Effects of an Impaired Fasting Glucose on the Left Atrial Strain Evaluated by Speckle Tracking Echocardiography
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Echocardiographic Assessment
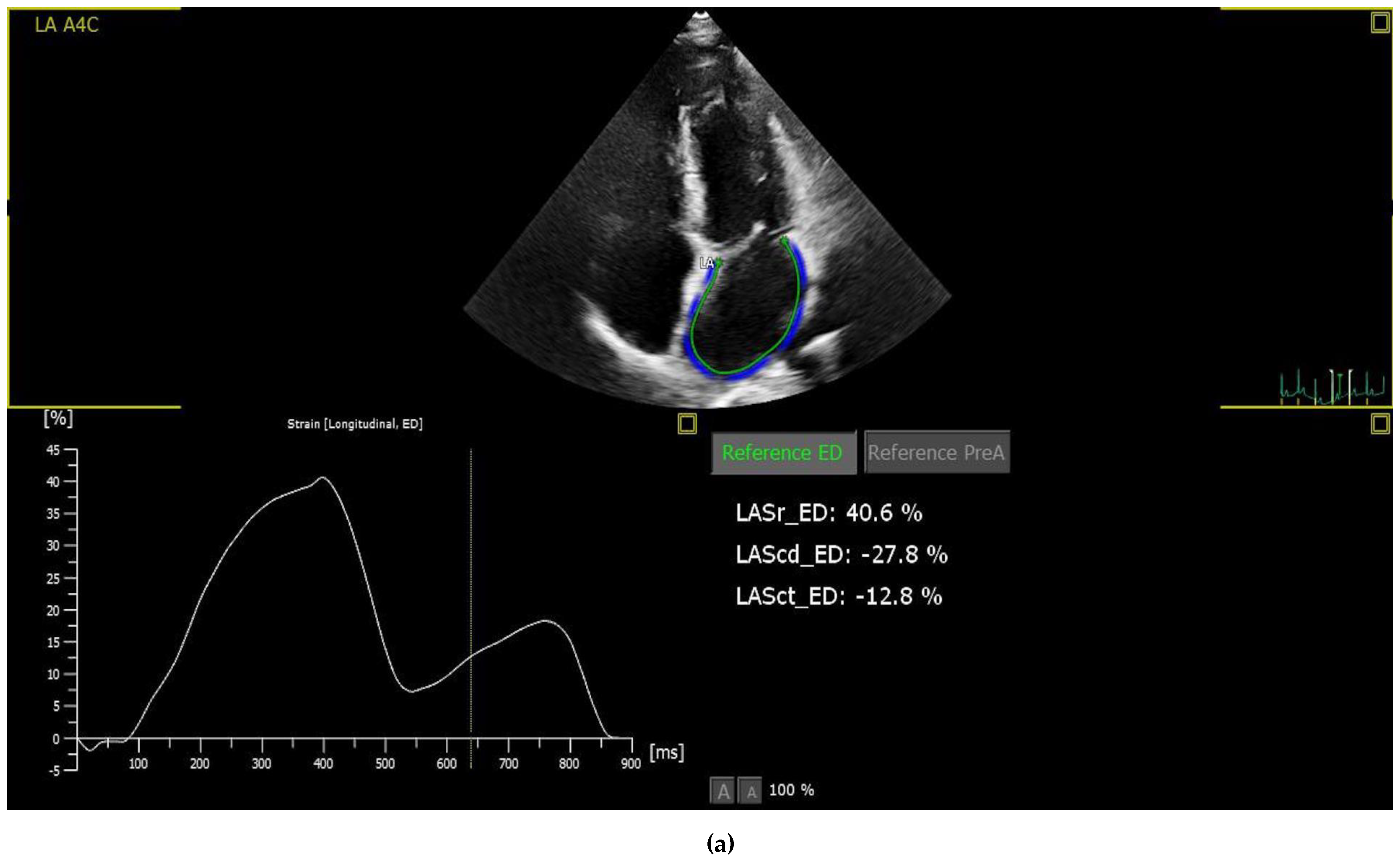
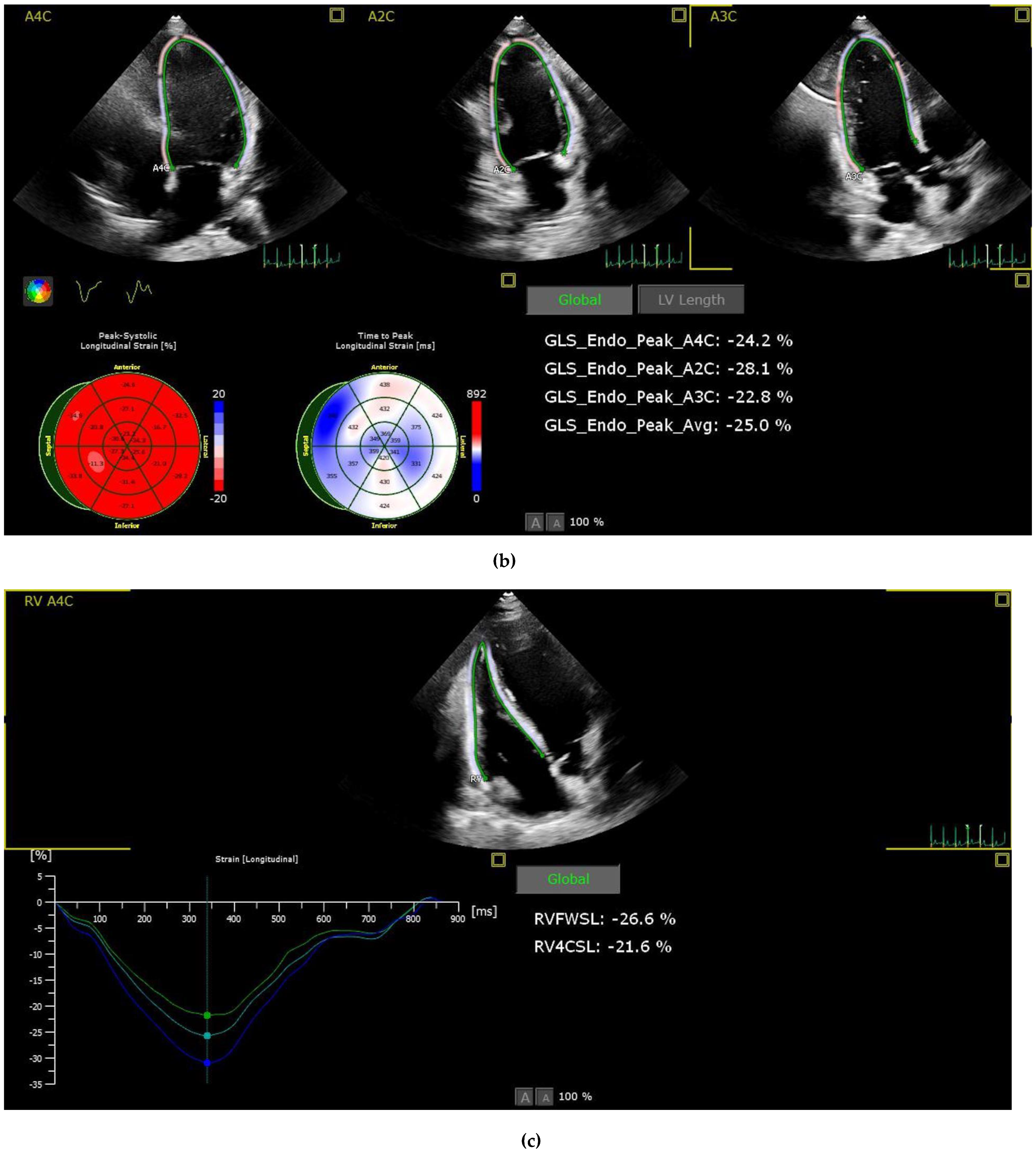
2.3. Strain Analysis
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
Left Atrium Function Differences between the Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Koro, C.E.; Kolatkar, N.S. The incidence of heart failure among nondiabetic patients with and without impaired fasting glucose. J. Diabetes Complicat. 2009, 23, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Milwidsky, A.; Maor, E.; Kivity, S.; Berkovitch, A.; Zekry, S.B.; Tenenbaum, A.; Fisman, E.Z.; Erez, A.; Segev, S.; Sidi, Y.; et al. Impaired fasting glucose and left ventricular diastolic dysfunction in middle-age adults: A retrospective cross-sectional analysis of 2971 subjects. Cardiovasc. Diabetol. 2015, 14, 119. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Martinis, F.; Pezzutto, F.; Sechi, L.A. Plasma glucose levels and left ventricular diastolic function in nondiabetic hypertensive patients. Am. J. Hypertens. 2013, 26, 1353–1361. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Dini, F.L.; Henein, M.; Mondillo, S. Left atrial strain: A new parameter for assessment of left ventricular filling pressure. Heart Fail. Rev. 2016, 21, 65–76. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Pastore, M.C.; Mandoli, G.E.; Santoro, C.; Cavigli, L.; Focardi, M.; D’ascenzi, F.; Cameli, M. Left atrial strain in cardiovascular diseases: An overview of clinical applications. Cardiol. Hung. 2021, 51, 11–17. [Google Scholar] [CrossRef]
- Corradi, D.; Callegari, S.; Benussi, S.; Maestri, R.; Pastori, P.; Nascimbene, S.; Bosio, S.; Dorigo, E.; Grassani, C.; Rusconi, R.; et al. Myocyte changes and their left atrial distribution in patients with chronic atrial fibrillation related to mitral valve disease. Hum. Pathol. 2005, 36, 1080–1089. