Vesicovaginal Fistulas: Prevalence, Impact, and Management Challenges
Abstract
:1. Introduction and Prevalence
2. Etiology
3. Impact
4. Management Challenges
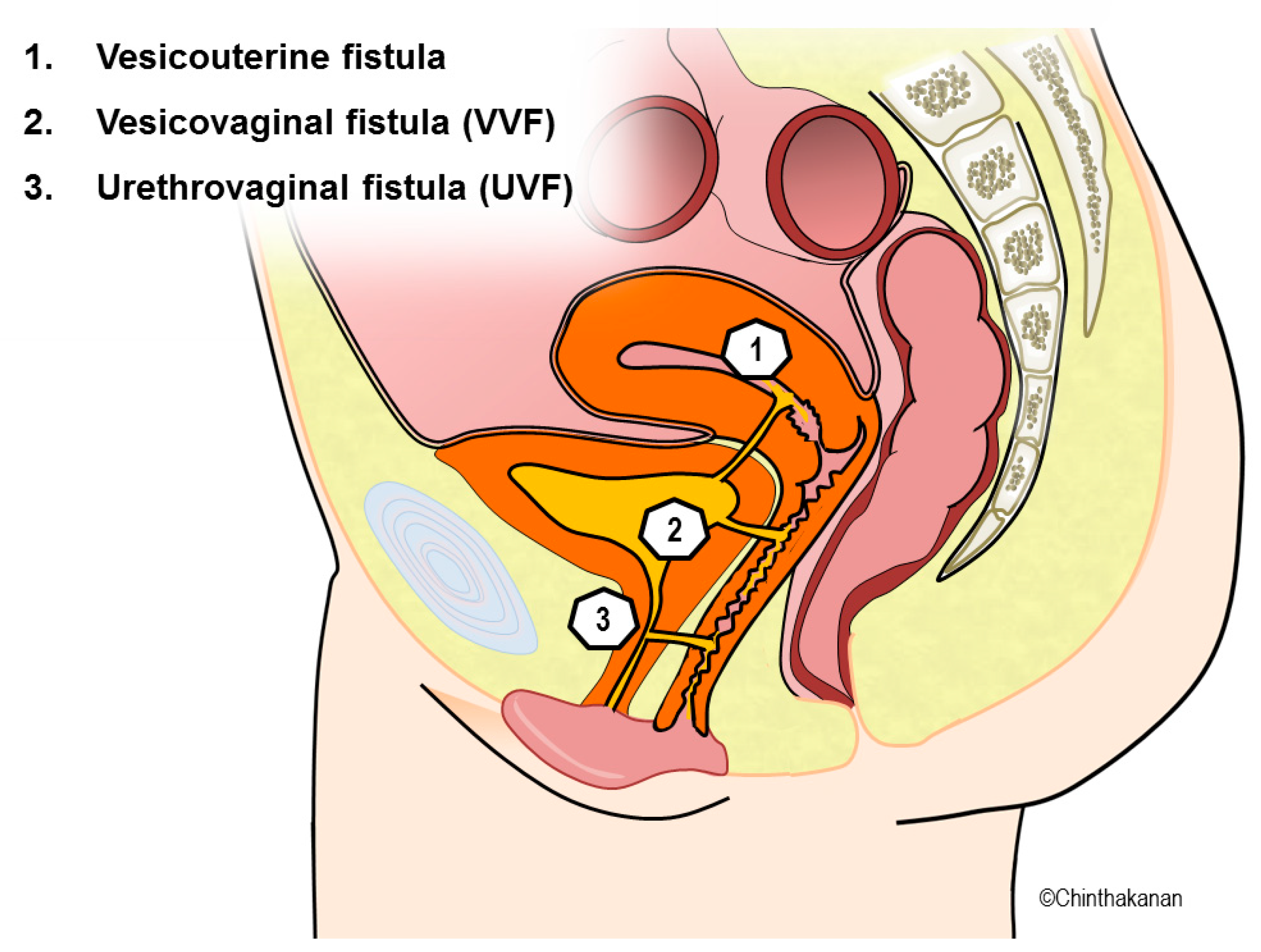
5. Diagnosis
6. Treatment of VVFs
6.1. Conservative Management
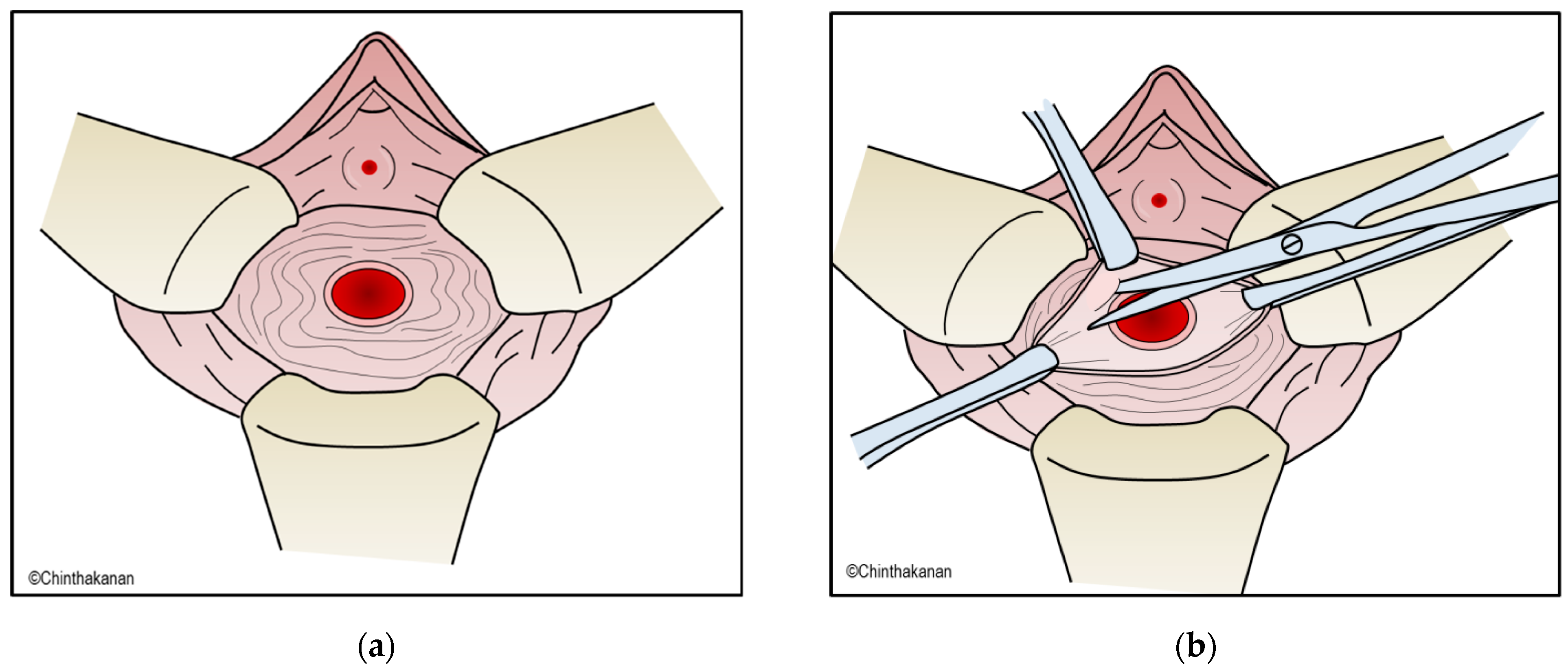
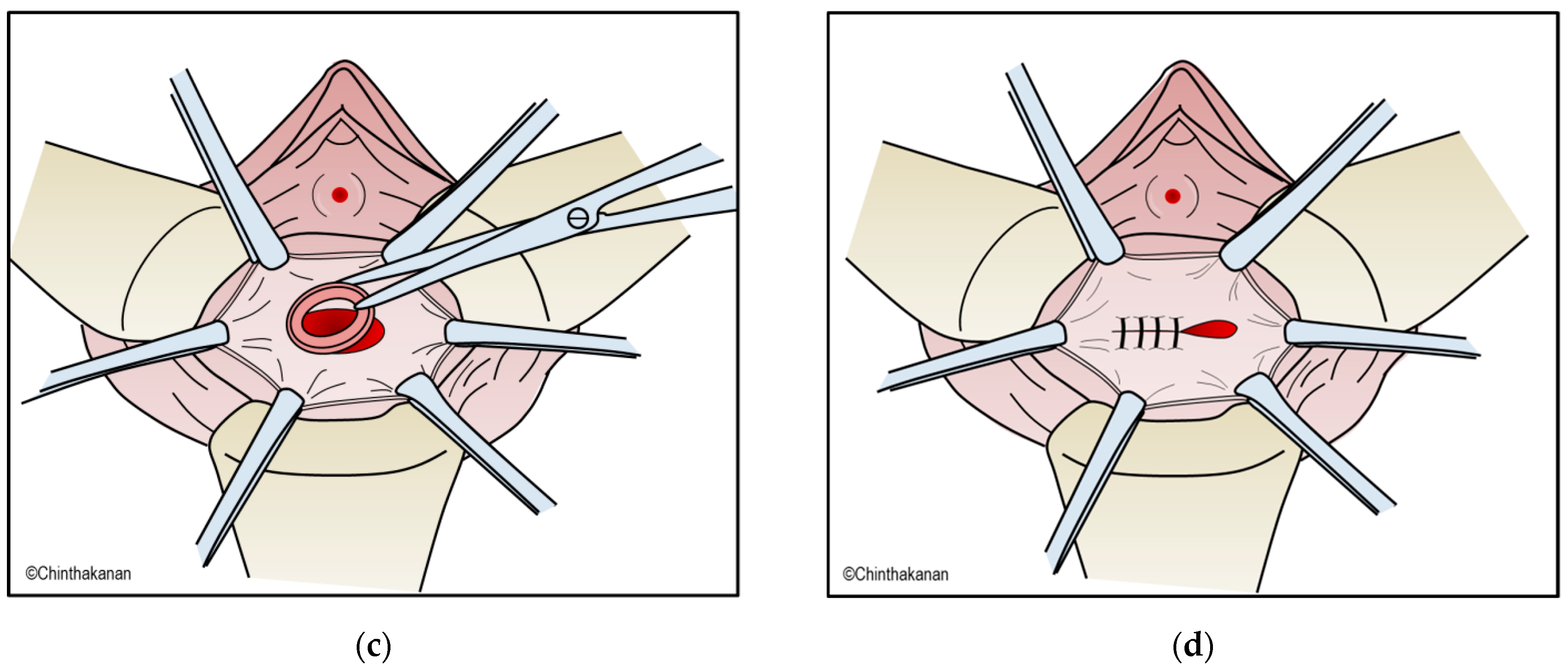
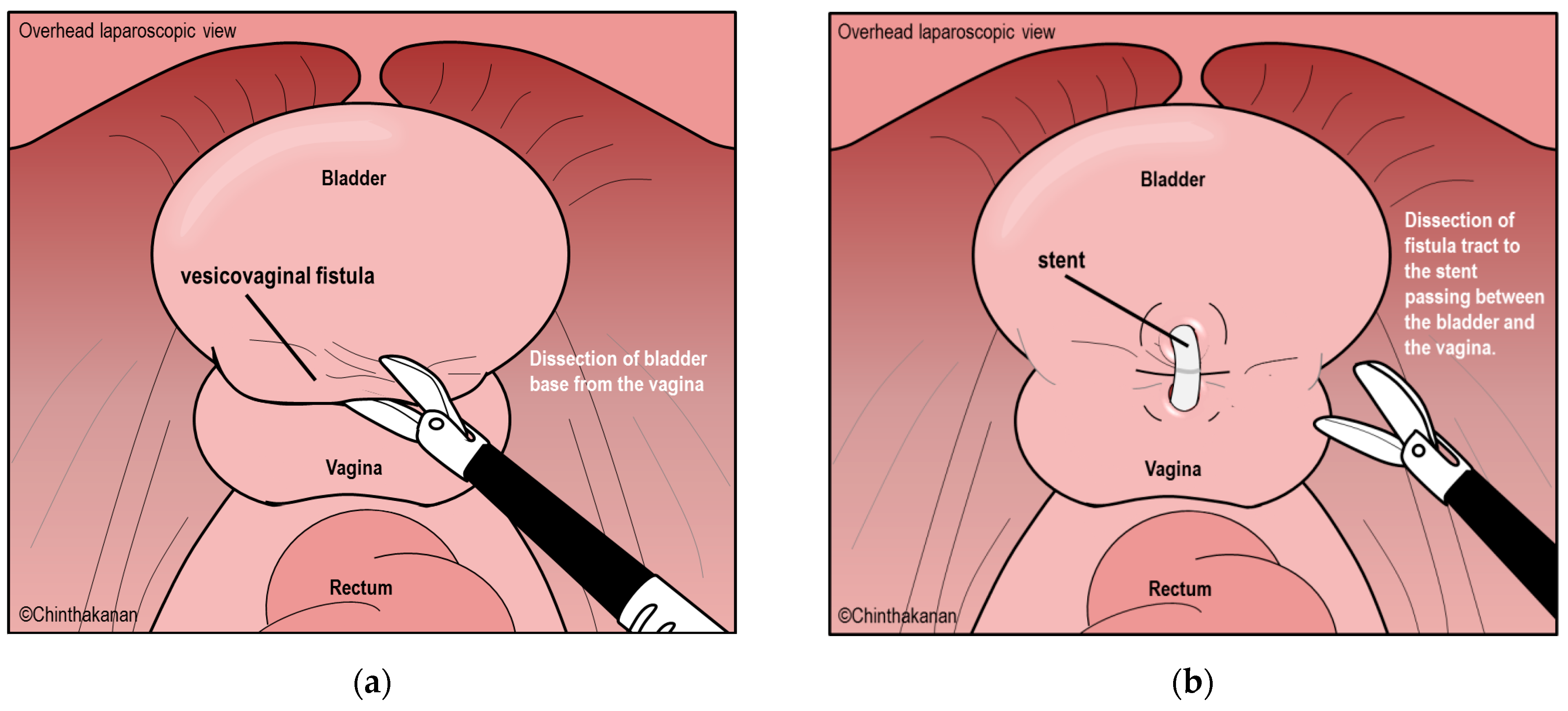
6.2. Surgical Management
6.3. Current Trends in Surgical Management and Surgical Technics
6.4. Timing of VVF Repair
6.5. Postoperative Care
6.6. Prevention
7. Novel Treatment of VVFs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrestha, D.B.; Budhathoki, P.; Karki, P.; Jha, P.; Mainali, G.; Dangal, G.; Baral, G.; Shrestha, M.; Gyawali, P. Vesico-Vaginal Fistula in Females in 2010–2020: A Systemic Review and Meta-analysis. Reprod. Sci. 2022, 29, 3346–3364. [Google Scholar] [CrossRef] [PubMed]
- Hadley, H.R. Vesicovaginal fistula. Curr. Urol. Rep. 2002, 3, 401–407. [Google Scholar] [CrossRef]
- Stamatakos, M.; Sargedi, C.; Stasinou, T.; Kontzoglou, K. Vesicovaginal fistula: Diagnosis and management. Indian J. Surg. 2014, 76, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Tahzib, F. Epidemiological determinants of vesicovaginal fistulas. Br. J. Obstet. Gynaecol. 1983, 90, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Rajaian, S.; Pragatheeswarane, M.; Panda, A. Vesicovaginal fistula: Review and recent trends. Indian J. Urol. 2019, 35, 250–258. [Google Scholar] [CrossRef]
- Zacharin, R.F. A History of Obstetric Vesicovaginal Fistula. Aust. N. Z. J. Surg. 2000, 70, 851–854. [Google Scholar] [CrossRef]
- Falk, H.C.; Tancer, M.L. Vesicovaginal Fistula: An historical survey. Obstet. Gynecol. 1954, 3, 337–341. [Google Scholar]
- Zacharin, R.F. Obstetric Fistula; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hilton, P. Vesico-vaginal fistula: New perspectives. Curr. Opin. Obstet. Gynecol. 2001, 13, 513–520. [Google Scholar] [CrossRef]
