The Impact of Infections on the Progression of Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
- Mild infections: did not require hospitalization for treatment, were self-limited or required a short course of antibiotic therapy (less than 5 days). The inflammatory syndrome was minimal or absent. Among these, the following were identified in our patients: asymptomatic bacteriuria, cystitis, dental infections, uncomplicated upper-respiratory-tract infections and gastritis.
- Moderate infections: inflammatory syndrome present (CRP between 10 and 30 UI/mL, leukocytosis), required hospitalization or occurred during hospitalization and had the potential for progression in the absence of antibiotic treatment. Among them, the following were identified in our group: orchiepididymitis, prostatitis and post-operative infections of soft tissue.
- Severe infections: had significant inflammatory syndrome (CRP over 30 UI/mL, leukocytosis/leukopenia), required hospitalization and prolonged antibiotic therapy, and patients often presented with altered general condition. Severe infections are also considered life-threatening. Among them, the following were identified in our patients: sepsis, endocarditis, cholecystitis, enterocolitis due to Clostridioides difficile, pyelonephritis and acute lower-respiratory-tract infections. In our study, we excluded viral infections such as those caused by hepatitis B or C or HIV due to the multitude of different possible patterns of glomerular injury associated with them, each with a different evolution toward end-stage kidney disease. On the other hand, viral infections causing upper- or lower-respiratory-tract infections were not excluded, but we could not properly identify which virus was involved specifically.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.S.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, J.; Matsushita, K. Clinical epidemiology of infectious disease among patients with chronic kidney disease. Clin. Exp. Nephrol. 2019, 23, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, J.; Taliercio, J.; I Feldman, H.; Srivastava, A.; Townsend, R.; Cohen, D.L.; Horwitz, E.; Rao, P.; Charleston, J.; Fink, J.C.; et al. Inflammatory Markers and Incidence of Hospitalization with Infection in Chronic Kidney Disease. Am. J. Epidemiol. 2020, 189, 433–444. [Google Scholar] [CrossRef]
