Common Complications and Cardiopulmonary Resuscitation in Patients with Left Ventricular Assist Devices: A Narrative Review
Abstract
:1. Introduction
2. Advanced Heart Failure
3. Classifications
4. Therapeutical Interventions
5. Life-Threatening Complications of LVAD Therapy Potentially Leading to Cardiac Arrest
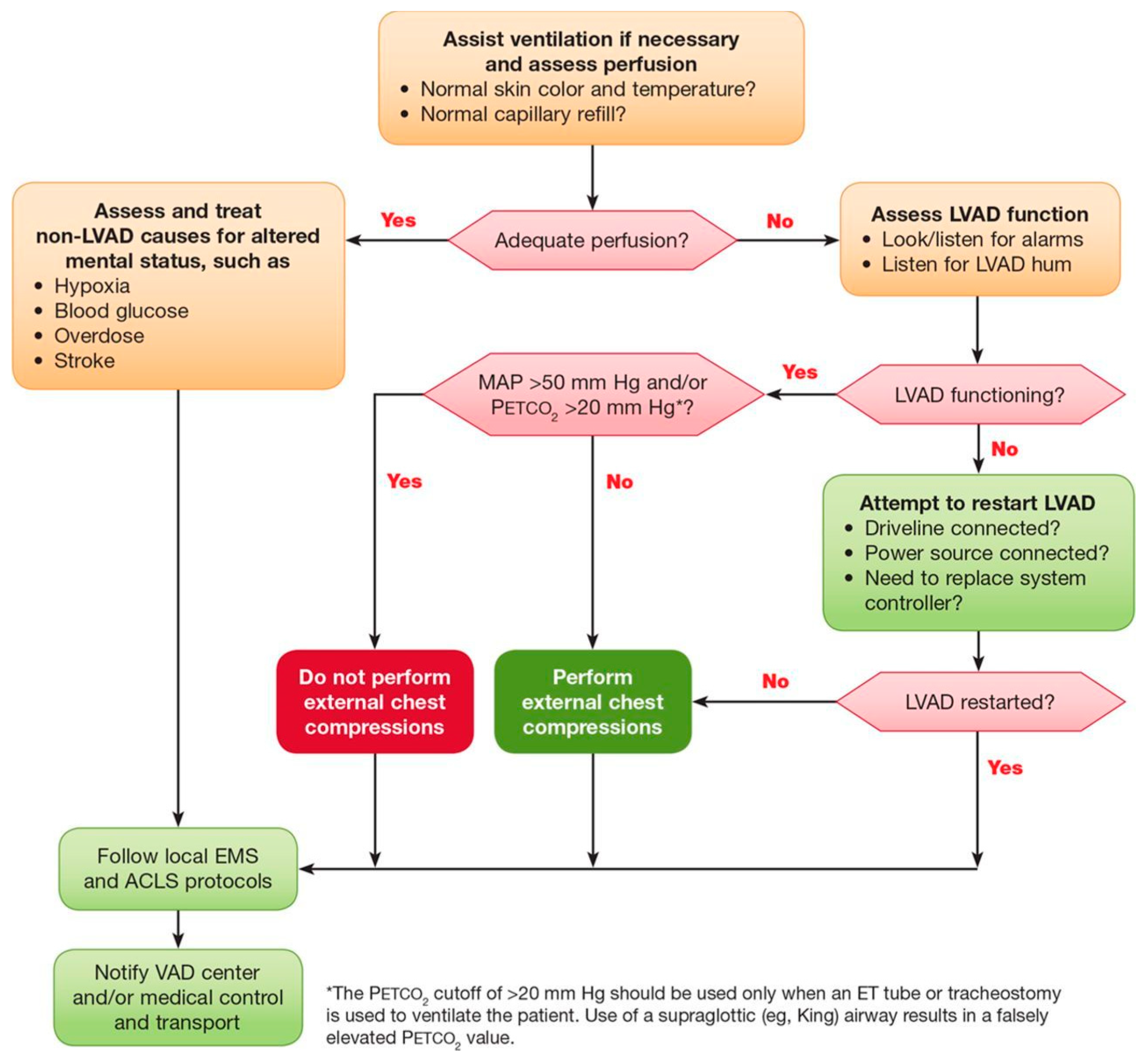
6. Approach to Cardiopulmonary Resuscitation in Patients with LVAD
6.1. Assessment of Flow and Perfusion
6.2. Assessment of Device
6.3. Chest Compressions and Defibrillation
6.4. Pump Alarms
6.5. Additional Diagnostic Assessment
6.6. Transfer to Implanting Facility
7. Long-Term Results
8. Future Challenges
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Saeed, D.; Feldman, D.; El Banayosy, A.; Birks, E.; Blume, E.; Cowger, J.; Hayward, C.; Jorde, U.; Kremer, J.; MacGowan, G.; et al. The 2023 International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support: A 10-Year Update. J. Heart Lung Transplant. 2023, 42, e1–e222. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, G.; Koprivanac, M.; Karimov, J.H.; Moazami, N.; Fukamachi, K. Current status of mechanical circulatory support for treatment of advanced end-stage heart failure: Successes, shortcomings and needs. Expert Rev. Cardiovasc. Ther. 2017, 15, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Ksela, J. Current status of long-term mechanical circulatory support. Transplant. Regen. Med. 2018, 2, 41–49. [Google Scholar]
- Velicki, L.; Frazier, O.H. Long-term ventricular assist devices in current clinical practice. Vojnosanit. Pregl. 2013, 70, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Truby, L.K.; Rogers, J.G. Advanced heart failure: Epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef]
- Kosir, G.; Jug, B.; Novakovic, M.; Bozic Mijovski, M.; Ksela, J. Endocan Is an Independent Predictor of Heart Failure-Related Mortality and Hospitalizations in Patients with Chronic Stable Heart Failure. Dis. Markers 2019, 2019, 9134096. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [PubMed]
- Kossman, C.E. Nomenclature and criteria for the diagnosis of cardiovascular diseases. Circulation 1964, 30, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.W.; Pagani, F.D.; Young, J.B.; Jessup, M.; Miller, L.; Kormos, R.L.; Naftel, D.C.; Ulisney, K.; Desvigne-Nickens, P.; Kirklin, J.K.; et al. INTERMACS profiles of advanced heart failure: The current picture. J. Heart Lung Transplant. 2009, 28, 535–541. [Google Scholar] [CrossRef]
- Mancini, D.; Colombo, P.C. Left Ventricular Assist Devices: A Rapidly Evolving Alternative to Transplant. J. Am. Coll. Cardiol. 2015, 65, 2542–2555. [Google Scholar] [CrossRef]
- Ambrozic, J.; Toplisek, J.; Knezevic, I.I.; Bunc, M.; Stajer, D. Chronic ischemic mitral regurgitation—A diagnostic and therapeutic challenge. Zdrav. Vestn. 2014, 83, 54–69. [Google Scholar]
