Pituitary-Related Adverse Events and Onset Patterns Caused by Immune Checkpoint Inhibitors: Analysis Using the Japanese Adverse Drug Event Report Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Target Adverse Events and Target Drugs
2.3. Subsection
2.3.1. Disproportionality Analysis
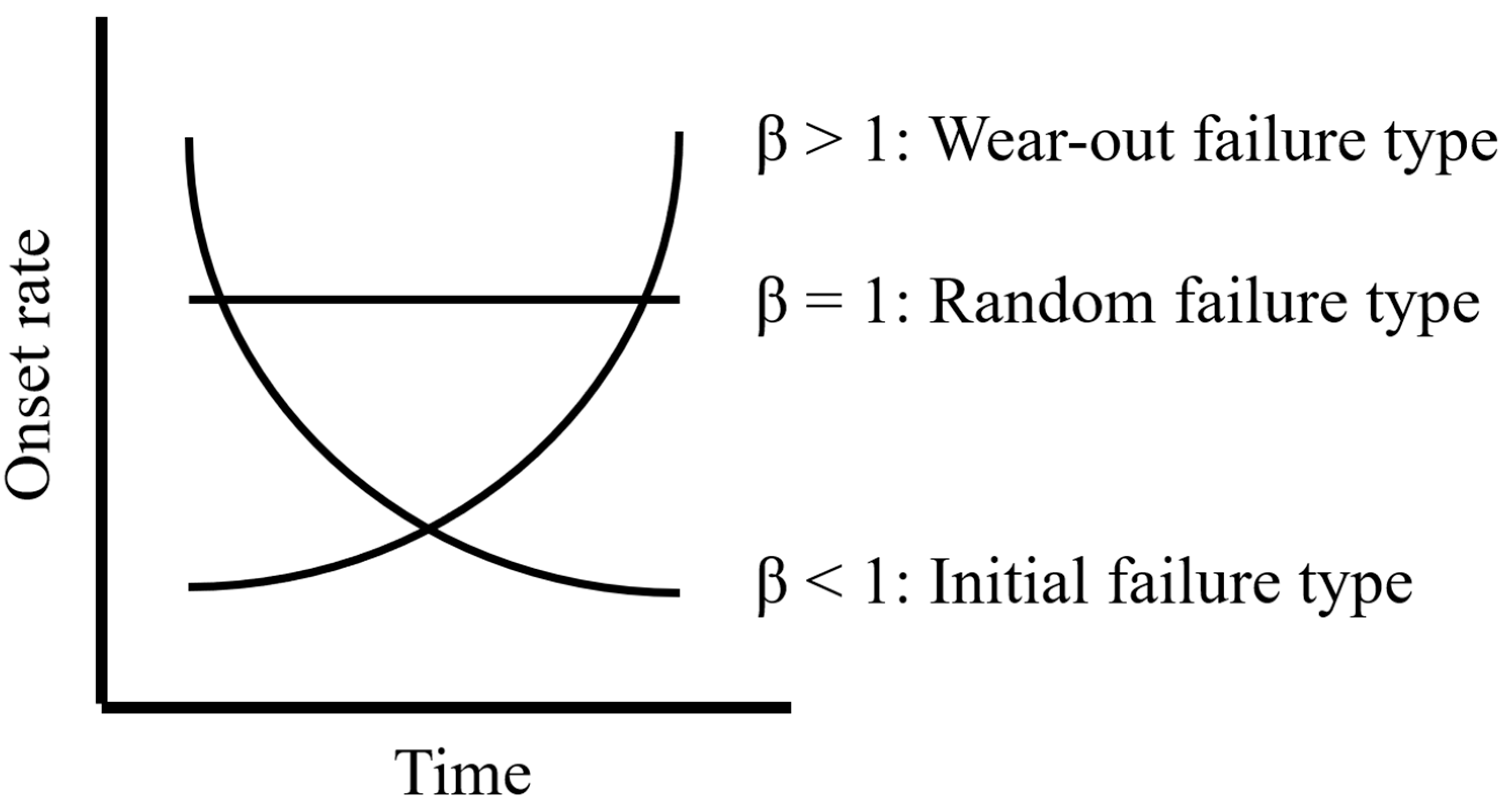
2.3.2. Weibull Analysis
3. Results
3.1. Signal Score for Pituitary-Related Adverse Events for Each ICI
3.2. Analysis of the Onset Pattern of Pituitary-Related Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gente, K.; Diekmann, L.; Daniello, L.; Will, J.; Feisst, M.; Olsavszky, V.; Günther, J.; Lorenz, H.M.; Souto-Carneiro, M.M.; Hassel, J.C.; et al. Sex and anti-inflammatory treatment affect outcome of melanoma and non-small cell lung cancer patients with rheumatic immune-related adverse events. J. Immunother. Cancer 2023, 11, e007557. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.; Thienen, H.; Blank, C.U. Toxicity patterns with immunomodulating antibodies and their combinations. Semin. Oncol. 2015, 42, 423–428. [Google Scholar] [CrossRef]
- Van der Kooij, M.K.; Suijkerbuijk, K.P.M.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; Blank, C.U.; Boers-Sonderen, M.J.; van Breeschoten, J.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Haanen, J.B.A.G.; et al. Safety and Efficacy of Checkpoint Inhibition in Patients with Melanoma and Preexisting Autoimmune Disease: A Cohort Study. Ann. Intern. Med. 2021, 174, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Buti, S.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Bersanelli, M.; Michiara, M.; Grassadonia, A.; Brocco, D.; et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. Oncologist 2019, 24, e327–e337. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Mangan, B.L.; McAlister, R.K.; Balko, J.M.; Johnson, D.B.; Moslehi, J.J.; Gibson, A.; Phillips, E.J. Evolving insights into the mechanisms of toxicity associated with immune checkpoint inhibitor therapy. Br. J. Clin. Pharmacol. 2020, 86, 778–1789. [Google Scholar] [CrossRef]
- Liu, M.; Christ, L.; Richters, A.; Özdemir, B.C. Toxicity, disease management and outcome of treatment with immune checkpoint inhibitors by sex in patients with cancer and preexisting autoimmune disease. Oncol. Lett. 2023, 26, 377. [Google Scholar] [CrossRef]
- Postow, M.A. Managing immune checkpoint-blocking antibody side effects. Am. Soc. Clin. Oncol. Educ. Book 2015, 76–83. [Google Scholar] [CrossRef]