- Ward, G.G. Marion Sims and the origin of modern gynecology. Bull. N. Y. Acad. Med. 1936, 12, 93. [Google Scholar]
- Stanford, E.; Romanzi, L. Vesicovaginal fistula: What is the preferred closure technique? Int. Urogynecology J. 2012, 23, 383–385. [Google Scholar] [CrossRef]
- Sims, J.M. On the treatment of vesico-vaginal fistula. Am. J. Med. Sci. 1852, 45, 59–82. [Google Scholar] [CrossRef]
- Ojanuga, D. The medical ethics of the’father of gynaecology’, Dr J Marion Sims. J. Med. Ethics 1993, 19, 28–31. [Google Scholar] [CrossRef]
- Wall, L.L. The medical ethics of Dr J Marion Sims: A fresh look at the historical record. J. Med. Ethics 2006, 32, 346–350. [Google Scholar] [CrossRef]
- Kumar, S.; Kekre, N.S.; Gopalakrishnan, G. Vesicovaginal fistula: An update. Indian J. Urol. 2007, 23, 187–191. [Google Scholar] [CrossRef]
- Husson, F.C., II. Trendelenberg on Operations for Vesico-Vaginal Fistula, and on the Elevation of the Pelvis During Operations in the Pelvic Cavity. Ann. Surg. 1890, 11, 463–466. [Google Scholar] [PubMed]
- Miklos, J.R.; Moore, R.D.; Chinthakanan, O. Laparoscopic and robotic-assisted vesicovaginal fistula repair: A systematic review of the literature. J. Minim. Invasive Gynecol. 2015, 22, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Nesrallah, L.J.; Srougi, M.; Gittes, R.F. The o’conor technique: The gold standard for supratrigonal vesicovaginal fistula repair. J. Urol. 1999, 161, 566–568. [Google Scholar] [CrossRef]
- Dalela, D.; Ranjan, P.; Sankhwar, P.L.; Sankhwar, S.N.; Naja, V.; Goel, A. Supratrigonal VVF repair by modified O’Connor’s technique: An experience of 26 cases. Eur. Urol. 2006, 49, 551–556. [Google Scholar] [CrossRef]
- Wall, L.L. Obstetric vesicovaginal fistula as an international public-health problem. Lancet 2006, 368, 1201–1209. [Google Scholar] [CrossRef]
