Anti-Inflammatory and Antipruritic Effects of Remote Ischaemic Postconditioning in a Mouse Model of Experimental Allergic Contact Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. Chemicals and Antibodies
2.3. Study Design
2.4. Measurement of Scratching Behaviour
2.5. Measurement of Ear Thickness
2.6. Measurement of Cytokine Levels
2.7. Histological Analysis
2.8. Immunohistochemical Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of Remote Ischemic Postconditioning on Ear Thickness and Itching Behaviour
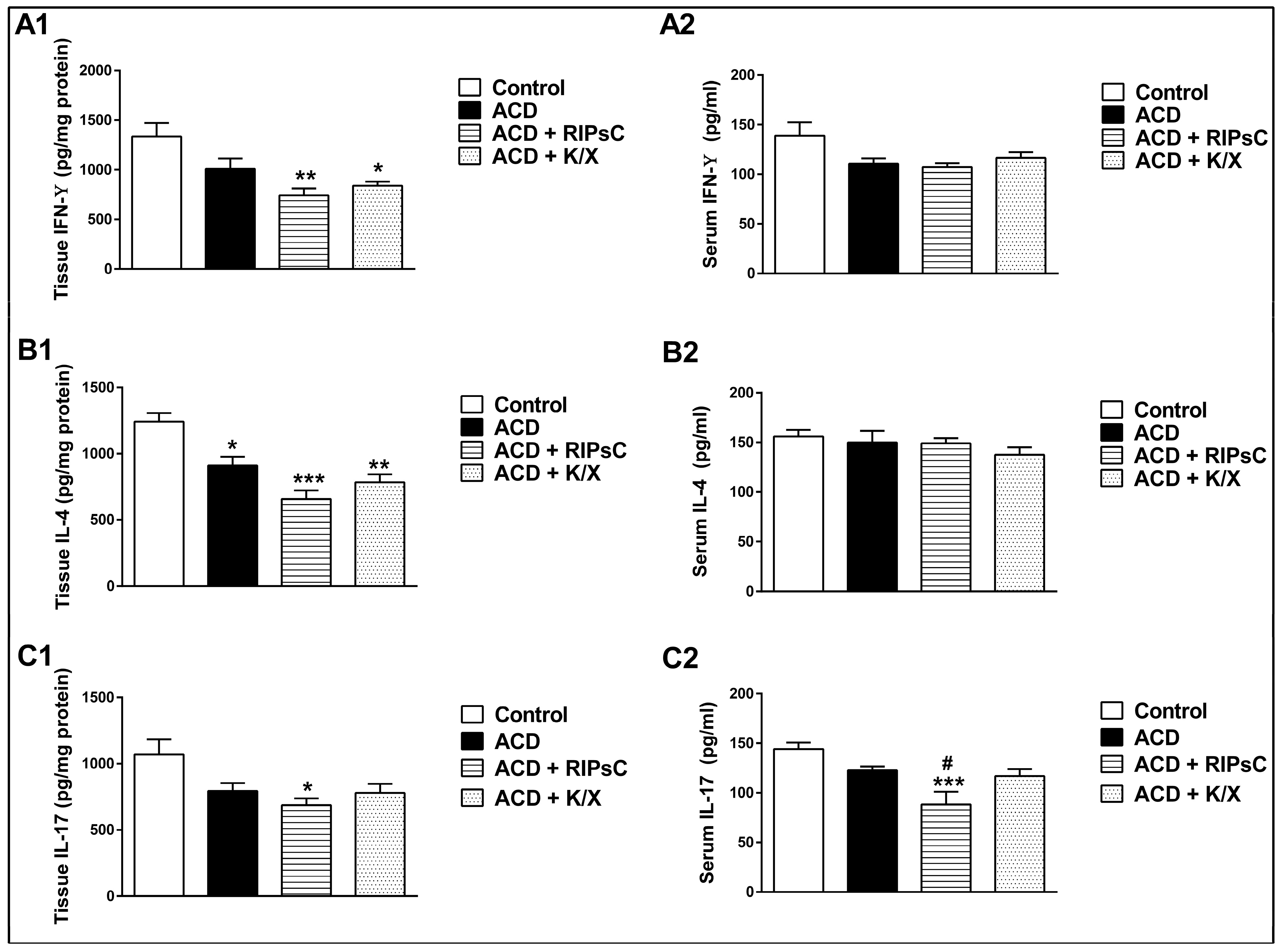
3.2. Effect of Remote Ischemic Postconditioning on Tissue and Serum Cytokine Concentrations
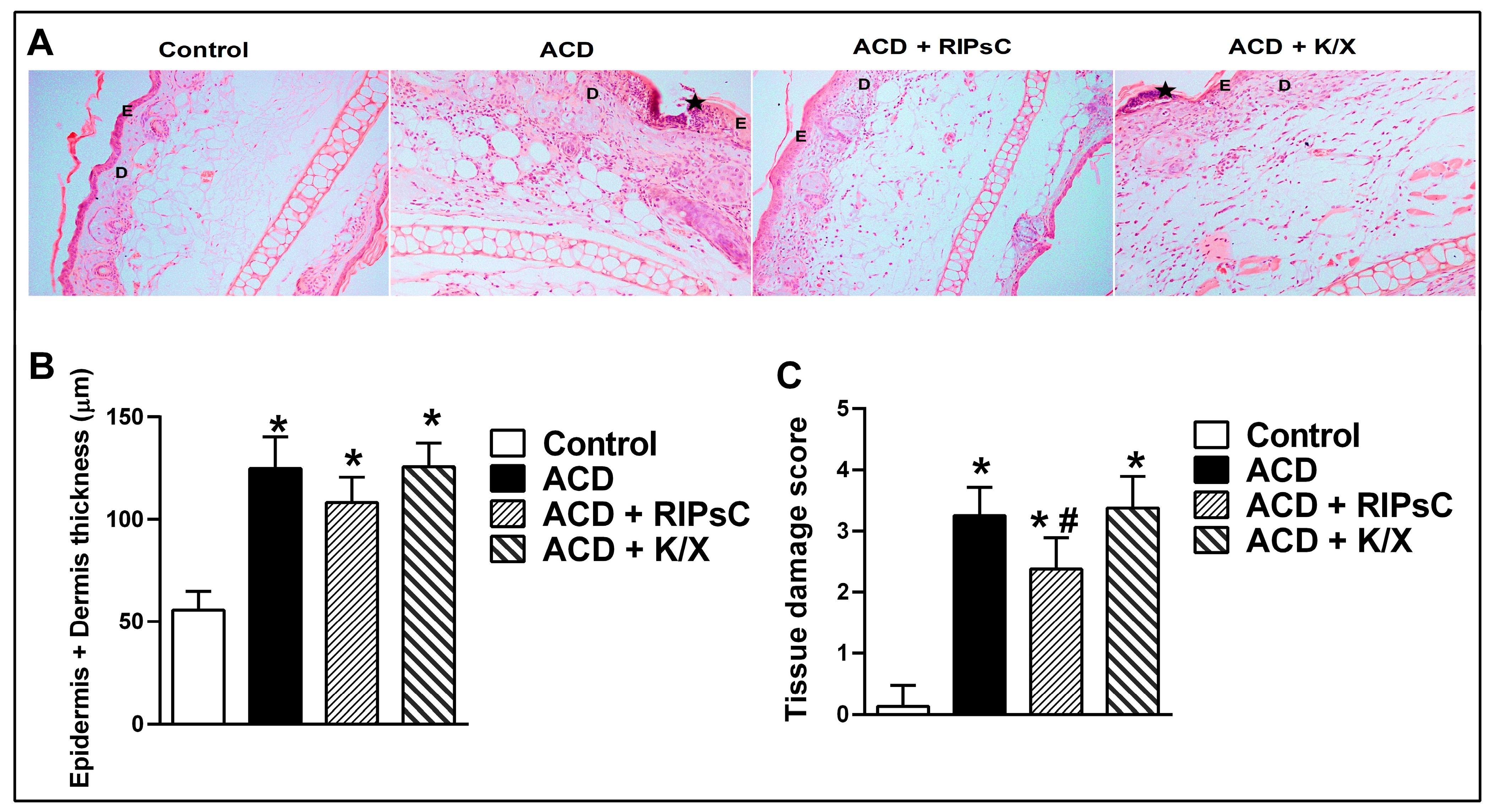
3.3. Effect of Remote Ischemic Postconditioning on Histological Changes
3.3.1. Haematoxylin & Eosin Staining
3.3.2. Toluidine Staining
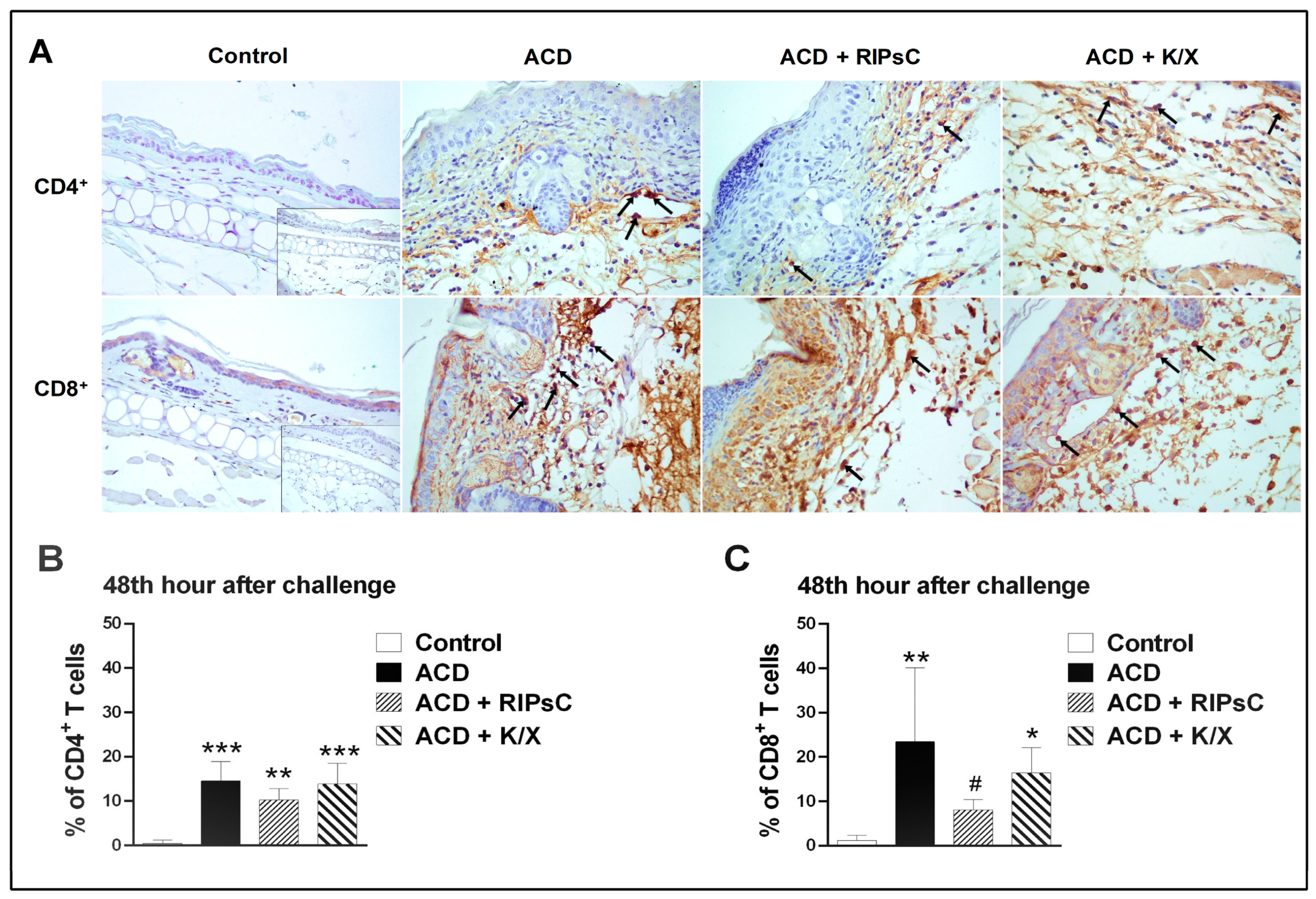
3.4. Effect of Remote Ischemic Postconditioning on T-Cell Infiltration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamson, A.S. The Economics Burden of Atopic Dermatitis. Adv. Exp. Med. Biol. 2017, 1027, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Tramontana, M.; Hansel, K.; Bianchi, L.; Sensini, C.; Malatesta, N.; Stingeni, L. Advancing the understanding of allergic contact dermatitis: From pathophysiology to novel therapeutic approaches. Front. Med. 2023, 10, 1184289. [Google Scholar] [CrossRef] [PubMed]
- Aye, A.; Song, Y.J.; Jeon, Y.D.; Jin, J.S. Xanthone suppresses allergic contact dermatitis in vitro and in vivo. Int. Immunopharmacol. 2020, 78, 106061. [Google Scholar] [CrossRef]
