The Benefits of Combining Bobath and Vojta Therapies in Infants with Motor Development Impairment—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
- The reflex roll from the supine position—Phase I, from side decubitus; phase II, III, and IV, with reflex activation of two or three points.
- Reflex crawling from the prone position with reflex activation of two or three points and the first position (crouching at the edge of the bed).
- Righting reactions—maintaining the normal position of the head in space and normal alignment with the trunk, and the trunk with the upper and lower limbs.
- Balancing reactions—the visual, vestibular, and proprioceptive pathways are trained through posture changes.
- Verticalization reactions—adopting positions that favour and facilitate movement.
- a.
- 1.
- Reflex rolling
- 2.
- Reflex crawling
- A.
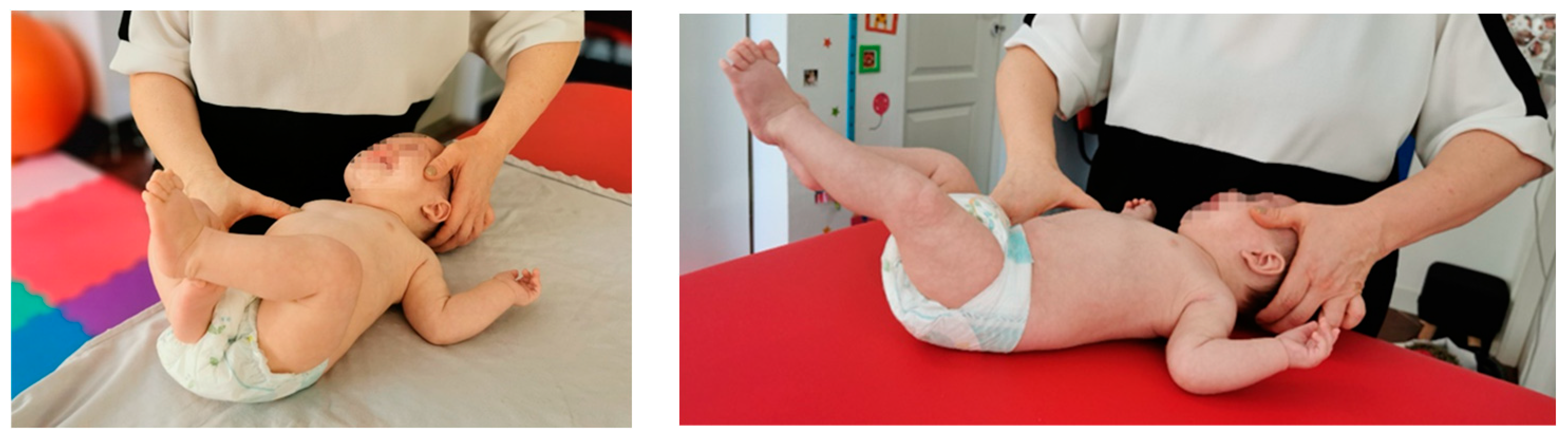
- Stimulation in “two points” of support on the facial side is the EMH area and on the occipital side is the distal part of the tibia. The epicondyle area is stimulated for 10–15 s until obtaining the kinesiological response of the lower limb on the occipital side, which achieves reverse supination and inversion of the abducted metatarsals with flexion of the fingers, and for the lower limb on the facial side, an eversion position with the abducted metatarsals and finger extension (Figure 2a).
- B.
- Stimulation in “three points” of support on the facial side is the EMH area and on the occipital side is the distal part of the tibia; the proximal part of the tibia on the facial side is a kinesiological response, and the lower limb on the occipital side. The flexion of the fingers is accentuated, and on the lower limb facial part, a median position is obtained in terms of flexion and extension—respectively, inversion and eversion. These activations are repeated 3 times on each side (Figure 2b,c).

- b.
- Bobath therapy
- Inhibitory reflex positions, in general, are partially opposite to the infant’s abnormal posture. First, the head and neck are positioned, then the trunk, shoulders, and hips, to achieve a redistribution of muscle tone as close to normal as possible.