- Lemery, S.; Keegan, P.; Pazdur, R. First FDA approval agnostic of cancer site-when a biomarker defines the indication. N. Engl. J. Med. 2017, 377, 1409–1412. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, T.; Fukuoka, H.; Takahashi, Y. Immune checkpoint inhibitor—Related hypophysitis. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101668. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 2021, 17, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Arlt, W.; Allolio, B. Adrenal insufficiency. Lancet 2003, 361, 1881–1893. [Google Scholar] [CrossRef]
- Bergthorsdottir, R.; Leonsson-Zachrisson, M.; Odén, A.; Johannsson, G. Premature mortality in patients with Addison’s disease: A population-based study. J. Clin. Endocrinol. Metab. 2006, 91, 4849–4853. [Google Scholar] [CrossRef]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef]
- Faje, A. Immunotherapy and hypophysitis: Clinical presentation, treatment, and biologic insights. Pituitary 2016, 19, 82–92. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Okuji, T.; Ito, M.; Onoue, T.; Goto, M.; Sugiyama, M.; Tsunekawa, T.; et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: A prospective study. J. Immunother. Cancer 2020, 8, e000779. [Google Scholar] [CrossRef]
- Labadzhyan, A.; Wentzel, K.; Hamid, O.; Chow, K.; Kim, S.; Piro, L.; Melmed, S. Endocrine Autoantibodies Determine Immune Checkpoint Inhibitor-induced Endocrinopathy: A Prospective Study. J. Clin. Endocrinol. Metab. 2022, 107, 1976–1982. [Google Scholar] [CrossRef]
- Kotwal, A.; Rouleau, S.G.; Dasari, S.; Kottschade, L.; Ryder, M.; Kudva, Y.C.; Markovic, S.; Erickson, D. Immune checkpoint inhibitor-induced hypophysitis: Lessons learnt from a large cancer cohort. J. Investig. Med. 2022, 70, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Yingying, H.; Jian, G.; Lifu, Z.; Xiaolin, L.; Xina, L.; Bin, Z.; Xin, H. Colitis following the use of immune checkpoint inhibitors: A real-world analysis of spontaneous reports submitted to the FDA adverse event reporting system. Int. Immunopharmacol. 2020, 84, 106601. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kozuka, Y.; Uno, H.; Utsumi, K.; Noyori, O.; Hosoki, R. Spontaneous and immune checkpoint inhibitor-induced autoimmune diseases: Analysis of temporal information by using the Japanese adverse drug event report database. Clin. Drug Investig. 2021, 41, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform. 2021, 22, bbab347. [Google Scholar] [CrossRef] [PubMed]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef]
- Norén, G.N.; Hopstadius, J.; Bate, A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat. Methods Med. Res. 2013, 22, 57–69. [Google Scholar] [CrossRef]