- Bour, H.; Peyron, E.; Gaucherand, M.; Garrigue, J.L.; Desvignes, C.; Kaiserlian, D.; Revillard, J.P.; Nicolas, J.F. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur. J. Immunol. 1995, 25, 3006–3010. [Google Scholar] [CrossRef]
- Gocinski, B.L.; Tigelaar, R.E. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J. Immunol. 1990, 144, 4121–4128. [Google Scholar] [CrossRef]
- He, D.; Wu, L.; Kim, H.K.; Li, H.; Elmets, C.A.; Xu, H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J. Immunol. 2006, 177, 6852–6858. [Google Scholar] [CrossRef]
- Liu, J.; Harberts, E.; Tammaro, A.; Girardi, N.; Filler, R.B.; Fishelevich, R.; Temann, A.; Licona-Limon, P.; Girardi, M.; Flavell, R.A.; et al. IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis. J. Investig. Dermatol. 2014, 134, 1903–1911. [Google Scholar] [CrossRef]
- Kostner, L.; Anzengruber, F.; Guillod, C.; Recher, M.; Schmid-Grendelmeier, P.; Navarini, A.A. Allergic Contact Dermatitis. Immunol. Allergy Clin. N. Am. 2017, 37, 141–152. [Google Scholar] [CrossRef]
- Niculet, E.; Bobeica, C.; Tatu, A.L. Glucocorticoid-Induced Skin Atrophy: The Old and the New. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1041–1050. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef]
- Na, H.S.; Kim, Y.I.; Yoon, Y.W.; Han, H.C.; Nahm, S.H.; Hong, S.K. Ventricular premature beat—Driven intermittent restoration of coronary blood flow reduces the incidence of reperfusion-induced ventricular fibrillation in a cat model of regional ischemia. Am. Heart J. 1996, 132, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Liauw, S.K.; Rubin, B.B.; Lindsay, T.F.; Romaschin, A.D.; Walker, P.M. Sequential ischemia/reperfusion results in contralateral skeletal muscle salvage. Am. J. Physiol. 1996, 270, H1407–H1413. [Google Scholar] [CrossRef]