- Adler, A.; Ronsmans, C.; Calvert, C.; Filippi, V. Estimating the prevalence of obstetric fistula: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2013, 13, 1. [Google Scholar] [CrossRef]
- Hogan, M.C.; Foreman, K.J.; Naghavi, M.; Ahn, S.Y.; Wang, M.; Makela, S.M.; Lopez, A.D.; Lozano, R.; Murray, C.J.L. Maternal mortality for 181 countries, 1980–2008: A systematic analysis of progress towards Millennium Development Goal 5. Lancet 2010, 375, 1609–1623. [Google Scholar] [CrossRef]
- Miller, E.A.; Webster, G.D. Current management of vesicovaginal fistulae. Curr. Opin. Urol. 2001, 11, 417–421. [Google Scholar] [CrossRef]
- Duong, T.H.; Taylor, D.P.; Meeks, G.R. A multicenter study of vesicovaginal fistula following incidental cystotomy during benign hysterectomies. Int. Urogynecology J. 2011, 22, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Muleta, M.; Rasmussen, S.; Kiserud, T. Obstetric fistula in 14,928 Ethiopian women. Acta Obstet. Gynecol. Scand. 2010, 89, 945–951. [Google Scholar] [CrossRef]
- Lewis Wall, L.; Karshima, J.A.; Kirschner, C.; Arrowsmith, S.D. The obstetric vesicovaginal fistula: Characteristics of 899 patients from Jos, Nigeria. Am. J. Obstet. Gynecol. 2004, 190, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.M.; Kopp, D.M.; Tang, J.H.; Rosenberg, N.E.; Chipungu, E.; Harfouche, M.; Moyo, M.; Mwale, M.; Wilkinson, J.P. Association between parity and fistula location in women with obstetric fistula: A multivariate regression analysis. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 831–836. [Google Scholar] [CrossRef]
- Duong, T.H.; Gellasch, T.L.; Adam, R.A. Risk factors for the development of vesicovaginal fistula after incidental cystotomy at the time of a benign hysterectomy. Am. J. Obstet. Gynecol. 2009, 201, 512.e1–512.e4. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.Y.; Paraiso, M.F.R. Vesicovaginal Fistula Due to an Unreported Foreign Body in an Adolescent. J. Pediatr. Adolesc. Gynecol. 2007, 20, 253–255. [Google Scholar] [CrossRef]