- Cravedi, P.; Remuzzi, G. Pathophysiology of proteinuria and its value as an outcome measure in CKD. Br. J. Clin. Pharmacol. 2013, 76, 516–523. [Google Scholar] [CrossRef]
- Iseki, K.; Iseki, C.; Ikemiya, Y.; Fukiyama, K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996, 49, 800–805. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
- Farag, A.; Garg, A.X.; Li, L.; Jain, A.K. Dosing Errors in Prescribed Antibiotics for Older Persons with CKD: A Retrospective Time Series Analysis. Am. J. Kidney Dis. 2014, 63, 422–428. [Google Scholar] [CrossRef]
- Oda, T.; Yoshizawa, N. Factors Affecting the Progression of Infection-Related Glomerulonephritis to Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 905. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, N.; Yamada, M.; Fujino, M.; Oda, T. Nephritis-Associated Plasmin Receptor (NAPlr): An Essential Inducer of C3-Dominant Glomerular Injury and a Potential Key Diagnostic Biomarker of Infection-Related Glomerulonephritis (IRGN). Int. J. Mol. Sci. 2022, 23, 9974. [Google Scholar] [CrossRef] [PubMed]
- Godaly, G.; Ambite, I.; Svanborg, C. Innate immunity and genetic determinants of urinary tract infection susceptibility. Curr. Opin. Infect. Dis. 2015, 28, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ambite, I.; Butler, D.; Wan, M.L.Y.; Rosenblad, T.; Tran, T.H.; Chao, S.M.; Svanborg, C. Molecular determinants of disease severity in urinary tract infection. Nat. Rev. Urol. 2021, 18, 468–486. [Google Scholar] [CrossRef]
- Behzadi, E.; Behzadi, P. The role of toll-like receptors (TLRs) in urinary tract infections (UTIs). Cent. Eur. J. Urol. 2016, 69, 404–410. [Google Scholar] [CrossRef]
- Hussein, A.; Askar, E.; Elsaeid, M.; Schaefer, F. Functional polymorphisms in transforming growth factor-beta-1 (TGFbeta-1) and vascular endothelial growth factor (VEGF) genes modify risk of renal parenchymal scarring following childhood urinary tract infection. Nephrol. Dial. Transpl. 2010, 25, 779–785. [Google Scholar] [CrossRef]
- Yang, W.-S.; Chang, Y.-C.; Hsieh, M.-L.; Wang, J.-L.; Wu, L.-C.; Chang, C.-H. Stratified risks of infection-related hospitalization in patients with chronic kidney disease—A prospective cohort study. Sci. Rep. 2020, 10, 4475. [Google Scholar] [CrossRef] [PubMed]