- Przyklenk, K.; Bauer, B.; Ovize, M.; Kloner, R.A.; Whittaker, P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993, 87, 893–899. [Google Scholar] [CrossRef]
- Souza Filho, M.V.; Loiola, R.T.; Rocha, E.L.; Simão, A.F.; Gomes, A.S.; Souza, M.H.; Ribeiro, R.A. Hind limb ischemic preconditioning induces an anti-inflammatory response by remote organs in rats. Braz. J. Med. Biol. Res. 2009, 42, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Alganabi, M.; Biouss, G.; Ganji, N.; Yamoto, M.; Lee, C.; Li, B.; Pierro, A. Remote ischemic conditioning causes CD4 T cells shift towards reduced cell-mediated inflammation. Pediatr. Surg. Int. 2022, 38, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Ma, X.T.; Ma, X.; Chen, M.; Chu, Y.H.; Wu, L.J.; Wang, W.; Qin, C.; Tian, D.S. Remote Limb Ischemic Postconditioning Protects Against Ischemic Stroke by Promoting Regulatory T Cells Thriving. J. Am. Heart Assoc. 2021, 10, e023077. [Google Scholar] [CrossRef] [PubMed]
- Motomura, A.; Shimizu, M.; Kato, A.; Motomura, K.; Yamamichi, A.; Koyama, H.; Ohka, F.; Nishikawa, T.; Nishimura, Y.; Hara, M.; et al. Remote ischemic preconditioning protects human neural stem cells from oxidative stress. Apoptosis 2017, 22, 1353–1361. [Google Scholar] [CrossRef]
- Fukunaga, A.; Horikawa, T.; Ogura, K.; Taguchi, K.; Yu, X.; Funasaka, Y.; Takeda, M.; Nakamura, H.; Yodoi, J.; Nishigori, C. Thioredoxin suppresses the contact hypersensitivity response by inhibiting leukocyte recruitment during the elicitation phase. Antioxid. Redox Signal. 2009, 11, 1227–1235. [Google Scholar] [CrossRef]