- Fourie, T.; Ramphal, S. Aerosol caps and vesicovaginal fistulas. Int. J. Gynecol. Obstet. 2001, 73, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Binstock, M.A.; Semrad, N.; Dubow, L.; Watring, W. Combined vesicovaginal-ureterovaginal fistulas associated with a vaginal foreign body. Obstet. Gynecol. 1990, 76, 918–921. [Google Scholar]
- Arikan, N.; Türkölmez, K.; Aytaç, S.; Gögüs, O. Vesicovaginal fistula associated with a vaginal foreign body. BJU Int. 2000, 85, 375–376. [Google Scholar] [CrossRef]
- Sinha, A.; Olah, K.S.J. Vesicovaginal fistula caused by a foreign body: Delayed presentation and repair with martius graft. J. Obstet. Gynaecol. 2005, 25, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Ghoniem, G.M.; Warda, H.A. The management of genitourinary fistula in the third millennium. Arab. J. Urol. 2014, 12, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, A.; Jauniaux, E.; Hussein, A.M.; Coutinho, C.; Tinari, S.; Khalil, A.; Shamshirsaz, A.; Palacios-Jaraquemada, J.; D’Antonio, F. Urological complications in women undergoing surgery for placenta accreta spectrum disorders: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2023. [Google Scholar] [CrossRef]
- Ahmed, S.; Holtz, S.A. Social and economic consequences of obstetric fistula: Life changed forever? Int. J. Gynecol. Obstet. 2007, 99 (Suppl. 1), S10–S15. [Google Scholar] [CrossRef]
- Umoiyoho, A.; Inyang-Etoh, E.; Abah, G.; Abasiattai, A.; Akaiso, O. Quality of life following successful repair of vesicovaginal fistula in Nigeria. Rural Remote Health 2011, 11, 1734. [Google Scholar] [CrossRef]