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Pastore, M.C.; Benfari, G.; Bisleri, G.; Maccherini, M.; Lisi, G.; Cameli, P.; Lisi, M.; Dokollari, A.; Carrucola, C.; et al. Left atrial strain as a pre-operative prognostic marker for patients with severe mitral regurgitation. Int. J. Cardiol. 2021, 324, 139–145. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Sparla, S.; Iardino, E.; Mondillo, S. Left atrial strain: A useful index in atrial fibrillation. Int. J. Cardiol. 2016, 220, 208–213. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Shi, K.; Yang, M.X.; Huang, S.; Yan, W.F.; Qian, W.L.; Li, Y.; Guo, Y.K.; Yang, Z.G. Effect of diabetes mellitus on the development of left ventricular contractile dysfunction in women with heart failure and preserved ejection fraction. Cardiovasc. Diabetol. 2021, 20, 185. [Google Scholar] [CrossRef]
- Georgievska-Ismail, L.; Zafirovska, P.; Hristovski, Z. Evaluation of the role of left atrial strain using two-dimensional speckle tracking echocardiography in patients with diabetes mellitus and heart failure with preserved left ventricular ejection fraction. Diabetes Vasc. Dis. Res. 2016, 13, 384–394. [Google Scholar] [CrossRef]
- Boyer, J.K.; Thanigaraj, S.; Schechtman, K.B.; Pérez, J.E. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am. J. Cardiol. 2004, 93, 870–875. [Google Scholar] [CrossRef]
- Iguchi, Y.; Kimura, K.; Aoki, J.; Kobayashi, K.; Terasawa, Y.; Sakai, K.; Shibazaki, K. Prevalence of atrial fibrillation in community-dwelling Japanese aged 40 years or older in Japan: Analysis of 41,436 non-employee residents in Kurashiki-city. Circ. J. Off. J. Jpn. Circ. Soc. 2008, 72, 909–913. [Google Scholar] [CrossRef]
- Nichols, G.A.; Hillier, T.A.; Erbey, J.R.; Brown, J.B. Congestive heart failure in type 2 diabetes: Prevalence, incidence, and risk factors. Diabetes Care 2001, 24, 1614–1619. [Google Scholar] [CrossRef]
- Polovina, M.; Lund, L.H.; Đikić, D.; Petrović-Đorđević, I.; Krljanac, G.; Milinković, I.; Veljić, I.; Piepoli, M.F.; Rosano, G.M.C.; Ristić, A.D.; et al. Type 2 diabetes increases the long-term risk of heart failure and mortality in patients with atrial fibrillation. Eur. J. Heart Fail. 2020, 22, 113–125. [Google Scholar] [CrossRef]