- Sandberg, L.; Taavola, H.; Aoki, Y.; Chandler, R.; Norén, G.N. Risk Factor Considerations in Statistical Signal Detection: Using Subgroup Disproportionality to Uncover Risk Groups for Adverse Drug Reactions in VigiBase. Drug Saf. 2020, 43, 999–1009. [Google Scholar] [CrossRef]
- Sauzet, O.; Carvajal, A.; Escudero, A.; Molokhia, M.; Cornelius, V.R. Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 2013, 36, 995–1006. [Google Scholar] [CrossRef]
- Arima, H.; Iwama, S.; Inaba, H.; Ariyasu, H.; Makita, N.; Otsuki, M.; Kageyama, K.; Imagawa, A.; Akamizu, T. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: Clinical guidelines of the Japan Endocrine Society. Endocr. J. 2019, 66, 581–586. [Google Scholar] [CrossRef]
- Available online: https://www.opdivo.jp/system/files/2023-03/OPD_guide.pdf (accessed on 26 September 2023).
- Hasegawa, S.; Ikesue, H.; Nakao, S.; Shimada, K.; Mukai, R.; Tanaka, M.; Matsumoto, K.; Inoue, M.; Satake, R.; Yoshida, Y.; et al. Analysis of immune-related adverse events caused by immune checkpoint inhibitors using the Japanese Adverse Drug Event Report database. Pharmacoepidemiol. Drug Saf. 2020, 29, 1279–1294. [Google Scholar] [CrossRef]
- Santini, F.C.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Lacouture, M.E.; Gambarin-Gelwan, M.; Wilkins, O.; Panora, E.; Halpenny, D.F.; Long, N.M.; et al. Safety and Efficacy of Re-Treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol. Res. 2018, 6, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhang, Y.; Wang, J.; Li, K.; Chen, X.; Heng, J.; Gao, Q.; Ye, Y.; Zhang, Z.; Liu, Y.; et al. Association Between Sex and Immune-Related Adverse Events During Immune Checkpoint Inhibitor Therapy. J. Natl. Cancer Inst. 2021, 113, 1396–1404. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Yoneyama, E.; Takaki, A.; Takahashi, N.; Ono, A.; Kato, A.; Adachi, I. Safety Evaluation of Retreatment with Immune Checkpoint Inhibitors. Jpn. J. Pharm. Health Care Sci. 2019, 45, 659–666. [Google Scholar] [CrossRef]
- Yang, F.; Shay, C.; Abousaud, M.; Tang, C.; Li, Y.; Qin, Z.; Saba, N.F.; Teng, Y. Patterns of toxicity burden for FDA-approved immune checkpoint inhibitors in the United States. J. Exp. Clin. Cancer Res. 2023, 42, 4. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Vaidya, R.; Albain, K.S.; LeBlanc, M.; Minasian, L.M.; Gotay, C.C.; Henry, N.L.; Fisch, M.J.; Lee, S.M.; Blanke, C.D.; et al. Sex Differences in Risk of Severe Adverse Events in Patients Receiving Immunotherapy, Targeted Therapy, or Chemotherapy in Cancer Clinical Trials. J. Clin. Oncol. 2022, 40, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Ippolito, S.; Lupi, I.; Caturegli, P. Hypophysitis induced by immune checkpoint inhibitors: A 10-year assessment. Expert. Rev. Endocrinol. Metab. 2019, 14, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Oh, J.M.; Kim, I.W. Drug repositioning prediction for psoriasis using the adverse event reporting database. Front. Med. 2023, 10, 1159453. [Google Scholar] [CrossRef]