- By positioning the head, we activate the tonic reflexes of the neck to favour the flexion or extension of the upper or lower limbs.
- To activate asymmetric cervical tonic reflexes, the head is positioned on the side of the interested limb, obtaining relaxation of the flexor tone; alternatively, we can mobilize it more easily by turning it to the opposite side.
- To activate the tonic labyrinthine reflex, the infant is positioned in the supine position (DD), and by anterior flexion of the head and neck, with the positioning of the upper limbs crossed on the chest, a relaxation of the lower limbs is obtained; thus, their movement becomes freer, without a spastic contraction.
- In the case of the opisthotonos position, to relax the extensor muscles of the neck, trunk, and limbs, the foetal position is adopted, with slight antero-posterior swings.
- Infants who showed a tendency to crouch were held by the palms and lifted, obtaining extension of the head and limbs, in a reflex inhibitory position, facilitating easy movement of the limbs. The same relaxation can be obtained from the ventral decubitus (DV) position, lifting the infant’s head off the bed with one hand and holding the abdomen with the other hand, ensuring stimulation of the Landau reflex.
- Exercise complexes are used to stimulate, by all means, the body’s equilibrium reactions, challenging and strengthening them through repetition.
- From the sitting position, from the knees, and from all fours, small and short pressures are applied to the infant’s shoulder, pushing it in all directions, thus being taught to react by raising the arm on the side towards which the infant is being pushed. It is performed in two sets of five repetitions, with a 1 min break between sets.
- For sitting up from DD, the return to the lateral decubitus is initiated, with support, on the body, on the forearm, then on the palm; the position is supported by the physiotherapist, with a socket at the level of the lower limbs. It is performed in two sets of five repetitions, with a 1 min break between sets.
- The transfer from sitting to all fours is performed by loading of the upper limbs, using left or right lateral movement using a downward inclined plane. It is performed in two sets of five repetitions, with a 1 min break between sets.
- Lifting in the quadrupedal position: from the DV, loaded support is performed on the upper limbs, gradually moving to the easy lifting of the pelvis by flexing the coxo-femoral joints, favouring support of the knees. Execution: 5 repetitions on the mat or with the help of the Bobath ball.
