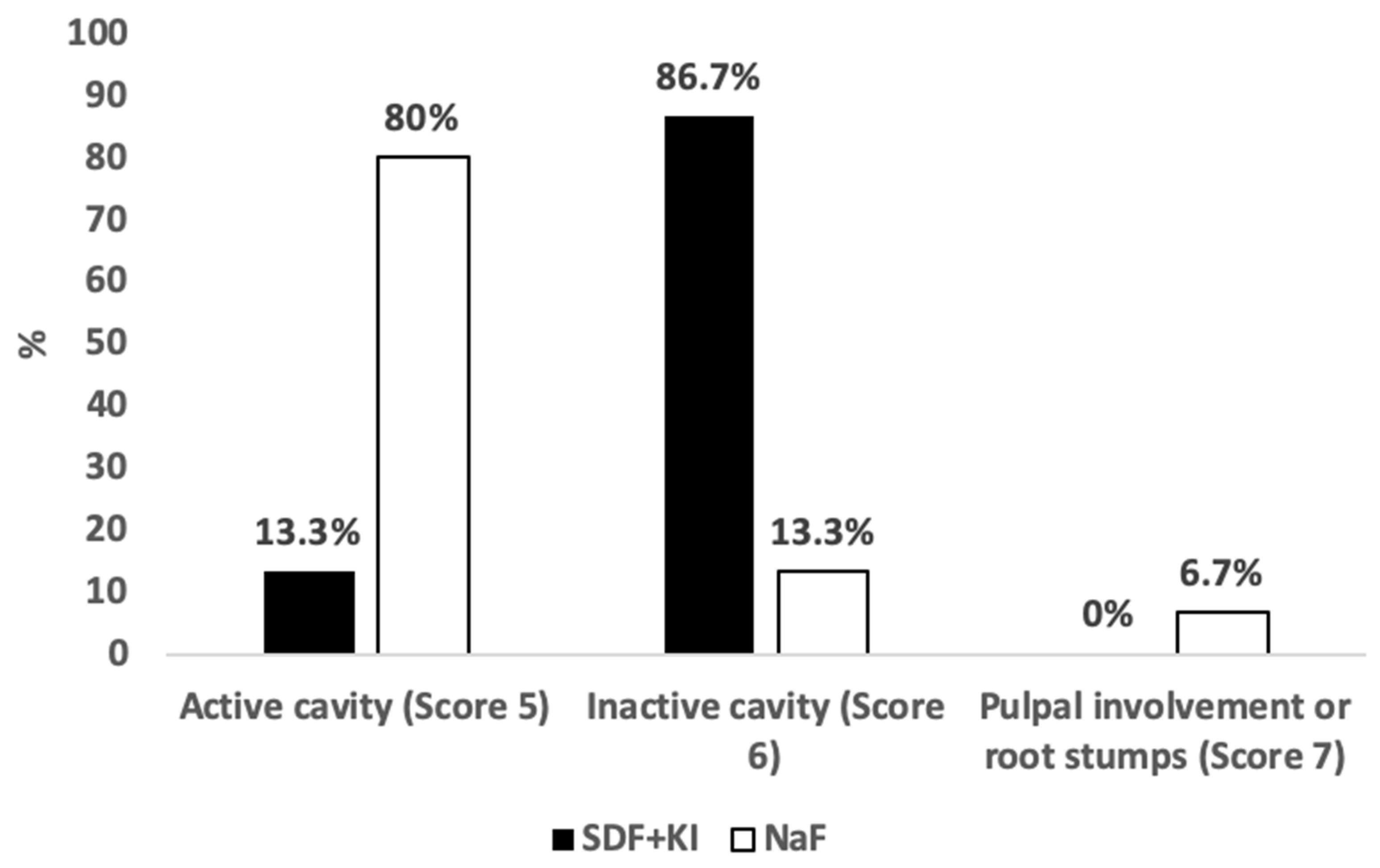
3.2. Caries Activity
3.3. Children’s Behavior
3.4. O’Leary Plaque Control Record (PCR)
3.5. Dentist’s Opinions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sivaramakrishnan, G.; Sridharan, K. Fluoride varnish versus glutaraldehyde for hypersensitive teeth: A randomized controlled trial, meta-analysis and trial sequential analysis. Clin. Oral Investig. 2019, 23, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Shiau, H.J. Dentin hypersensitivity. J. Evid. Based Dent. Pract. 2012, 12, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Splieth, C.H.; Tachou, A. Epidemiology of dentin hypersensitivity. Clin. Oral Investig. 2013, 17 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Soni, A.; Khan, M.; Kauser, A.; Charan, V.S.; Akula, S. Efficacy of remineralizing agents to occlude dentinal tubules in primary teeth subjected to dentin hypersensitivity in vitro: SEM study. J. Fam. Med. Prim. Care 2020, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2019, 54, 7–14. [Google Scholar] [CrossRef]
- Çolak, H.; Dülgergil, Ç.; Dalli, M.; Hamidi, M. Early childhood caries update: A review of causes, diagnoses, and treatments. J. Nat. Sci. Biol. Med. 2013, 4, 29. [Google Scholar] [CrossRef]
- Cummins, D. Dentin hypersensitivity: From diagnosis to a breakthrough therapy for everyday sensitivity relief. J. Clin. Dent. 2009, 20, 1–9. [Google Scholar]
- Ritter, A.V.; Dias, W.D.L.; Miguez, P.; Caplan, D.J.; Swift, E.J. Treating cervical dentin hypersensitivity with fluoride varnish: A randomized clinical study. J. Am. Dent. Assoc. 2006, 137, 1013–1020. [Google Scholar] [CrossRef]
- Milgrom, P.; Rothen, M.; Spadafora, A.; Skaret, E. A case report: Arresting dental caries. J. Dent. Hyg. JDH 2001, 75, 241–243. [Google Scholar]
- FDA. Food and Drug Administration. Diammine Silver Fluoride Dental Hypersensitivity Varnish. 510(k) Premarket Notification. 2017. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K102973 (accessed on 20 January 2020).
- Gao, S.S.; Amarquaye, G.; Arrow, P.; Bansal, K.; Bedi, R.; Campus, G.; Chen, K.J.; Chibinski, A.C.R.; Chinzorig, T.; Crystal, Y.O.; et al. Global Oral Health Policies and Guidelines: Using Silver Diamine Fluoride for Caries Control. Front. Oral Health 2021, 2, 685557. [Google Scholar] [CrossRef]
- Slayton, R.L.; Urquhart, O.; Araujo, M.W.; Fontana, M.; Guzmán-Armstrong, S.; Nascimento, M.M.; Nový, B.B.; Tinanoff, N.; Weyant, R.J.; Wolff, M.S.; et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J. Am. Dent. Assoc. 2018, 149, 837–849. [Google Scholar] [CrossRef]