- Amazue, L.O.; Eze, J.E.; Essien, N.F.; Nnadozie, E.E.; Onu, D.U. Health disclosure mediates stigma-psychological well-being link among Vesico-Vaginal fistula patients. Psychol. Health Med. 2023, 28, 336–343. [Google Scholar] [CrossRef]
- Kavoussi, L.R.; Wein, A.J. Campbell-Walsh Urology; Saunders Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Morita, T.; Tokue, A. Successful endoscopic closure of radiation induced vesicovaginal fistula with fibrin glue and bovine collagen. J. Urol. 1999, 162, 1689. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, Y.; Hirai, K.; Ishiko, O.; Ogita, S. Vesicovaginal fistula treated with fibrin glue. Int. J. Gynecol. Obstet. 2001, 73, 147–149. [Google Scholar] [CrossRef]
- Evans, L.A.; Ferguson, K.H.; Foley, J.P.; Rozanski, T.A.; Morey, A.F. Fibrin sealant for the management of genitourinary injuries, fistulas and surgical complications. J. Urol. 2003, 169, 1360–1362. [Google Scholar] [CrossRef]
- Dogra, P.; Nabi, G. Laser welding of vesicovaginal fistula. Int. Urogynecology J. 2001, 12, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.N.; Saini, A.K. Laser welding of vesicovaginal fistula—Outcome analysis and long-term outcome: Single-centre experience. Int. Urogynecology J. 2011, 22, 981–984. [Google Scholar] [CrossRef] [PubMed]
- FALK, H.C.; ORKIN, L.A. Nonsurgical closure of vesicovaginal fistulas. Obstet. Gynecol. 1957, 9, 538–541. [Google Scholar] [PubMed]
- Stovsky, M.; Ignatoff, J.; Blum, M.; Nanninga, J.; O’Conor, V.; Kursh, E. Use of electrocoagulation in the treatment of vesicovaginal fistulas. J. Urol. 1994, 152, 1443–1444. [Google Scholar] [CrossRef]
- Muto, G.; D’URSO, L.; Castelli, E.; Formiconi, A.; Bardari, F. Cyanoacrylic glue: A minimally invasive nonsurgical first line approach for the treatment of some urinary fistulas. J. Urol. 2005, 174, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Aycinena, J.F. Small vesicovaginal fistula. Urology 1977, 9, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Baumrucker, G.O. Ball repair of vesicovaginal fistula. Urology 1974, 3, 333–336. [Google Scholar] [CrossRef]