- Michler, R.E.; Smith, P.K.; Parides, M.K.; Ailawadi, G.; Thourani, V.; Moskowitz, A.J.; Acker, M.A.; Hung, J.W.; Chang, H.L.; Perrault, L.P.; et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N. Engl. J. Med. 2016, 374, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Castelvecchio, S.; Garatti, A.; Gagliardotto, P.V.; Menicanti, L. Surgical ventricular reconstruction for ischaemic heart failure: State of the art. Eur. Heart J. Suppl. 2016, 18 (Suppl. E), E8–E14. [Google Scholar] [CrossRef]
- Krabatsch, T.; Drews, T.; Potapov, E.; Weng, Y.; Pasic, M.; Hetzer, R. Different surgical strategies for implantation of continuous-flow VADs-Experience from Deutsches Herzzentrum Berlin. Ann. Cardiothorac. Surg. 2014, 3, 472–474. [Google Scholar]
- Salas De Armas, I.A.; Patel, J.A.; Akay, M.A.; Patel, M.K.; Rajagopal, K.; Karabulut, M.N.; Kar, B.; Gregoric, I.D. Off-Pump Continuous-Flow Left Ventricular Assist Device Implantation. Tex. Heart Inst. J. 2021, 48, e197033. [Google Scholar] [CrossRef]
- Yuzefpolskaya, M.; Schroeder, S.E.; Houston, B.A.; Robinson, M.R.; Gosev, I.; Reyentovich, A.; Koehl, D.; Cantor, R.; Jorde, U.P.; Kirklin, J.K.; et al. The Society of Thoracic Surgeons Intermacs 2022 Annual Report: Focus on the 2018 Heart Transplant Allocation System. Ann. Thorac. Surg. 2023, 115, 311–327. [Google Scholar] [CrossRef]
- Desai, S.R.; Hwang, N.C. Advances in Left Ventricular Assist Devices and Mechanical Circulatory Support. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1193–1213. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M. Anticoagulation management of left ventricular assist devices. Am. J. Hematol. 2015, 90, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, N.J.; Yalcin, Y.C.; Bax, H.I.; Constantinescu, A.A.; Brugts, J.J.; Manintveld, O.C.; Birim, O.; Croughs, P.D.; Bogers, A.J.J.C.; Caliskan, K.; et al. Single-Center Experience With Protocolized Treatment of Left Ventricular Assist Device Infections. Front. Med. 2022, 9, 835765. [Google Scholar] [CrossRef] [PubMed]
- Peberdy, M.A.; Gluck, J.A.; Ornato, J.P.; Bermudez, C.A.; Griffin, R.E.; Kasirajan, V.; Kerber, R.E.; Lewis, E.F.; Link, M.S.; Miller, C.; et al. Cardiopulmonary Resuscitation in Adults and Children With Mechanical Circulatory Support: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1115–e1134. [Google Scholar] [CrossRef]
- Pavlovic, N.V.; Randell, T.; Madeira, T.; Hsu, S.; Zinoviev, R.; Abshire, M. Risk of left ventricular assist device driveline infection: A systematic literature review. Heart Lung 2019, 48, 90–104. [Google Scholar] [CrossRef]
- Szymanski, T.W.; Weeks, P.A.; Patel, C.J.; Jezovnik, M.K.; Gulbis, B.; Nathan, S.S.; Jumean, M.F.; Radovancevic, R.; Kar, B.; Gregoric, I.D. Risk of pump thrombosis and stroke in patients with continuous-flow left ventricular assist devices and gastrointestinal bleeding. Artif. Organs 2020, 44, 1171–1175. [Google Scholar] [CrossRef]
- Kadono, Y.; Nakamura, H.; Saito, S.; Nishida, T.; Takagaki, M.; Shigematsu, T.; Asai, K.; Murakami, T.; Todo, K.; Fujinaka, T.; et al. Endovascular treatment for large vessel occlusion stroke in patients with ventricular assist devices. J. Neurointerv. Surg. 2019, 11, 1205–1209. [Google Scholar] [CrossRef]