- Pareek, M.; Nielsen, M.L.; Gerke, O.; Leósdóttir, M.; Møller, J.E.; Hindersson, P.; Sehestedt, T.B.; Wachtell, K.; Nilsson, P.M.; Olsen, M.H. Worsening diastolic function is associated with elevated fasting plasma glucose and increased left ventricular mass in a supra-additive fashion in an elderly, healthy, Swedish population. Int. J. Cardiol. 2015, 184, 466–472. [Google Scholar] [CrossRef]
- Pareek, M.; Vaduganathan, M.; Bhatt, D.L.; Leósdóttir, M.; Olsen, M.H. Prognostic implications of fasting plasma glucose in subjects with echocardiographic abnormalities. Int. J. Cardiol. 2017, 241, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.J.; Tajik, A.J.; Nishimura, R.A.; Oh, J.K.; Khandheria, B.K.; Seward, J.B. Unlocking the mysteries of diastolic function: Deciphering the Rosetta Stone 10 years later. J. Am. Coll. Cardiol. 2008, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.J.; Park, J.H. Echocardiographic Measurement of Left Atrial Strain—A Key Requirement in Clinical Practice. Circ. J. Off. J. Jpn. Circ. Soc. 2021, 86, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. Left atrial strain in left ventricular diastolic dysfunction: Have we finally found the missing piece of the puzzle? Heart Fail. Rev. 2020, 25, 409–417. [Google Scholar] [CrossRef]
- Levy, F.; Iacuzio, L.; Schouver, E.D.; Essayagh, B.; Civaia, F.; Dommerc, C.; Maréchaux, S. Performance of a new fully automated transthoracic three-dimensional echocardio-graphic software for quantification of left cardiac chamber size and function: Comparison with 3 Tesla cardiac magnetic resonance. J. Clin. Ultrasound 2019, 47, 546–554. [Google Scholar] [CrossRef]
- Cameli, M.; Sparla, S.; Losito, M.; Righini, F.M.; Menci, D.; Lisi, M.; D’Ascenzi, F.; Focardi, M.; Favilli, R.; Pierli, C.; et al. Correlation of Left Atrial Strain and Doppler Measurements with Invasive Measurement of Left Ventricular End-Diastolic Pressure in Patients Stratified for Different Values of Ejection Fraction. Echocardiography 2016, 33, 398–405. [Google Scholar] [CrossRef]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef]