- Ozlen, N.; Ercetin, D.; Sapmaz-Metin, M.; Gunduz, O. Anti-inflammatory effect of hydrogen sulfide donor sodium-sulfide in an experimental mouse model of contact hypersensitivity. Dermatol. Sin. 2023, 41, 94–102. [Google Scholar] [CrossRef]
- Nadeem, M.; Kindelin, A.; Mahady, L.; Bhatia, K.; Hoda, M.N.; Ducruet, A.F.; Ahmad, S. Remote Ischemic Post-Conditioning Therapy is Protective in Mouse Model of Traumatic Optic Neuropathy. Neuromol. Med. 2021, 23, 371–382. [Google Scholar] [CrossRef]
- Rohailla, S.; Clarizia, N.; Sourour, M.; Sourour, W.; Gelber, N.; Wei, C.; Li, J.; Redington, A.N. Acute, delayed and chronic remote ischemic conditioning is associated with downregulation of mTOR and enhanced autophagy signaling. PLoS ONE 2014, 9, e111291. [Google Scholar] [CrossRef] [PubMed]
- Todurga, Z.G.; Gunduz, O.; Karadag, C.H.; Ulugol, A. Descending serotonergic and noradrenergic systems do not regulate the antipruritic effects of cannabinoids. Acta Neuropsychiatr. 2016, 28, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Manresa, M.C. Animal Models of Contact Dermatitis: 2,4-Dinitrofluorobenzene-Induced Contact Hypersensitivity. In Animal Models of Allergic Disease Methods and Protocols; Nagamoto-Combs, K., Ed.; Humana Press: New York, NY, USA, 2021; pp. 87–100. [Google Scholar]
- Moreno-Sosa, T.; Sánchez, M.B.; Pietrobon, E.O.; Fernandez-Muñoz, J.M.; Zoppino, F.C.M.; Neira, F.J.; Germanó, M.J.; Cargnelutti, D.E.; Innocenti, A.C.; Jahn, G.A.; et al. Desmoglein-4 Deficiency Exacerbates Psoriasiform Dermatitis in Rats While Psoriasis Patients Displayed a Decreased Gene Expression of DSG4. Front. Immunol. 2021, 12, 625617. [Google Scholar] [CrossRef]