- Chu, C.H.; Lo, E.C.M.; Lin, H.C. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J. Dent. Res. 2002, 81, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Crystal, Y.O.; Janal, M.N.; Hamilton, D.S.; Niederman, R. Parental perceptions and acceptance of silver diamine fluoride staining. J. Am. Dent. Assoc. 2017, 148, 510–518.e4. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.M.; McIntyre, J.M.; Craig, G.G. Ion uptake into demineralized dentine from glass ionomer cement following pretreatment with silver fluoride and potassium iodide. Aust. Dent. J. 2006, 51, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.M.; McIntyre, J.M.; Craig, G.G.; Mulyani Zilm, P.S.; Gully, N.J. An in vitro model to measure the effect of a silver fluoride and potassium iodide treatment on the permeability of demineralized dentine to Streptococcus mutans. Aust. Dent. J. 2005, 50, 242–245. [Google Scholar] [CrossRef]
- Hamama, H.H.; Yiu, C.K.; Burrow, M.F. Effect of silver diamine fluoride and potassium iodide on residual bacteria in dentinal tubules. Aust. Dent. J. 2015, 60, 80–87. [Google Scholar] [CrossRef]
- Koizumi, H.; Hamama, H.H.; Burrow, M.F. Effect of a silver diamine fluoride and potassium iodide-based desensitizing and cavity cleaning agent on bond strength to dentine. Int. J. Adhes. Adhes. 2016, 68, 54–61. [Google Scholar] [CrossRef]
- Turton, B.; Horn, R.; Durward, C. Caries arrest and lesion appearance using two different silver fluoride therapies on primary teeth with and without potassium iodide: 12-month results. Clin. Exp. Dent. Res. 2021, 7, 609–619. [Google Scholar] [CrossRef]
- de Souza-e-Silva, C.M.; Parisotto, T.M.; Steiner-Oliveira, C.; Gavião, M.B.D.; Nobre-dos-Santos, M. Oral rehabilitation of primary dentition affected by amelogenesis imperfecta: A case report. J. Contemp. Dent. Pract. 2010, 11, 71–77. [Google Scholar] [CrossRef]
- dos Santos, M.; Brito, D.; Buzanscki, A.; Vieira, T.; Maia, L. A Minimally Invasive Approach to Manage Dental Pain in a Child with Enamel Dental Defects: 18-Month Results. J. Bacteriol. Parasitol. 2013, 2, 596. [Google Scholar] [CrossRef]
- Bjorndal, L.; Larsen, T.; Thylstrup, A. A clinical and microbiological study of deep carious lesions during stepwise excavation using long treatment intervals. Caries Res. 1997, 31, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Ekstrand, K.R. International Caries Detection and Assessment System (ICDAS) and its International Caries Classification and Management System (ICCMS)—Methods for staging of the caries process and enabling dentists to manage caries. Community Dent. Oral Epidemiol. 2013, 41, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.C.C.M.; Andrade, A.K.M.; Montes, M.A.J.R. Diagnosis and treatment of dentinal hypersensitivity. J. Oral Sci. 2009, 51, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Gillam, D.G.; Orchardson, R. Advances in the treatment of root dentine sensitivity: Mechanisms and treatment principles. Endod. Top. 2006, 13, 13–33. [Google Scholar] [CrossRef]
- Franck, L.S.; Greenberg, C.S.; Stevens, B. Pain assessment in infants and children. Pediatr. Clin. N. Am. 2000, 47, 487–512. [Google Scholar] [CrossRef]
- Frankl, S.; Shiere, F.; Fogels, H. Should the parent remain with the child in the dental operatory? J. Dent. Child. 1962, 29, 150–163. [Google Scholar]
- Versloot, J.; Craig, K.D. The communication of pain in paediatric dentistry. Eur. Arch. Paediatr. Dent. Off. J. Eur. Acad. Paediatr. Dent. 2009, 10, 61–66. [Google Scholar] [CrossRef]