- Initiating the verticalization by performing the “Servant Knight” position: the initial position is with support of both knees and support at the level of the upper limbs, by pulling up a lower limb that is bent (triple flexion—in the hip, knee, and foot) and pushing for verticalization, it is reached with support of both lower limbs to stabilize and balance the position. Execution: 2 sets of 5 repetitions on each lower limb, with a 1 min break between sets (Figure 3).
- In the orthostatism position, using the inflatable disc, balance is stimulated; as an indispensable reaction to walking, the infant is easily unbalanced by antero-posterior and lateral pushing manoeuvres.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maitre, N.L.; Slaughter, J.C.; Aschner, J.L. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum. Dev. 2013, 89, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Căciulan, E.; Stanca, D. Infantile Cerebral Palsy—Cerebral Motor Disability; BREN: Bucharest, Romania, 2018; pp. 133–141. [Google Scholar]
- Brogna, C.; Romeo, D.M.; Cervesi, C.; Scrofani, L.; Romeo, M.G.; Mercuri, E.; Guzzetta, A. Prognostic value of the qualitative assessments of general movements in late-preterm infants. Early Hum. Dev. 2013, 89, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Zanon, M.A.; Pacheco, R.L.; Latorraca, C.O.C.; Cabrera Martimbianco, A.L.; Pachito, S.V.; Riera, R. Neurodevelopmental Treatment (Bobath) for Children With Cerebral Palsy: A Systematic Review. J. Child. Neurol. 2019, 34, 679–686. [Google Scholar] [CrossRef]
- Kiebzak, W.; Żurawski, A.; Głuszek, S.; Kosztołowicz, M.; Białek, W.A. Cortisol Levels in Infants with Central Coordination Disorders during Vojta Therapy. Children 2021, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.; Orton, J.; Anderson, P.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes post-hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst. Rev. 2012, 12, CD005495. [Google Scholar] [PubMed]
- Malmqvist, O.; Ohlin, A.; Ågren, J.; Jonsson, M. Seizures in newborn infants without hypoxic ischemic encephalopathy—Antenatal and labor-related risk factors: A case-control study. J. Matern. Fetal. Neonatal. Med. 2020, 33, 799–805. [Google Scholar] [CrossRef]
- Peebles, P.J.; Duello, T.M.; Eickhoff, J.C.; McAdams, R.M. Antenatal and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J. Perinatol. 2020, 40, 63–69. [Google Scholar] [CrossRef]
- Parker, S.J.; Kuzniewicz, M.; Niki, H.; Wu, Y.W. Antenatal and Intrapartum Risk Factors for Hypoxic-Ischemic Encephalopathy in a US Birth Cohort. J. Pediatr. 2018, 203, 163–169. [Google Scholar] [CrossRef]