- Lind, V.; Hammar, N.; Lundman, P.; Friberg, L.; Talbäck, M.; Walldius, G.; Norhammar, A. Impaired fasting glucose: A risk factor for atrial fibrillation and heart failure. Cardiovasc. Diabetol. 2021, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef]
| FBG 70–100 (n = 95) | FBG 100–125 (n = 53) | p Value | |
|---|---|---|---|
| Age (year) | 33.2 ± 9 | 38.5 ± 10.1 | 0.001 |
| Gender (f, %) | 45 (47.4) | 29 (54.7) | 0.493 |
| Height (cm) | 168.3 ± 9.3 | 168.9 ± 10.1 | 0.697 |
| Weight (kg) | 71.1 ± 13.4 | 74.9 ± 13.3 | 0.089 |
| Body mass index (kg/m2) | 25.01 ± 3.7 | 26.24 ±3.7 | 0.058 |
| Current smoker (n, %) | 41 (43.1) | 18 (51.4) | 0.273 |
| Serum fasting glucose (mg/dL) | 89.6 ± 14.4 | 110.8 ± 15.8 | <0.001 |
| Haemoglobin (g/dL) | 14 ± 2.2 | 14 ± 1.8 | 0.928 |
| WBC count (103 μL) | 7.4 ± 1.7 | 7.3 ± 1.7 | 0.625 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.222 |
| GFR (mL/min per 1.73 m2) | 108.1 ± 18.6 | 108.2 ± 19.5 | 0.986 |
| ALT (U/L) | 19.6 ± 18.7 | 17.7 ± 7.9 | 0.469 |
| AST (U/L) | 20.3 ± 23.2 | 20.4 ± 19.2 | 0.975 |
| Total cholesterol (mg/dL) | 173 ± 35.8 | 180.4 ± 32.5 | 0.207 |
| LDL cholesterol (mg/dL) | 103.5 ± 32.2 | 110 ± 26 | 0.201 |
| HDL cholesterol (mg/dL) | 47.5 ± 13.3 | 45.6 ± 11.1 | 0.372 |
| Triglyceride (mg/dL) | 112.7 ± 63 | 124 ± 72.2 | 0.316 |
| TSH (mU/L) | 2 ± 1.2 | 2.1 ± 1.1 | 0.599 |
| FBG 70–100 (n = 95) | FBG 100–125 (n = 53) | p Value | |
|---|---|---|---|
| LV-GLS | −20.8 ± 4.1 | −20.9 ± 2.6 | 0.803 |
| LAS-r | 52.3 ± 15 | 44.5 ± 10.7 | 0.001 |
| LAS-cd | 36.9 ± 11.7 | 28.4 ± 9.7 | <0.001 |
| LAS-ct | 15.4 ± 9.4 | 16.1 ± 7.3 | 0.653 |
| RV-FWSL | −29.5 ± 5.5 | −27.9 ± 5.9 | 0.096 |
| RV-GLS | −25.5 ± 6.9 | −24.4 ± 5.2 | 0.297 |
| LVEF (%) | 65 ± 4.1 | 65.4 ± 4.2 | 0.594 |
| LA diameter (cm) | 3.1 ± 0.5 | 3.3 ± 0.4 | 0.036 |
| RA diameter (cm) | 3.3 ± 0.5 | 3.4 ± 0.5 | 0.050 |
| LVEDD (cm) | 4.6 ± 0.4 | 4.6 ± 0.5 | 0.925 |
| LVESD (cm) | 2.7 ± 0.4 | 2.6 ± 0.5 | 0.602 |
| Mitral E (m/s) | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.037 |
| Mitral A (m/s) | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.065 |