- Versloot, J.; Veerkamp, J.S.J.; Hoogstraten, J. Dental Discomfort Questionnaire: Assessment of dental discomfort and/or pain in very young children. Community Dent. Oral Epidemiol. 2006, 34, 47–52. [Google Scholar] [CrossRef]
- Boeira, G.; Correa, M.; Peres, K.; Peres, M.; Santos, I.; Matijasevich, A.; Barros, A.; Demarco, F. Caries is the main cause for dental pain in childhood: Findings from a birth cohort. Caries Res. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Ferreira-Júnior, O.M.; Freire, M.d.C.M.; Moreira, R.d.S.; Costa, L.R. Contextual and individual determinants of dental pain in preschool children. Community Dent. Oral Epidemiol. 2015, 43, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Paepegaey, A.-M.; Barker, M.L.; Bartlett, D.W.; Mistry, M.; West, N.X.; Hellin, N.; Brown, L.J.; Bellamy, P.G. Measuring enamel erosion: A comparative study of contact profilometry, non-contact profilometry and confocal laser scanning microscopy. Dent. Mater. 2013, 29, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Rivera, S.; Aparicio, T.; Lazo, R.; Aw, T.-C.; Mancl, L.; Milgrom, P. The short-term effects of diammine silver fluoride on tooth sensitivity: A randomized controlled trial. J. Dent. Res. 2011, 90, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Craig, G.G.; Knight, G.M.; McIntyre, J.M. Clinical evaluation of diamine silver fluoride/potassium iodide as a dentine desensitizing agent: A pilot study. Aust. Dent. J. 2012, 57, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, J.D.; Pashley, D.H. The Effects of Desensitizing Agents on the Hydraulic Conductance of Human Dentin in vitro. J. Dent. Res. 1981, 60, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Thrash, W.J.; Jones, D.L.; Dodds, W.J. Effect of a fluoride solution on dentinal hypersensitivity. Am. J. Dent. 1992, 5, 299–302. [Google Scholar]
- Sihra, R.; Schroth, R.J.; Bertone, M.; Martin, H.; Mittermuller, B.-A.; Lee, V.; Patterson, B.; Moffatt, M.E.; Klus, B.; Fontana, M.; et al. The Effectiveness of Silver Diamine Fluoride and Fluoride Varnish in Arresting Caries in Young Children and Associated Oral Health-Related Quality of Life. J. Can. Dent. Assoc. 2020, 86, 1488–2159. [Google Scholar]
- Duangthip, D.; Chu, C.H.; Lo, E.C.M. A randomized clinical trial on arresting dentine caries in preschool children by topical fluorides—18 month results. J. Dent. 2016, 44, 57–63. [Google Scholar] [CrossRef]
- Trieu, A.; Mohamed, A.; Lynch, E. Silver diamine fluoride versus sodium fluoride for arresting dentine caries in children: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 2115. [Google Scholar] [CrossRef]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.L.; Chu, C.H.; Lo, E.C.M. The inhibitory effects of silver diamine fluorides on cysteine cathepsins. J. Dent. 2014, 42, 329–335. [Google Scholar] [CrossRef]