- Hirfanoglu, T.; Ozturk, Z.; Gokdogan, G.S.; Hirfanoglu, I.M.; Onal, E.E.; Turkyilmaz, C.; Ergenekon, E.; Koc, E. Neonatal Seizures and Future Epilepsy: Predictive Value of Perinatal Risk Factors, Electroencephalography, and Imaging. J. Pediatr. Neurosci. 2020, 15, 190–198. [Google Scholar] [CrossRef]
- Accordino, F.; Consonni, S.; Fedeli, T.; Kullman, G.; Moltrasio, F.; Ghidini, A.; Locatelli, A. Risk factors for cerebral palsy in PPROM and preterm delivery with intact membranes. J. Matern. Fetal. Neonatal. Med. 2016, 29, 3854–3859. [Google Scholar] [CrossRef]
- Kooiker, M.J.G.; Swarte, R.M.C.; Smit, L.S.; Reiss, I.K.M. Perinatal risk factors for visuospatial attention and processing dysfunctions at 1 year of age in children born between 26 and 32 weeks. Early Hum. Dev. 2019, 130, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Mendler, M.R.; Mendler, I.; Hassan, M.A.; Mayer, B.; Bode, H.; Hummler, H.D. Predictive Value of Thompson-Score for Long-Term Neurological and Cognitive Outcome in Term Newborns with Perinatal Asphyxia and Hypoxic-Ischemic Encephalopathy Undergoing Controlled Hypothermia Treatment. Neonatology 2018, 114, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Anuriev, A.M.; Gorbachev, V.I. Hypoxic-ischemic brain damage in premature newborns. Zh. Nevrol. Psikhiatr. Im. SS Korsakova 2019, 119, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Shah, V.; Patwardhan, A.; Sattar, A.; Wang, S.; Raffay, T.; Martin, R.J.; Abu Jawdeh, E.G. Prematurity and postnatal alterations in intermittent hypoxaemia. Arch. Dis. Child Fetal. Neonatal. Ed. 2021, 106, 557–559. [Google Scholar] [CrossRef]
- Stomnaroska, O.; Petkovska, E.; Jancevska, S.; Danilovski, D. Neonatal Hypoglycemia: Risk Factors and Outcomes. Prilozi 2017, 38, 97–101. [Google Scholar] [CrossRef]
- Kavurt, S.; Celik, K. Incidence and risk factors of postnatal growth restriction in preterm infants. J. Matern. Fetal. Neonatal. Med. 2018, 31, 1105–1107. [Google Scholar] [CrossRef]
- Stark, A.; Cantrell, S.; Greenberg, R.G.; Permar, S.R.; Weimer, K.E.D. Long-term Outcomes after Postnatal Cytomegalovirus Infection in Low Birthweight Preterm Infants: A Systematic Review. Pediatr. Infect. Dis. J. 2021, 40, 571–581. [Google Scholar] [CrossRef]
- Vaughan-Graham, J.; Patterson, K.; Zabjek, K.; Cott, C. Conceptualizing movement by expert Bobath instructors in neurological rehabilitation. J. Eval. Clin. Pract. 2017, 23, 1153–1163. [Google Scholar] [CrossRef]
- Vaughan-Graham, J.; Cott, C.; Holland, A.; Michielsen, M.; Magri, A.; Suzuki, M.; Brooks, D. Developing a revised definition of the Bobath concept. Physiother. Res. Int. 2018, 24, e1762. [Google Scholar] [CrossRef]