- Ray, A.; Esen, U.; Nwabineli, J. Iatrogenic vesico-vaginal fistula caused by shelf pessary. J. Obstet. Gynaecol. 2006, 26, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G.P.; Narayansingh, G.V.; Parkin, D.E.; Swami, S. Vesicovaginal fistula occurring 48 hours after insertion of a ring pessary. J. Obstet. Gynaecol. 2000, 20, 637. [Google Scholar] [CrossRef]
- Goldstein, I.; Wise, G.J.; Tancer, M.L. A vesicovaginal fistula and intravesical foreign body. A rare case of the neglected pessary. Am. J. Obstet. Gynecol. 1990, 163, 589–591. [Google Scholar] [CrossRef]
- Park, S.H.; Park, W.H.; Kim, H.G. Successful Case of Conservative Treatment of Vesicovaginal Fistula by Hodge Pessary Insertion. Low. Urin. Tract Symptoms 2015, 7, 118–120. [Google Scholar] [CrossRef]
- Kumar, U.; Albala, D.M. Fibrin glue applications in urology. Curr. Urol. Rep. 2001, 2, 79–82. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Cui, L.; Wang, J.; Gong, F.; Jia, G. Conservative treatment of patients with bladder genital tract fistula: Three case reports. Medicine 2020, 99, e21430. [Google Scholar] [CrossRef]
- Waaldijk, K. The immediate management of fresh obstetric fistulas. Am. J. Obstet. Gynecol. 2004, 191, 795–799. [Google Scholar] [CrossRef]
- Tavares, M.A.; Campagne Lpiseau, S.; Canis, M.; Botchorishvili, R. Intravesical repair of vesicovaginal fistula guided by cystoscopy. Facts Views Vis. Obgyn 2021, 13, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, M.; Lengauer, L.; Rochat, C.H.; Ploumidis, A.; Kröpfl, D.; Rassweiler, J.; Buffi, N.M.; Wiklund, P.; Mottrie, A.; John, H. Best Practices in Robotic-assisted Repair of Vesicovaginal Fistula: A Consensus Report from the European Association of Urology Robotic