- Mei, M.L.; Li, Q.L.; Chu, C.H.; Yiu, C.K.Y.; Lo, E.C.M. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent. Mater. 2012, 28, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Holmen, L.; Mejare, I.; Malmgren, B.; Thvlstrup, A. The effect of regular professional plaque removal on dental caries in vivo a polarized light and scanning electron microscope study: A Polarized light and Scanning Electron Microscope Study. Caries Res. 1988, 22, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Farahani, M.R.D.; Marino, G.; Matera, C.; Baena, R.R.Y.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. Biomed. Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [PubMed]
- Alkilzy, M.; Qadri, G.; Splieth, C.H.; Santamaría, R.M. Biomimetic Enamel Regeneration Using Self-Assembling Peptide P(11)-4. Biomimetics 2023, 8, 290. [Google Scholar] [CrossRef]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.; Brookes, S.; Aggeli, A. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef]
- Kirkham, J.; Brookes, S.J.; Shore, R.C.; Wood, S.R.; Smith, D.; Zhang, J.; Chen, H.; Robinson, C. Physico-chemical properties of crystal surfaces in matrix–mineral interactions during mammalian biomineralisation. Curr. Opin. Colloid. Interface Sci. 2002, 7, 124–132. [Google Scholar] [CrossRef]
- Sindhura, V.; Uloopi, K.S.; Vinay, C.; Chandrasekhar, R. Evaluation of enamel remineralizing potential of self-assembling peptide P(11)-4 on artificially induced enamel lesions in vitro. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 352–356. [Google Scholar] [CrossRef]
- Seifo, N.; Cassie, H.; Radford, J.; Innes, N. “It’s really no more difficult than putting on fluoride varnish”: A qualitative exploration of dental professionals’ views of silver diamine fluoride for the management of carious lesions in children. BMC Oral Health 2020, 20, 257. [Google Scholar] [CrossRef]
| Score | Definition |
|---|---|
| 0 | Sound |
| 1 | Active lesion in enamel, without cavity (bright surface with brown discoloration) |
| 2 | Active cavity in enamel (opaque enamel surface and loss of substance) |
| 3 | Active cavity in enamel (bright surface, brown discoloration, wet dentin) |
| 4 | Inactive cavity in enamel (bright surface, brown discoloration, and loss of substance) |
| 5 | Active cavity in enamel/dentin (yellow or light brown discoloration, wet dentin) |
| 6 | Inactive cavity in enamel/dentin (dark brown discoloration, hard and dry dentin) |
| 7 | Pulpal involvement or root stumps |
| 8 | Filled tooth |
| 9 | Missing tooth |
| Test (Riva Star®, n = 15) | Control (Duraphat®, n = 15) | Total | p-Value | ||
|---|---|---|---|---|---|
| Age, mean ± SD | 3.58 ± 0.95 | 3.76 ± 1.18 | 3.67 ± 1.06 | 0.65 | |
| Gender, n (%) | Males | 9 (60%) | 7 (46.7%) | 16 (53.3%) | 0.46 |
| Females | 6 (40%) | 8 (53.3%) | 14 (46.7%) | ||
| Caries index, tooth level, mean ± SD | dt | 6.73 ± 2.43 | 6.87 ± 3.14 | 6.80 ± 2.56 | 0.90 |
| mt | 0.13 ± 0.52 | 0.00 ± 0.00 | 0.07 ± 0.37 | 0.78 | |
| ft | 1.20 ± 1.86 | 0.53 ± 0.92 | 0.87 ± 1.48 | 0.54 | |
| dmft | 8.07 ± 2.79 | 7.40 ± 3.56 | 7.73 ± 3.16 | 0.57 | |
| Caries index, surface level, mean ± SD | ds | 18.73 ± 10.48 | 14.40 ± 8.34 | 16.57 ± 9.56 | 0.25 |