- Orton, J.; Spittle, A.; Doyle, L.; Anderson, P.; Boyd, R. Do early intervention programmes improve cognitive and motor outcomes for preterm infants after discharge? A systematic review. Dev. Med. Child Neurol. 2009, 51, 851–859. [Google Scholar] [CrossRef]
- Bukhovets, B.; Romanchuk, A. The physical development of children with cerebral palsy in use of Bobat’s method in physical therapy course. Phys. Educ. Sport Kinesither. Res. J. 2017, 2, 82–88. [Google Scholar]
- Varadharajulu, G.; Shetty, L.; Sahoo, K. The Effect of Bobath Concept and Conventional Approach on the Functional Outcome in the Post Stroke Hemiplegic Individuals. J. Phys. Educ. Sport 2017, 4, 10–14. [Google Scholar] [CrossRef]
- Vojta, V.; Peters, A. Das Vojta Prinzip, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Vojta, V. Reflex rotation as a pathway to human locomotion. Zeitschrift Orthopädie Ihre Grenzgebiete 1970, 108, 446–452. [Google Scholar]
- Pásztai, Z. Kinetoterapie în Neuropediatrie; Editura Arionda: Galați, Romania, 2004; pp. 117–123. [Google Scholar]
- Bayley, N. Bayley Scales of Infant and Toddler Development—Third Edition: Administration Manual; Harcourt Assessment: San Antonio, TX, USA, 2006. [Google Scholar]
- Sung, Y.H.; Ha, S.Y. The Vojta approach changes thicknesses of abdominal muscles and gait in children with spastic cerebral palsy: A randomized controlled trial, pilot study. Technol. Health Care 2020, 28, 293–301. [Google Scholar] [CrossRef]
- Ungureanu, A.; Rusu, L.; Rusu, M.R.; Marin, M.I. Balance Rehabilitation Approach by Bobath and Vojta Methods in Cerebral Palsy: A Pilot Study. Children 2022, 9, 1481. [Google Scholar] [CrossRef]
- Khan, M.H.; Grzegorzek, M. Vojta-Therapy: A Vision-Based Framework to Recognize the Movement Patterns. Int. J. Softw. Innov. IJSI 2017, 5, 18–32. [Google Scholar] [CrossRef]
- Khan, M.H.; Grzegorzek, M. Research Anthology on Rehabilitation Practices and Therapy; IGI Global: Hershey, PA, USA, 2021; pp. 383–398. [Google Scholar]
- Khan, M.H.; Helsper, J.; Farid, M.S.; Grzegorzek, M. A computer vision-based system for monitoring Vojta therapy. Int. J. Med. Inform. 2018, 113, 85–95. [Google Scholar] [CrossRef]
- Lim, H.; Kim, T. Effects of Vojta Therapy on Gait of Children with Spastic Diplegia. J. Phys. Therapy Sci. 2014, 25, 1605–1608. [Google Scholar] [CrossRef]
- Ha, S.Y.; Sung, Y.H. Effects of Vojta approach on diaphragm movement in children with spastic cerebral palsy. J. Exerc. Rehabil. 2018, 14, 1005–1009. [Google Scholar] [CrossRef]
- Kashuba, V.; Dolynskyi, B.; Todorova, V.; Bukhovets, B.; Andrieieva, O.; Shankovskyi, A.; Salatenko, I.; Lutskyi, V.; Kovalchuk, L. Physical Rehabilitation of Children with Cerebral Palsy by Bobath-Therapy Method. Int. J. Appl. Exerc. Physiol. Maz. 2020, 9, 6–13. [Google Scholar]