- Flammia, R.S.; Tufano, A.; Proietti, F.; Gerolimetto, C.; De Nunzio, C.; Franco, G.; Leonardo, C. Renal surgery for kidney cancer: Is preoperative proteinuria a predictor of functional and survival outcomes after surgery? A systematic review of the literature. Minerva Urol. Nephrol. 2022, 74, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Matsushita, K.; Ärnlöv, J.; Inker, L.A.; Katz, R.; Polkinghorne, K.R.; Rothenbacher, D.; Sarnak, M.J.; Astor, B.C.; Coresh, J.; et al. Cystatin C versus Creatinine in Determining Risk Based on Kidney Function. N. Engl. J. Med. 2013, 369, 932–943. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, B.; Yuan, H.; Zhao, W. Evaluation of serum neutrophil gelatinase-associated lipocalin in older patients with chronic kidney disease. Aging Med. 2020, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood Kidney Injury Molecule-1 Is a Biomarker of Acute and Chronic Kidney Injury and Predicts Progression to ESRD in Type I Diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, A.; Sugaya, T.; Hikawa, A.; Yamanouchi, M.; Hirata, Y.; Ishimitsu, T.; Numabe, A.; Takagi, M.; Hayakawa, H.; Tabei, F.; et al. Clinical evaluation of urinary excretion of liver-type fatty acid-binding protein as a marker for the monitoring of chronic kidney disease: A multicenter trial. J. Lab. Clin. Med. 2005, 145, 125–133. [Google Scholar] [CrossRef] [PubMed]

| Total (238) | With Infections (117) | No Infections (121) | p-Value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 60 (18) | 62 (20) | 57 (18) | 0.004 |
| Sex M, n (%) | 130 (54.6) | 54 (46.1) | 76 (62.8) | 0.01 |
| Hemoglobin, g/dL, mean (SD) | 11.7 (2.20) | 11.3 (2.18) | 12.05 (2.18) | 0.03 |
| Leukocytes/mL, median (IQR) | 7700 (3025) | 7700 (3500) | 8000 (2800) | 0.47 |
| Neutrophils/mL, median (IQR) | 5100 (2525) | 4900 (2850) | 5400 (2050) | 0.19 |
| Lymphocytes/mL, median (IQR) | 1850 (800) | 1800 (800) | 2000 (800) | 0.02 |
| CRP, UI/mL, median (IQR) | 5 (9) | 5 (10) | 5 (9) | 0.31 |
| Fibrinogen, mg/dL, median (IQR) | 573 (224) | 586 (234) | 560 (201) | 0.06 |
| Serum albumin, g/dL, median (IQR) | 4.27 (0.59) | 4.28 (0.62) | 4.22 (0.59) | 0.87 |
| Hematuria, n (%) | 149 (62.6) | 82 (70) | 67 (55.3) | 0.01 |
| Leukocyturia, n (%) | 126 (52.9) | 83 (70.9) | 43 (35.5) | <0.001 |
| SCr_M0, mg/dL, median (IQR) | 2.3 (1.55) | 2.17 (1.77) | 2.39 (1.32) | 0.708 |
| eGFR_M0, mL/min, median (IQR) | 27 (21) | 27 (24.2) | 29 (18.8) | 0.431 |
| SCr_inf1, mg/dL, median (IQR) | 2.83 (3.22) | |||
| eGFR_inf1, mL/min, median (IQR) | 19.1 (19.93) | |||
| SCr_inf2, mg/dL, median (IQR) | 3.35 (3.21) | |||