Urology Section Scientific Working Group for Reconstructive Urology. Eur. Urol. 2020, 78, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, R.; Spagnol, G.; Marchetti, M.; Montan, G.; Saccardi, C.; Noventa, M. Vaginal-Laparoscopic Repair (VLR) of Primary and Persistent Vesico-Vaginal Fistula: Description of a New Technique and Surgical Outcomes. J. Clin. Med. 2023, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, A.; Sönmez, G.; Tombul, Ş.T.; Golbasi, A.; Demirtaş, T. Natural Orifice Transurethral Endoscopic Vesicovaginal Fistula (NOTE-VVF) Treatment for Early and Small Fistulas: Case Report and the Point of Technique. Cureus 2022, 14, e23786. [Google Scholar] [CrossRef]
- Blaivas, J.G.; Heritz, D.M.; Romanzi, L.J. Early versus late repair of vesicovaginal fistulas: Vaginal and abdominal approaches. J. Urol. 1995, 153, 1110–1113. [Google Scholar] [CrossRef]
- Angioli, R.; Penalver, M.; Muzii, L.; Mendez, L.; Mirhashemi, R.; Bellati, F.; Crocè, C.; Panici, P.B. Guidelines of how to manage vesicovaginal fistula. Crit. Rev. Oncol. /Hematol. 2003, 48, 295–304. [Google Scholar] [CrossRef]
- Vakili, B.; Chesson, R.R.; Kyle, B.L.; Shobeiri, S.A.; Echols, K.T.; Gist, R.; Zheng, Y.T.; Nolan, T.E. The incidence of urinary tract injury during hysterectomy: A prospective analysis based on universal cystoscopy. Am. J. Obstet. Gynecol. 2005, 192, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Ibeanu, O.A.; Chesson, R.R.; Echols, K.T.; Nieves, M.; Busangu, F.; Nolan, T.E. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet. Gynecol. 2009, 113, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Saguan, D.; Northington, G.; Chinthakanan, O.; Hudson, C.; Karp, D. Iatrogenic lower urinary tract injury at the time of pelvic reconstructive surgery: Does previous pelvic surgery increase the risk? Int. Urogynecology J. 2014, 25, 1041–1046. [Google Scholar] [CrossRef]