- Barnabei, A.; Corsello, A.; Paragliola, R.M.; Iannantuono, G.M.; Falzone, L.; Corsello, S.M.; Torino, F. Immune Checkpoint Inhibitors as a Threat to the Hypothalamus-Pituitary Axis: A Completed Puzzle. Cancers 2022, 14, 1057. [Google Scholar] [CrossRef]
- Jo, K., III. Hypothalamic Syndrome: Key Points and Precautions for Diagnosis and Treatment. Nihon Naika Gakkai Zasshi 1994, 83, 2052–2507. [Google Scholar] [CrossRef][Green Version]
- Tshuma, N.; Glynn, N.; Evanson, J.; Powles, T.; Drake, W.M. Hypothalamitis and severe hypothalamic dysfunction associated with anti-programmed cell death ligand 1 antibody treatment. Eur. J. Cancer 2018, 104, 247–249. [Google Scholar] [CrossRef]
- Available online: https://www.opdivo.jp/system/files/2023-06/side_effect.pdf (accessed on 30 September 2023).
- Weber, J. Epidemiology of adverse reactions to nonsteroidal anti-inflammatory drugs. Adv. Inflamm. Res. 1984, 6, 1–7. [Google Scholar]
- McAdams, M.A.; Governale, L.A.; Swartz, L.; Hammad, T.A.; Dal Pan, G.J. Identifying patterns of adverse event reporting for four members of the angiotensin II receptor blockers class of drugs: Revisiting the Weber effect. Pharmacoepidemiol. Drug Saf. 2008, 17, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Neha, R.; Subeesh, V.; Beulah, E.; Gouri, N.; Maheswari, E. Existence of Notoriety Bias in FDA Adverse Event Reporting System Database and Its Impact on Signal Strength. Hosp. Pharm. 2021, 56, 152–158. [Google Scholar] [CrossRef] [PubMed]
| Target Adverse Event | Other Adverse Events | Total | ||
|---|---|---|---|---|
| Target drug | N11 | N10 | N1+ | |
| Other drugs | N01 | N00 | N0+ | |
| Total | N+1 | N+0 | N++ | |
| Target Adverse Event | Other Adverse Events | Total | ||
|---|---|---|---|---|
| Women | Target drug | Nwomen11 | Nwomen10 | Nwomen1+ |
| Other drugs | Nwomen01 | Nwomen00 | Nwomen0+ | |
| Men | Target drug | Nmen11 | Nmen10 | Nmen1+ |
| Other drugs | Nmen 01 | Nmen00 | Nmen0+ |
| Class | Drug | Anterior Pituitary Hypofunction | Anterior Pituitary Hyperfunction | Posterior Pituitary Disorder | Pituitary Neoplasm | ||||
|---|---|---|---|---|---|---|---|---|---|
| N11 | IC (95%CrI) | N11 | IC (95%CrI) | N11 | IC (95%CrI) | N11 | IC (95%CrI) | ||
| anti-CTLA-4 antibody | ipilimumab | 213 | 5.53 (5.30 *–5.69) | 0 | NA | 5 | −1.24 (−2.80–−0.26 †) | 1 | 0.62 (−3.17–2.30) |
| anti-PD-1 antibody | nivolumab | 400 | 4.96 (4.79 *–5.08) | 1 | −0.94 (−4.72–0.75) | 20 | −0.89 (−1.64–−0.37 †) | 1 | −0.37 (−4.15–1.32) |