| eGFR_inf2, mL/min, median (IQR) | 15 (19.5) | |||
| SCr_inf3, mg/dL, median (IQR) | 3.43 (2.9) | |||
| eGFR_inf3, mL/min, median (IQR) | 14 (15) | |||
| Final eGFR, mL/min, median (IQR) | 9 (14) | 69 (8.5) | 13 (18) | 0.017 |
| Time M0_endpoint, months, median (IQR) | 60 (19) | 57 (23) | 60 (11) | 0.007 |
| Time M0_inf1, months, median (IQR) | 4 (23) | |||
| Time inf1_inf2, months, median (IQR) | 17 (23) | |||
| Time inf2_inf3, months, median (IQR) | 6 (14) | |||
| Time inf3_endpoint, months, median (IQR) | 15 (20) | |||
| Severity of infection, n (%) | 1. Mild—60 (51.2) 2. Moderate—5 (4.2) 3. Severe—52 (44.4) | |||
| At least two severe infections, n (%) | 11 (9.4) | |||
| More than three infections, n (%) | 24 (20.5) | |||
| Septic AKI, n (%) | 0 episodes—103 (88) 1 episode—14 (11.9) | |||
| KRT during the follow-up period, n (%) | 102 (42.8) | 61 (52.1) | 41 (33.8) | 0.004 |
| Etiology of CKD (%) | 1. Benign nephrosclerosis (28.1) 2. Primary glomerulopathies (22.6) 3. Diabetic nephropathy (13.4) | 1. Benign nephrosclerosis (26.4) 2. Primary glomerulopathies (17.9) 3. Diabetic nephropathy (10.2) | 1. Benign nephrosclerosis (29.7) 2. Primary glomerulopathies (27.2) 3. Diabetic nephropathy (16.5) | 0.10 |
| Stage of CKD at the first visit, n (%) | G1: 3 (1.2) | G1: 0 (0) | G1: 3 (2.4) | 0.46 |
| G2: 16 (6.7) | G2: 9 (7.7) | G2: 7 (5.8) | ||
| G3a: 23 (9.6) | G3a: 14 (11.9) | G3a: 9 (7.4) | ||
| G3b: 62 (26) | G3b: 25 (21.3) | G3b: 37 (30.5) | ||
| G4: 96 (40.3) | G4: 49 (41.8) | G4: 47 (38.8) | ||
| G5: 38 (15.9) | G5: 20 (17) | G5: 18 (14.8) | ||
| Risk group, n (%) | 1. Low—5 (2.1) 2. Moderate—5 (2.1) 3. High—25 (10.5) 4. Very high—203 (85.2) | 1. Low—2 (0.8) 2. Moderate—3 (1.2) 3. High—13 (5.4) 4. Very high—99 (84.6) | 1. Low—3 (2.4) 2. Moderate—2 (1.6) 3. High—12 (9.9) 4. Very high—104 (85.9) | 0.92 |
| Hypertension, n (%) | 220 (92.4) | 110 (94) | 110 (90.9) | 0.36 |
| Diabetes mellitus, n (%) | 89 (37.3) | 41 (35) | 48 (39.6) | 0.46 |
| Stroke, n (%) | 19 (7.9) | 10 (8.5) | 9 (7.4) | 0.75 |
| Malignancies, n (%) | 46 (19.32) | 20 (17.09) | 26 (21.4) | 0.39 |
| COPD, n (%) | 16 (6.8) | 13 (11.1) | 3 (2.4) | 0.008 |
| Heart failure, n (%) | 73 (30.6) | 33 (28.2) | 40 (33) | 0.41 |
| Chronic venous insufficiency, n (%) | 22 (9.2) | 8 (6.8) | 14 (11.5) | 0.20 |
| Charlson score, median (IQR) | 6 (4) | 6 (4) | 6 (4) | 0.46 |
| Total: 117 | Mild Infections-60 | Moderate Infections-5 | Severe Infections-52 |