- Gilmour, D.T.; Dwyer, P.L.; Carey, M.P. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet. Gynecol. 1999, 94, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Hilton, P. Vesico-vaginal fistulas in developing countries. Int. J. Gynecol. Obstet. 2003, 82, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Dutta, A.; Gupta, N.; Pal, D.K. Interposing layer of fibrin glue: A new horizon in vesico-vaginal fistula repair. Urologia 2022, 89, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Streit-Ciećkiewicz, D.; Futyma, K.; Miotła, P.; Grzybowska, M.E.; Rechberger, T. Platelet-Rich Plasma as Adjuvant Therapy for Recurrent Vesicovaginal Fistula: A Prospective Case Series. J. Clin. Med. 2019, 8, 2122. [Google Scholar] [CrossRef] [PubMed]
- Shirvan, M.K.; Alamdari, D.H.; Ghoreifi, A. A novel method for iatrogenic vesicovaginal fistula treatment: Autologous platelet rich plasma injection and platelet rich fibrin glue interposition. J. Urol. 2013, 189, 2125–2129. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinthakanan, O.; Sirisreetreerux, P.; Saraluck, A. Vesicovaginal Fistulas: Prevalence, Impact, and Management Challenges. Medicina 2023, 59, 1947. https://doi.org/10.3390/medicina59111947
Chinthakanan O, Sirisreetreerux P, Saraluck A. Vesicovaginal Fistulas: Prevalence, Impact, and Management Challenges. Medicina. 2023; 59(11):1947. https://doi.org/10.3390/medicina59111947
Chicago/Turabian StyleChinthakanan, Orawee, Pokket Sirisreetreerux, and Apisith Saraluck. 2023. "Vesicovaginal Fistulas: Prevalence, Impact, and Management Challenges" Medicina 59, no. 11: 1947. https://doi.org/10.3390/medicina59111947
APA StyleChinthakanan, O., Sirisreetreerux, P., & Saraluck, A. (2023). Vesicovaginal Fistulas: Prevalence, Impact, and Management Challenges. Medicina, 59(11), 1947. https://doi.org/10.3390/medicina59111947