- Muñoz, F.C.; Cervantes, M.M.; Cervantes-García, D.; Jiménez, M.; Ventura-Juárez, J.; Salinas, E. Glycomacropeptide Attenuates Inflammation, Pruritus, and Th2 Response Associated with Atopic Dermatitis Induced by 2,4-Dinitrochlorobenzene in Rat. J. Immunol. Res. 2017, 2017, 6935402. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, F.; Bourgeois, C. Animal Models of Contact Dermatitis; Young Suck, R., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 2; pp. 23–38. [Google Scholar] [CrossRef][Green Version]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the murine contact hypersensitivity model: Toward the understanding of allergic contact dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Saika, A.; Nagatake, T.; Hirata, S.I.; Sawane, K.; Adachi, J.; Abe, Y.; Isoyama, J.; Morimoto, S.; Node, E.; Tiwari, P.; et al. ω3 fatty acid metabolite, 12-hydroxyeicosapentaenoic acid, alleviates contact hypersensitivity by downregulation of CXCL1 and CXCL2 gene expression in keratinocytes via retinoid X receptor α. Faseb J. 2021, 35, e21354. [Google Scholar] [CrossRef]
- Zaladonis, A.; Zhang, X.; Manupipatpong, K.K.; Kalaiselvan, S.; Alvarez, P.; Jensen, L.E. Interleukin-36 (IL-36) system in the 1-fluoro-2,4-dinitrobenzene (DNFB) mouse model of allergic contact dermatitis. Allergy 2020, 75, 2078–2081. [Google Scholar] [CrossRef] [PubMed]
- Lent-Schochet, D.; Jialal, I. Physiology, Edema. [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537065/ (accessed on 5 June 2023).