- Kashuba, V.; Bukhovets, B. Indicators of Cerebral Blood Flow Changes in Venous Vessels of Children with ICP in the Course of Physical Rehabilitation Using the Bobath Therapy Method. Cxiднoєвpoпeйcький нaцioнaльний yнiвepcитeт iмeнi Лeci Укpaїнки 2017, 28, 156–163. [Google Scholar]
- Jung, M.W.; Landenberger, M.; Jung, T.; Lindenthal, T.; Philippi, H. Vojta therapy and neurodevelopmental treatment in children with infantile postural asymmetry: A randomised controlled trial. J. Phys. Ther. Sci. 2017, 29, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; Sakuma, K.; Takahashi, T. Follow-up study of children with cerebral coordination disturbance (CCD, Vojta). Brain Dev. 1983, 5, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, M.; Sutkowska, E.; Kuciel, N. The rehabilitation of a child with a Sotos syndrome. case report. Wiad. Lek. 2018, 71, 1849–1853. [Google Scholar]


| Variable | Bobath Therapy (n = 36) | Vojta Therapy (n = 36) | Bobath + Vojta Therapy (n = 36) | p |
|---|---|---|---|---|
| Male Female | 19 (52.78%) 17 (47.22%) | 22 (61.11%) 14 (38.89%) | 23 (63.89%) 13 (36.11%) | 0.337 |
| Urban Rural | 26 (72.22%) 10 (27.78%) | 32 (88.89%) 4 (11.11%) | 26 (72.22%) 10 (27.78%) | 0.145 |
| APGAR score <8 ≥8 | 6 (16.67%) 30 (83.33%) | 8 (22.22%) 28 (77.78%) | 7 (19.44%) 29 (80.56%) | 0.837 |
| Birth weight <3000 g ≥3000 g | 19 (52.78%) 17 (47.22%) | 13 (36.11%) 23 (63.89%) | 12 (33.33%) 24 (66.67%) | 0.192 |
| Premature | 1 (2.78%) | 3 (8.33%) | 4 (11.11%) | |
| Age mean (SD) | 3.64 (1.42) | 3.83 (1.50) | 3.81 (1.47) | |
| Birth weight Mean (SD) | 2898 (747.49) | 3229 (658.22) | 3241 (654.47) |
| Variable | Bobath Therapy (n = 36) | Vojta Therapy (n = 36) | Bobath + Vojta Therapy (n = 36) |
|---|---|---|---|
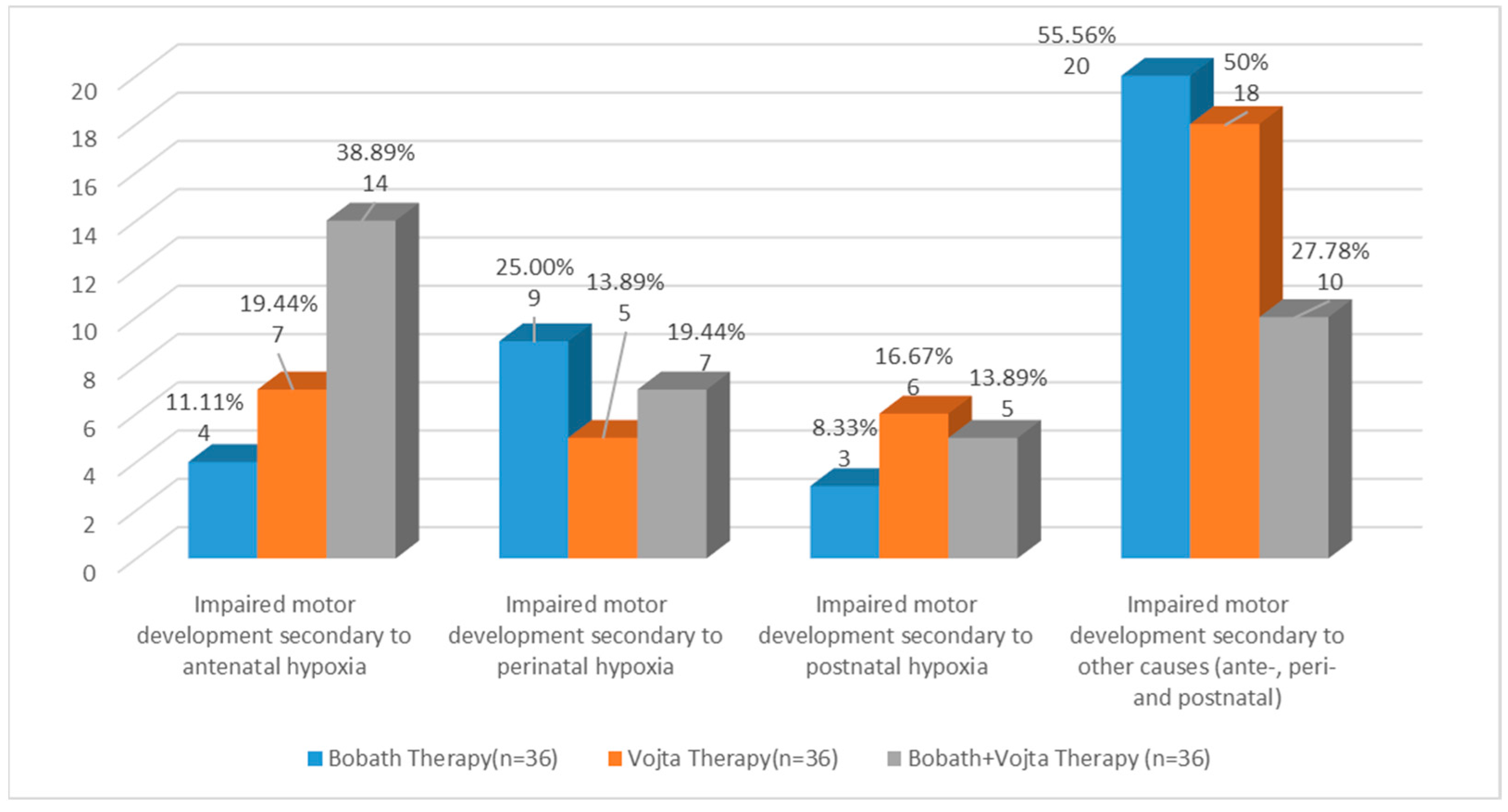
| Antenatal factors | 18 (50.00%) | 14 (38.89%) | 10 (27.78%) |
| Perinatal factors | 11 (30.55%) | 6 (16.67%) | 16 (44.44%) |
| Postnatal factors | 7 (19.45%) | 16 (44.44%) | 10 (27.78%) |
| Variable | Bobath Therapy (n = 36) | Vojta Therapy (n = 36) | Bobath + Vojta Therapy (n = 36) |
|---|---|---|---|
| Recovery after three months | 3 (8.33%) | 3 (8.33%) | 10 (27.77%) |
| Recovery after four months | 8 (22.22%) | 9 (25.00%) | 18 (50.00%) |
| Recovery after five months | 12 (33.33%) | 11 (30.56%) | 7 (19.44%) |
| Recovery after six months | 9 (25.00%) | 9 (25.00%) | 1 (2.78%) |
| Recovery after seven months | 4 (11.11%) | 4 (11.11%) | - |