| pembrolizumab | 134 | 4.04 (3.76 *–4.25) | 0 | NA | 26 | 0.16 (−0.49–0.63) | 0 | NA | |
| anti-PD-L1 antibody | atezolizumab | 15 | 2.40 (1.53 *–3.00) | 0 | NA | 11 | 0.55 (−0.48–1.24) | 0 | NA |
| avelumab | 0 | NA | 0 | NA | 0 | NA | 0 | NA | |
| durvalumab | 3 | 0.69 (−1.38–1.90) | 0 | NA | 0 | NA | 0 | NA | |
| Class | Drug | Women | Men | Women Versus Men | ||
|---|---|---|---|---|---|---|
| Nwomen11 | IC (95%CrI) | Nmen11 | IC (95%CrI) | ICΔ (95%CrI) | ||
| anti-CTLA-4 antibody | ipilimumab | 73 | 5.77 (5.39 *–6.05) | 137 | 5.01 (4.73 *–5.21) | 1.24 (0.85 **–1.52) |
| anti-PD−1 antibody | nivolumab | 105 | 5.35 (5.03 *–5.59) | 292 | 4.50 (4.31 *–4.64) | 1.09 (0.77 **–1.33) |
| pembrolizumab | 28 | 4.14 (3.51 *–4.58) | 104 | 3.65 (3.32 *–3.88) | 0.89 (0.26 **–1.34) | |
| anti-PD-L1 antibody | atezolizumab | 5 | 2.60 (1.04 *–3.58) | 9 | 1.86 (0.72 *–2.62) | 1.30 (−0.26–2.28) |
| Class | Drug | N11 | α (95%CI) | β (95%CI) |
|---|---|---|---|---|
| anti-CTLA-4 antibody | ipilimumab | 131 | 97.3 (86.3–109.4) | 1.54 (1.36–1.73) |
| anti-PD-1 antibody | nivolumab | 164 | 158.5 (141.9–176.6) | 1.50 (1.34–1.67) |
| pembrolizumab | 63 | 201.6 (174.6–231.7) | 1.87 (1.54–2.22) | |
| anti-PD-L1 antibody | atezolizumab | 5 | 115.7 (67.6–194.7) | 2.29 (0.92–4.49) |
| avelumab | 0 | NA | NA | |
| durvalumab | 0 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asano, H.; Noguchi, Y.; Kimura, M.; Usami, E.; Yoshimura, T. Pituitary-Related Adverse Events and Onset Patterns Caused by Immune Checkpoint Inhibitors: Analysis Using the Japanese Adverse Drug Event Report Database. Medicina 2023, 59, 1963. https://doi.org/10.3390/medicina59111963
Asano H, Noguchi Y, Kimura M, Usami E, Yoshimura T. Pituitary-Related Adverse Events and Onset Patterns Caused by Immune Checkpoint Inhibitors: Analysis Using the Japanese Adverse Drug Event Report Database. Medicina. 2023; 59(11):1963. https://doi.org/10.3390/medicina59111963
Chicago/Turabian StyleAsano, Hiroki, Yoshihiro Noguchi, Michio Kimura, Eiseki Usami, and Tomoaki Yoshimura. 2023. "Pituitary-Related Adverse Events and Onset Patterns Caused by Immune Checkpoint Inhibitors: Analysis Using the Japanese Adverse Drug Event Report Database" Medicina 59, no. 11: 1963. https://doi.org/10.3390/medicina59111963
APA StyleAsano, H., Noguchi, Y., Kimura, M., Usami, E., & Yoshimura, T. (2023). Pituitary-Related Adverse Events and Onset Patterns Caused by Immune Checkpoint Inhibitors: Analysis Using the Japanese Adverse Drug Event Report Database. Medicina, 59(11), 1963. https://doi.org/10.3390/medicina59111963