| ms | 0.67 ± 2.58 | 0.00 ± 0.00 | 0.33 ± 1.83 | 0.78 | |
| fs | 4.53 ± 7.61 | 1.87 ± 3.87 | 3.20 ± 6.09 | 0.57 | |
| dmfs | 23.93 ± 14.75 | 16.27 ± 8.96 | 20.10 ± 12.61 | 0.17 | |
| Characteristics | Test (Riva Star®, n = 15) | Control (Duraphat®, n = 15) | Mann–Whitney p-Value | |
|---|---|---|---|---|
| Baseline | Definitely negative | 1 (6.7%) | 3 (20%) | 0.54 |
| Negative | 3 (20%) | 3 (20%) | ||
| Positive | 8 (53.3%) | 6 (40%) | ||
| Definitely positive | 3 (20%) | 3 (20%) | ||
| During treatment | Definitely negative | 1 (6.7%) | 0 (0%) | 0.29 |
| Negative | 8 (53.3%) | 5 (33.3%) | ||
| Positive | 3 (20%) | 7 (46.7%) | ||
| Definitely positive | 3 (20%) | 3 (20%) | ||
| After treatment | Definitely negative | 1 (6.7%) | 0 (0%) | 0.62 |
| Negative | 4 (26.7%) | 2 (13.3%) | ||
| Positive | 6 (40%) | 10 (66.7%) | ||
| Definitely positive | 4 (26.7%) | 3 (20%) | ||
| Friedman Test p-value | 0.07 | 0.36 | ||
| Questions | Test (Riva Star®) | Control (Duraphat®) | p-Value | |
|---|---|---|---|---|
| n (%) | ||||
| Procedure Undertaken | Very Easy | 4 (26.7%) | 9 (60%) | 0.08 |
| Easy | 8 (53.3%) | 5 (33.3%) | ||
| Manageable | 1 (6.7%) | 1 (6.7%) | ||
| Difficult | 2 (13.3%) | 0 (0%) | ||
| Very Difficult | 0 (0%) | 0 (0%) | ||
| Materials Used in this Procedure | Very Easy to Handle | 9 (60%) | 11 (73.3%) | 0.62 |
| Easy to Handle | 6 (40%) | 3 (20%) | ||
| Manageable | 0 (0%) | 1 (6.7%) | ||
| Difficult to Handle | 0 (0%) | 0 (0%) | ||
| Very Difficult to Handle | 0 (0%) | 0 (0%) | ||
| Procedure Duration | Very Short/Short | 10 (66.7%) | 14 (93.3%) | 0.17 |
| Time Efficient | 4 (26.7%) | 1 (6.7%) | ||
| Long | 1 (6.7%) | 0 (0%) | ||
| Very Long | 0 (0%) | 0 (0%) | ||
| Child’s Discomfort with the Procedure | No Apparent Discomfort | 4 (26.7%) | 8 (53.3%) | 0.10 |
| Very Mild Discomfort | 2 (13.3%) | 2 (13.3%) | ||
| Mild Non-significant Discomfort | 5 (33.3%) | 4 (26.7%) | ||
| Moderate Discomfort | 3 (20%) | 1 (6.7%) | ||
| Significant Unacceptable Discomfort | 1 (6.7%) | 0 (0%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudrya, M.; Splieth, C.H.; Mourad, M.S.; Santamaría, R.M. Efficacy of Different Fluoride Therapies on Hypersensitive Carious Lesions in Primary Teeth. Medicina 2023, 59, 2042. https://doi.org/10.3390/medicina59112042
Abudrya M, Splieth CH, Mourad MS, Santamaría RM. Efficacy of Different Fluoride Therapies on Hypersensitive Carious Lesions in Primary Teeth. Medicina. 2023; 59(11):2042. https://doi.org/10.3390/medicina59112042
Chicago/Turabian StyleAbudrya, Mohamed, Christian H. Splieth, Mhd Said Mourad, and Ruth M. Santamaría. 2023. "Efficacy of Different Fluoride Therapies on Hypersensitive Carious Lesions in Primary Teeth" Medicina 59, no. 11: 2042. https://doi.org/10.3390/medicina59112042
APA StyleAbudrya, M., Splieth, C. H., Mourad, M. S., & Santamaría, R. M. (2023). Efficacy of Different Fluoride Therapies on Hypersensitive Carious Lesions in Primary Teeth. Medicina, 59(11), 2042. https://doi.org/10.3390/medicina59112042