| Mitral E/A ratio | 1.5 ± 0.5 | 1.3 ± 0.4 | 0.005 |
| Septal e′/a ratio | 1.7 ± 0.5 | 1.2 ± 0.4 | 0.000 |
| Lateral e′/a ratio | 2.2 ± 0.9 | 1.8 ± 0.7 | 0.008 |
| e′ (average) | 12.9 ± 0.5 | 11.5 ± 2.4 | 0.004 |
| E/e’ ratio | 6.2 ± 1.4 | 6.6 ± 1.5 | 0.229 |
| IVRT (ms) | 82.7 ± 19.9 | 93.4 ± 22.6 | 0.005 |
| DT (ms) | 129.4 ± 33 | 134.8 ± 40 | 0.412 |
| LA volume (mL/m2) | 39.7 ± 16 | 48.1 ± 17.8 | 0.006 |
| LVEDV (mL) | 73.8 ± 20.1 | 80.5 ± 18.9 | 0.055 |
| LVESV (mL) | 26.2 ± 8.2 | 27.5 ± 7.1 | 0.326 |
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| r | p-Value | R2 | p-Value | |
| BMI (kg/m2) | −0.249 | 0.002 | ||
| Age (Year) | −0.214 | 0.008 | 0.500 | 0.15 |
| LVEF (%) | −0.087 | 0.165 | 0.001 | |
| LV GLS | −0.235 | 0.004 | 0.002 | |
| Serum fasting glucose (mg/dL) | −0.268 | 0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bingöl, G.; Avcı Demir, F.; Özmen, E.; Ünlü, S.; Özden, Ö.; Tokdil, K.O.; Arsoy, L.B.; Özpamuk Karadeniz, F.; Ökçün, B. Effects of an Impaired Fasting Glucose on the Left Atrial Strain Evaluated by Speckle Tracking Echocardiography. Medicina 2023, 59, 1982. https://doi.org/10.3390/medicina59111982
Bingöl G, Avcı Demir F, Özmen E, Ünlü S, Özden Ö, Tokdil KO, Arsoy LB, Özpamuk Karadeniz F, Ökçün B. Effects of an Impaired Fasting Glucose on the Left Atrial Strain Evaluated by Speckle Tracking Echocardiography. Medicina. 2023; 59(11):1982. https://doi.org/10.3390/medicina59111982
Chicago/Turabian StyleBingöl, Gülsüm, Fulya Avcı Demir, Emre Özmen, Serkan Ünlü, Özge Özden, Kardelen Ohtaroğlu Tokdil, Leyla Bulut Arsoy, Fatma Özpamuk Karadeniz, and Barış Ökçün. 2023. "Effects of an Impaired Fasting Glucose on the Left Atrial Strain Evaluated by Speckle Tracking Echocardiography" Medicina 59, no. 11: 1982. https://doi.org/10.3390/medicina59111982
APA StyleBingöl, G., Avcı Demir, F., Özmen, E., Ünlü, S., Özden, Ö., Tokdil, K. O., Arsoy, L. B., Özpamuk Karadeniz, F., & Ökçün, B. (2023). Effects of an Impaired Fasting Glucose on the Left Atrial Strain Evaluated by Speckle Tracking Echocardiography. Medicina, 59(11), 1982. https://doi.org/10.3390/medicina59111982