|---|---|---|---|
| Inflammatory syndrome | Minimum/Absent | Moderate | Severe |
| Antibiotics | Short course (5 days) | Sometimes a prolonged course | Often prolonged course |
| Hospitalization | No need | Can be required Infections that occurred during hospitalization | Required |
| Evolution/Risks | Self-limited | Potential for progression | Potentially life-threatening in the absence of treatment |
| -asymptomatic bacteriuria | -orchiepididymitis | -sepsis | |
| -cystitis | -prostatitis | -endocarditis | |
| -dental infections | -post-operatory infections of soft tissue | -cholecystitis | |
| -upper-respiratory-tract infections | -enterocolitis | ||
| -gastritis | -pyelonephritis | ||
| -lower-respiratory-tract infections |
| First Infectious Episode (117 Cases) | N (%) | The Most Common Microorganism Identified (%) |
|---|---|---|
| Lower UTIs | 67 (57.2) | E. coli (43.2) |
| Upper UTIs | 20 (17) | Klebsiella spp. (20) |
| Acute lower-respiratory-tract infections | 15 (12.8) | Candida spp. (26.6) |
| Enterocolitis | 4 (3.4) | Cl. difficile (75) |
| Sepsis | 2 (1.7) | Klebsiella spp. (100) |
| Soft tissue | 2 (1.7) | Staphylococcus spp. (50) |
| Acute upper-respiratory-tract infection | 1 (0.8) | |
| Gastritis | 1 (0.8) | H. pylori (100) |
| Endocarditis | 1 (0.8) | |
| Dental infection | 1 (0.8) | |
| Orchiepididymitis | 1 (0.8) | |
| Cholecystitis | 1 (0.8) | |
| Second infectious episode (49 cases) | N (%) | The most common microorganism identified (%) |
| Lower UTIs | 28 (57) | E. coli (35) |
| Upper UTIs | 12 (24) | E. coli (58) |
| Acute lower-respiratory-tract infections | 4 (8) | Klebsiella spp. (50) |
| Enterocolitis | 2 (4) | Cl. difficile (50) |
| Acute upper-respiratory-tract infection | 1 (2) | |
| Soft tissue | 1 (2) | |
| Dental infection | 1 (2) | |
| Third infectious episode (27 cases) | N (%) | The most common microorganism identified (%) |
| Lower UTIs | 21 (7) | E. coli (33) |
| Enterocolitis | 2 (7) | Cl. difficile (100) |
| Upper UTIs | 1 (3) | |
| Acute lower-respiratory-tract infections | 1 (3) | Klebsiella spp. (100) |
| Soft tissue | 1 (3) | |
| Acute upper-respiratory-tract infections | 1 (3) |
| Variable | HR | CI | p-Value |
|---|---|---|---|
| Age (M0) | 1.021 | 1.005–1.036 | 0.009 |
| Hemoglobin (M0) | 0.861 | 0.786–0.943 | 0.001 |
| Hematuria (M0) | 1.604 | 1.047–2.457 | 0.03 |
| Proteinuria (M0) | 1.122 | 1.028–1.224 | 0.01 |