- Bouzas-Cruz, N.; Gonzalez-Fernandez, O.; Ferrera-Duran, C.; Woods, A.; Robinson-Smith, N.; Tovey, S.; Jungschleger, J.; Booth, K.; Shah, A.; Parry, G.; et al. Initial conservative management strategy of HeartWare left ventricular assist device thrombosis with intravenous heparin or bivalirudin. Int. J. Artif. Organs 2020, 43, 444–451. [Google Scholar] [CrossRef]
- Kortekaas, K.A.; den Exter, P.L.; Beeres, S.L.M.A.; Palmen, M.; Wouter Jukema, J.; Huisman, M.V.; Tops, L.F. Systemic thrombolysis in the management of pump thrombosis in patients with left ventricular assist devices. Front. Cardiovasc. Med. 2022, 9, 969766. [Google Scholar] [CrossRef]
- Li, S.; Beckman, J.A.; Welch, N.G.; Bjelkengren, J.; Masri, S.C.; Minami, E.; Stempien-Otero, A.; Levy, W.C.; O’Brien, K.D.; Lin, S.; et al. Accuracy of Doppler blood pressure measurement in continuous-flow left ventricular assist device patients. ESC Heart Fail. 2019, 6, 793–798. [Google Scholar] [CrossRef]
- Alvarez, P.A.; Ponnapureddy, R.; Voruganti, D.; Ruiz Duque, E.; Briasoulis, A. Noninvasive measurement of arterial blood pressure in patients with continuous-flow left ventricular assist devices: A systematic review. Heart Fail. Rev. 2021, 26, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Grmec, S.; Prosen, G. Continuous capnography and focused echocardiographic evaluation during resuscitation--additional criteria for cessation of treatment out-of-hospital-cardiac arrest. Resuscitation 2010, 81, 1731. [Google Scholar] [CrossRef] [PubMed]
- Ornato, J.P.; Garnett, A.R.; Glauser, F.L. Relationship between cardiac output and the end-tidal carbon dioxide tension. Ann. Emerg. Med. 1990, 19, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Krahn, L.E.; Petersen, A.; Miller, B.W.; Lizak, M.; Lyng, P. Abnormal Pulse Oximetry Signal. J. Clin. Sleep Med. 2018, 14, 1255–1256. [Google Scholar] [CrossRef]
- Iwashita, Y.; Ito, A.; Sasaki, K.; Suzuki, K.; Fujioka, M.; Maruyama, K.; Imai, H. Cardiopulmonary resuscitation of a cardiac arrest patient with left ventricular assist device in an out-of-hospital setting. Medicine 2020, 99, e18658. [Google Scholar] [CrossRef]
- Shinar, Z.; Bellezo, J.; Stahovich, M.; Cheskes, S.; Chillcott, S.; Dembitsky, W. Chest compressions may be safe in arresting patients with left ventricular assist devices (LVADs). Resuscitation 2014, 85, 702–704. [Google Scholar] [CrossRef]
- Singhvi, A.; Trachtenberg, B. Left Ventricular Assist Devices 101: Shared Care for General Cardiologists and Primary Care. J. Clin. Med. 2019, 8, 1720. [Google Scholar] [CrossRef]
- Sen, A.; Larson, J.S.; Kashani, K.B.; Libricz, S.L.; Patel, B.M.; Guru, P.K.; Alwardt, C.M.; Pajaro, O.; Farmer, J.C. Mechanical circulatory assist devices: A primer for critical care and emergency physicians. Crit. Care 2016, 20, 153. [Google Scholar] [CrossRef]