| Recovery | Hypotonia n = 40 | Hypertonia n = 58 | Mixt n = 10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bobath Therapy (n = 11) | Vojta Therapy (n = 14) | Bobath + Vojta Therapy (n = 15) | Bobath Therapy (n = 23) | Vojta Therapy (n = 19) | Bobath + Vojta Therapy (n = 16) | Bobath Therapy (n = 2) | Vojta Therapy (n = 3) | Bobath + Vojta Therapy (n = 5) | |
| Three months | - | 4 (28.57%) | 3 (20%) | 2 (8.69%) | - | 4 (25%) | 1 (50%) | - | 3 (60%) |
| Four months | 3 (27.27%) | 5 (35.71%) | 8 (53.53%) | 5 (21.73%) | 2 (10.52%) | 8 (50%) | - | 2 (66.6%) | 2 (40%) |
| Five months | 5 (45.45%) | 5 (35.71%) | 3 (20%) | 6 (26.08%) | 4 (21.05%) | 4 (25%) | 1 (50%) | 1 (33.3%) | - |
| Six months | 1 (9.09%) | - | 1 (6.66%) | 8 (34.78%) | 9 (47.36%) | - | - | - | - |
| Seven months | 2 (18.18%) | - | - | 2 (8.69%) | 4 (21.05%) | - | - | - | - |
| Bobat + Vojta Therapy vs. Bobat Therapy | Bobat + Vojta Therapy vs. Vojta Therapy | |||||
|---|---|---|---|---|---|---|
| p | OR | 95% CI | p | OR | 95% CI | |
| Antenatal factors | 0.87 | 1.11 | 0.26–4.63 | 0.91 | 1.11 | 0.17–7.10 |
| Perinatal factors | 0.30 | 0.43 | 0.09–2.08 | 0.23 | 2.95 | 0.50–17.48 |
| Hypertonia | 0.33 | 2.00 | 0.48–8.30 | 0.30 | 0.47 | 0.11–1.99 |
| Mixt | 0.19 | 0.18 | 0.01–2.38 | 0.92 | 1.10 | 0.13–9.14 |
| Recovery after 4 (months) | 0.0002 * | 6.66 | 2.43–18.23 | 0.0001 * | 0.14 | 0.05–0.38 |
| Bobath Therapy | Vojta Therapy | Bobath + Vojta Therapy | |
|---|---|---|---|
| Mean | 5.11 | 5.02 | 3.97 |
| Std. Deviation | 1.19 | 1.13 | 0.77 |
| 95% CI | 4.70–5.51 | 4.64–5.41 | 3.71–4.23 |
| Bobath + Vojta Therapy vs. Bobath Therapy | Bobath + Vojta Therapy vs. Vojta Therapy | |
|---|---|---|
| p | p < 0.0001 | p < 0.0001 |
| 95% CI | 0.66–1.61 | 0.59–1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parau, D.; Todoran, A.B.; Barcutean, L.; Avram, C.; Balasa, R. The Benefits of Combining Bobath and Vojta Therapies in Infants with Motor Development Impairment—A Pilot Study. Medicina 2023, 59, 1883. https://doi.org/10.3390/medicina59101883
Parau D, Todoran AB, Barcutean L, Avram C, Balasa R. The Benefits of Combining Bobath and Vojta Therapies in Infants with Motor Development Impairment—A Pilot Study. Medicina. 2023; 59(10):1883. https://doi.org/10.3390/medicina59101883
Chicago/Turabian StyleParau, Daniela, Anamaria Butila Todoran, Laura Barcutean, Calin Avram, and Rodica Balasa. 2023. "The Benefits of Combining Bobath and Vojta Therapies in Infants with Motor Development Impairment—A Pilot Study" Medicina 59, no. 10: 1883. https://doi.org/10.3390/medicina59101883
APA StyleParau, D., Todoran, A. B., Barcutean, L., Avram, C., & Balasa, R. (2023). The Benefits of Combining Bobath and Vojta Therapies in Infants with Motor Development Impairment—A Pilot Study. Medicina, 59(10), 1883. https://doi.org/10.3390/medicina59101883