| Serum creatinine (M0) | 1.262 | 1.141–1.396 | <0.001 |
| Serum creatinine at inf1 | 1.150 | 1.081–1.224 | <0.001 |
| Serum creatinine at inf2 | 1.413 | 1.188–1.679 | <0.001 |
| Serum creatinine at inf3 | 1.506 | 1.126–2.015 | 0.006 |
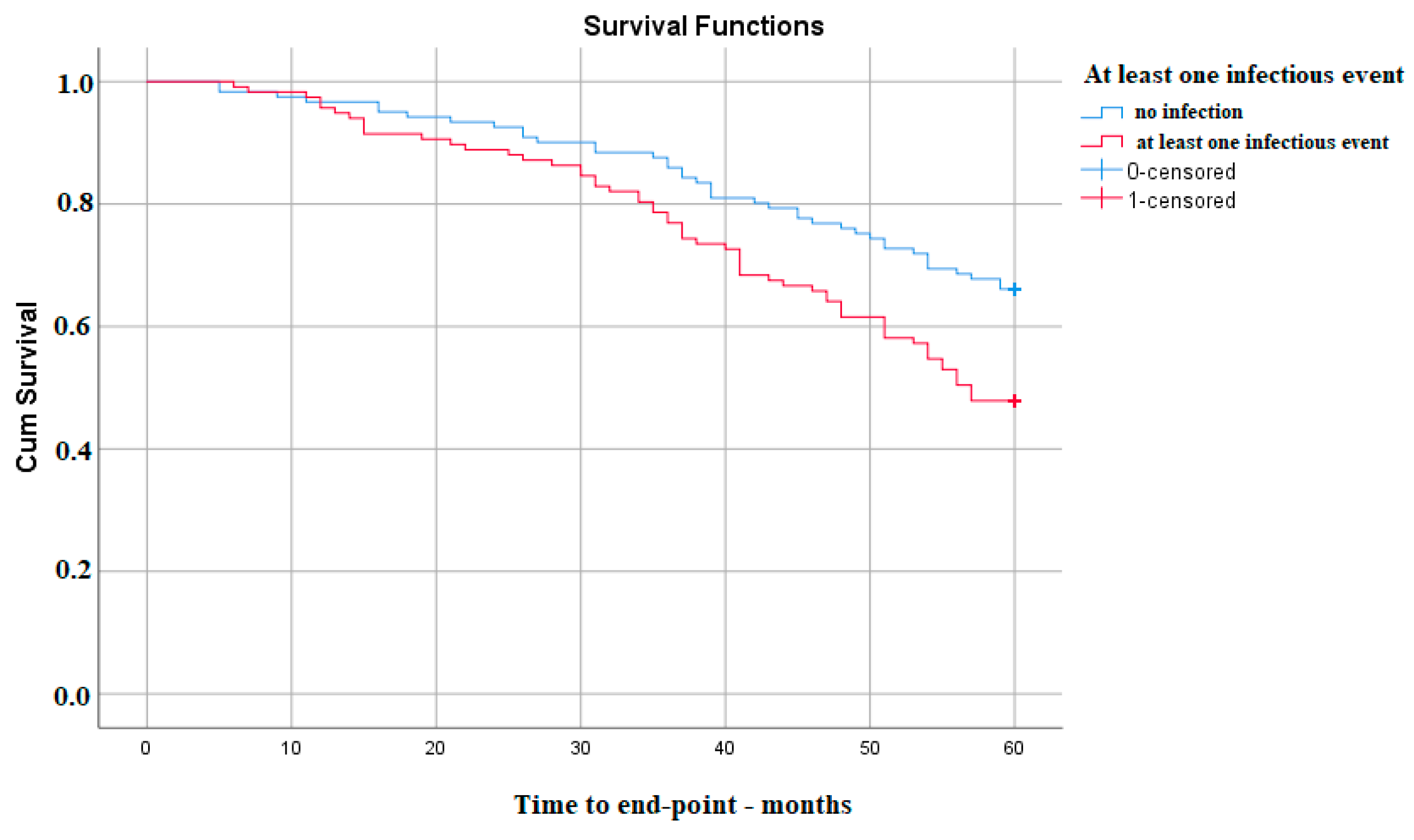
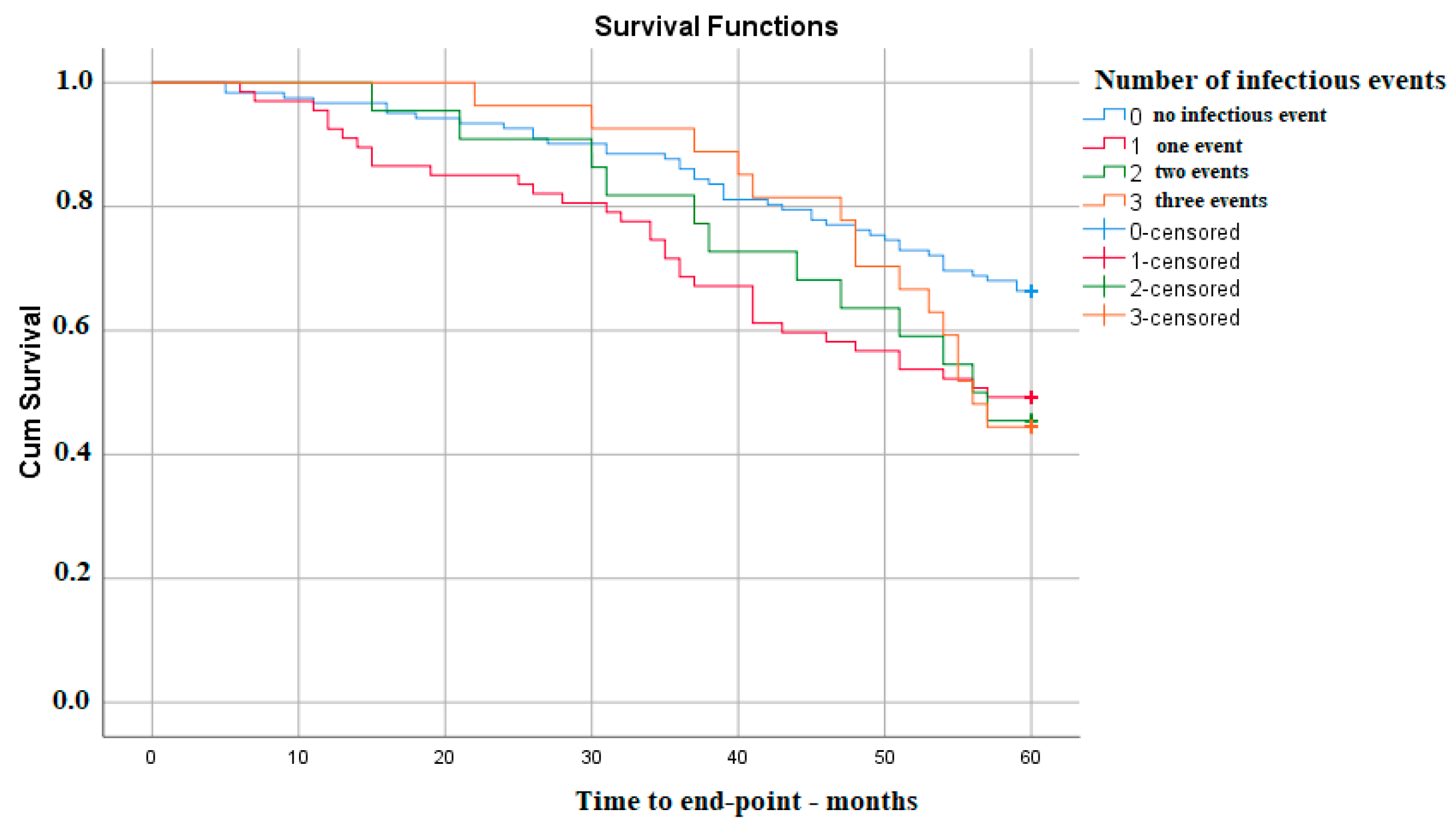
| Presence of an infectious event | 1.748 | 1.176–2.598 | 0.006 |
| Lower UTI at inf1 | 1.923 | 1.232–3.001 | 0.004 |
| Lower respiratory tract infection at inf1 | 3.631 | 1.904–6.924 | 0.001 |
| COPD | 2.467 | 1.376–4.424 | 0.002 |
| Variable | HR | CI | p-Value | HR | CI | p-Value |
|---|---|---|---|---|---|---|
| Age | 1.021 | 1.005–1.036 | 0.009 | 1.034 | 1.010–1.058 | 0.004 |
| Serum creatinine (M0) | 1.262 | 1.141–1.396 | <0.001 | 1.421 | 1.203–1.678 | <0.001 |
| Proteinuria (M0) | 1.122 | 1.028–1.224 | 0.01 | 1.241 | 1.126–1.369 | <0.001 |
| Presence of an infectious event | 1.748 | 1.176–2.598 | 0.006 | 1.705 | 1.013–2.868 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicu-Andreescu, I.; Căpușă, C.; Gârneață, L.; Ciurea, O.-A.; Dicu-Andreescu, I.-G.; Ungureanu, E.-A.; Vlad, D.-V.; Vișan, A.-C.; Ungureanu, V.-G.; Vlad, V.-V.; et al. The Impact of Infections on the Progression of Chronic Kidney Disease. Medicina 2023, 59, 1836. https://doi.org/10.3390/medicina59101836
Dicu-Andreescu I, Căpușă C, Gârneață L, Ciurea O-A, Dicu-Andreescu I-G, Ungureanu E-A, Vlad D-V, Vișan A-C, Ungureanu V-G, Vlad V-V, et al. The Impact of Infections on the Progression of Chronic Kidney Disease. Medicina. 2023; 59(10):1836. https://doi.org/10.3390/medicina59101836
Chicago/Turabian StyleDicu-Andreescu, Ioana, Cristina Căpușă, Liliana Gârneață, Otilia-Andreea Ciurea, Irinel-Gabriel Dicu-Andreescu, Elena-Alexandra Ungureanu, Denis-Valentin Vlad, Antonia-Constantina Vișan, Victor-Gabriel Ungureanu, Violeta-Valentina Vlad, and et al. 2023. "The Impact of Infections on the Progression of Chronic Kidney Disease" Medicina 59, no. 10: 1836. https://doi.org/10.3390/medicina59101836
APA StyleDicu-Andreescu, I., Căpușă, C., Gârneață, L., Ciurea, O.-A., Dicu-Andreescu, I.-G., Ungureanu, E.-A., Vlad, D.-V., Vișan, A.-C., Ungureanu, V.-G., Vlad, V.-V., Vasioiu, P.-C., Ciutacu, E.-M., Neicu, M., Penescu, M., & Verzan, C. (2023). The Impact of Infections on the Progression of Chronic Kidney Disease. Medicina, 59(10), 1836. https://doi.org/10.3390/medicina59101836