- Nathan, S.; Ghotra, A.S.; Rajagopal, K.; Patel, C.; Kumar, S.; Patel, M.; Salas de Armas, I.; Jumean, M.; Akay, M.H.; Akkanti, B.; et al. Left Ventricular Assist Device Outflow Graft Obstruction: A Case Series. ASAIO J. 2020, 66, 657–662. [Google Scholar] [CrossRef]
- Gregoric, I.D.; Poredos, P.; Jezovnik, M.K.; Ilic, M.; Shoukat, S.; Radovancevic, R.; Sharma, T.; Akay, M.H.; Matejin, S.; Nathan, S.; et al. Use of Transthoracic Echocardiogram to Detect Left Ventricular Thrombi. Ann. Thorac. Surg. 2021, 111, 556–560. [Google Scholar] [CrossRef]
- Loring, Z.; Sen, S.; Black-Maier, E.; Atwater, B.D.; Russell, S.D.; DeVore, A.D.; Piccini, J.P. Reducing ECG Artifact From Left Ventricular Assist Device Electromagnetic Interference. J. Am. Heart Assoc. 2020, 9, e017563. [Google Scholar] [CrossRef] [PubMed]
- Shroff, G.S.; Ocazionez, D.; Akkanti, B.; Vargas, D.; Garza, A.; Gupta, P.; Patel, J.A.; Patel, M.K.; Gregoric, I.D. CT Imaging of Complications Associated with Continuous-Flow Left Ventricular Assist Devices (LVADs). Semin. Ultrasound CT MRI 2017, 38, 616–628. [Google Scholar] [CrossRef]
- Mathew, R.P.; Alexander, T.; Patel, V.; Low, G. Chest radiographs of cardiac devices (Part 2): Ventricular assist devices. S. Afr. J. Radiol. 2019, 23, 1732. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, M.; Pizanis, N.; Tsourelis, L.; Koch, A.; Kamler, M.; Rassaf, T.; Luedike, P. Dynamics and prognostic value of B-type natriuretic peptide in left ventricular assist device recipients. J. Thorac. Dis. 2019, 11, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Barssoum, K.; Patel, H.; Rai, D.; Kumar, A.; Hassib, M.; Othman, H.F.; Thakkar, S.; El Karyoni, A.; Idemudia, O.; Ibrahim, F.; et al. Outcomes of Cardiac Arrest and Cardiopulmonary Resuscitation in Patients With Left Ventricular Assist Device; an Insight From a National Inpatient Sample. Heart Lung Circ. 2022, 31, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Saeed, D.; Stark, C.; Otto, W.; Loforte, A.; Zimpfer, D.; Bernhardt, A.M.; Potapov, E.; Morshius, M.; Schibilsky, D.; Albert, A.; et al. Outcome of Patients supported with HeartMate 3 after Extra-corporeal Life Support. On behalf of Durable MCS after ECLS Study Group. J. Thorac. Cardiovasc. Surg. 2023; online ahead of print. [Google Scholar] [CrossRef]
| 1. | Severe and persistent symptoms of HF; |
| 2. | Severe cardiac dysfunction: LVEF < 30% or inoperable valve abnormalities or inoperable congenital malformations or severe LV diastolic dysfunction; |
| 3. | Episodes of pulmonary/systemic congestion or low output requiring vasoactive support or malignant arrhythmias; |
| 4. | Severe impairment of exercise capacity. |
| Class 1 | No limitation of ordinary physical activity. |
| Class 2 | Slight limitation of ordinary physical activity. |
| Class 3 | Marked limitation of ordinary physical activity, but comfortable at rest. |
| Class 4 | Unable to carry out physical activity, symptomatic at rest. |
| INTERMACS Profile | Description | Time Frame for Intervention |
|---|---|---|
| 1 | Critical cardiogenic shock (life-threatening hypotension, critical organ hypoperfusion). “Crash and burn”. | Within hours |
| 2 | Progressive decline (worsening renal function, nutritional depletion). “Sliding on inotropes”. | Within days |
| 3 | Stable but inotrope-dependent (repeated failure to wean from inotrope support due to recurrent hypotension or renal failure). “Dependent stability”. | Within weeks |
| 4 | Resting symptoms (daily symptoms of congestion at rest or during activities). “Frequent flyer”. | Within months |
| 5 | Exertion intolerant (comfortable at rest, unable to engage in any other activity). “Housebound”. | Variable urgency, depends on maintenance of nutrition, organ function, and activity. |
| 6 | Exertion limited (comfortable at rest and with activities of daily living, but fatigue shortly after commencing any meaningful activity). “Walking wounded”. | Variable urgency, depends on maintenance of nutrition, organ function, and activity. |
| 7 | Intermediate NYHA III to IV. | MCS may not currently be indicated. |
| Bridge to decision | Short-term MCS until hemodynamics and end-organ perfusion are stabilized. |
| Bridge to candidacy | Long-term MCS to improve end-organ function to make an ineligible patient eligible for a heart transplant. |
| Bridge to transplantation | Long-term MCS to keep a patient without contraindications for heart transplant alive until a donor’s heart is available. |
| Bridge to recovery | Short- or long-term MCS to keep a patient alive until cardiac function recovers sufficiently. |
| Destination therapy | Long-term MCS as an alternative to heart transplant in patients with contraindications for transplant. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaloznik Djordjevic, J.; Özkan, T.; Göncz, E.; Ksela, J.; Möckel, M.; Strnad, M. Common Complications and Cardiopulmonary Resuscitation in Patients with Left Ventricular Assist Devices: A Narrative Review. Medicina 2023, 59, 1981. https://doi.org/10.3390/medicina59111981
Zaloznik Djordjevic J, Özkan T, Göncz E, Ksela J, Möckel M, Strnad M. Common Complications and Cardiopulmonary Resuscitation in Patients with Left Ventricular Assist Devices: A Narrative Review. Medicina. 2023; 59(11):1981. https://doi.org/10.3390/medicina59111981
Chicago/Turabian StyleZaloznik Djordjevic, Jerica, Timur Özkan, Eva Göncz, Jus Ksela, Martin Möckel, and Matej Strnad. 2023. "Common Complications and Cardiopulmonary Resuscitation in Patients with Left Ventricular Assist Devices: A Narrative Review" Medicina 59, no. 11: 1981. https://doi.org/10.3390/medicina59111981
APA StyleZaloznik Djordjevic, J., Özkan, T., Göncz, E., Ksela, J., Möckel, M., & Strnad, M. (2023). Common Complications and Cardiopulmonary Resuscitation in Patients with Left Ventricular Assist Devices: A Narrative Review. Medicina, 59(11), 1981. https://doi.org/10.3390/medicina59111981