- Nakae, S.; Komiyama, Y.; Nambu, A.; Sudo, K.; Iwase, M.; Homma, I.; Sekikawa, K.; Asano, M.; Iwakura, Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002, 17, 375–387. [Google Scholar] [CrossRef]
- Balaha, M.F.; Ahmed, N.J.; Almalki, Z.S.; Alahmari, A.K.; Alshehri, A.M.; Soliman, G.A.; Hamad, A.M. Epimedin A ameliorates DNFB-induced allergic contact dermatitis in mice: Role of NF-κB/NLRP3-driven pyroptosis, Nrf2/HO-1 pathway, and inflammation modulation. Life Sci. 2022, 302, 120653. [Google Scholar] [CrossRef]
- Gomez de Agüero, M.; Vocanson, M.; Hacini-Rachinel, F.; Taillardet, M.; Sparwasser, T.; Kissenpfennig, A.; Malissen, B.; Kaiserlian, D.; Dubois, B. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. J. Clin. Investig. 2012, 122, 1700–1711. [Google Scholar] [CrossRef]
- Jung, S.H.; Sun, X.; Ryu, W.S.; Yang, B.S. Topical administration of the pan-Src kinase inhibitors, dasatinib and LCB 03-0110, prevents allergic contact dermatitis in mice. Br. J. Dermatol. 2013, 168, 112–119. [Google Scholar] [CrossRef]
- de Souza Oliveira, V.H.; Amorim, M.A.; de Oliveira, J.R.J.M.; Soley, B.S.; Rocha, F.G.; de Mello Bandenburg, M.; Lejeune, V.B.P.; de Lima Silva, A.H.B.; Witherden, D.A.; Havran, W.L.; et al. Anti-proliferative and anti-inflammatory effects of the application of baclofen cream, a GABAB receptor agonist, on skin inflammation in mice. Eur. J. Pharmacol. 2023, 955, 175910. [Google Scholar] [CrossRef]
- Niculet, E.; Radaschin, D.S.; Nastase, F.; Draganescu, M.; Baroiu, L.; Miulescu, M.; Arbune, M.; Tatu, A.L. Influence of phytochemicals in induced psoriasis (Review). Exp. Ther. Med. 2020, 20, 3421–3424. [Google Scholar] [CrossRef]
- Chen, H.S.; Cui, Y.; Li, X.Q.; Wang, X.H.; Ma, Y.T.; Zhao, Y.; Han, J.; Deng, C.Q.; Hong, M.; Bao, Y.; et al. Effect of Remote Ischemic Conditioning vs Usual Care on Neurologic Function in Patients With Acute Moderate Ischemic Stroke: The RICAMIS Randomized Clinical Trial. JAMA 2022, 328, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Bu, Y.; Li, M.; Han, R.; Zhang, N.; Hao, J.; Jiang, W. Remote ischemic conditioning improves cognition in patients with subcortical ischemic vascular dementia. BMC Neurol. 2019, 19, 206. [Google Scholar] [CrossRef] [PubMed]
 : anaesthesia procedure;
: anaesthesia procedure;  : ischemic postconditioning (5 min ischemia, 5 min reperfusion/3 cycles); ♦: euthanasia; ACD: allergic contact dermatitis; RIPsC: remote ischemic postconditioning; K/X: ketamine/xylazine. * p < 0.0001 compared with control group; # p < 0.0001 compared with ACD group. Two-way analysis of variance, post hoc Bonferroni test. Data are expressed as mean ± SD (n = 16 ears, n = 8 mice).
: ischemic postconditioning (5 min ischemia, 5 min reperfusion/3 cycles); ♦: euthanasia; ACD: allergic contact dermatitis; RIPsC: remote ischemic postconditioning; K/X: ketamine/xylazine. * p < 0.0001 compared with control group; # p < 0.0001 compared with ACD group. Two-way analysis of variance, post hoc Bonferroni test. Data are expressed as mean ± SD (n = 16 ears, n = 8 mice).
 : anaesthesia procedure;
: anaesthesia procedure;  : ischemic postconditioning (5 min ischemia, 5 min reperfusion/3 cycles); ♦: euthanasia; ACD: allergic contact dermatitis; RIPsC: remote ischemic postconditioning; K/X: ketamine/xylazine. * p < 0.0001 compared with control group; # p < 0.0001 compared with ACD group. Two-way analysis of variance, post hoc Bonferroni test. Data are expressed as mean ± SD (n = 16 ears, n = 8 mice).
: ischemic postconditioning (5 min ischemia, 5 min reperfusion/3 cycles); ♦: euthanasia; ACD: allergic contact dermatitis; RIPsC: remote ischemic postconditioning; K/X: ketamine/xylazine. * p < 0.0001 compared with control group; # p < 0.0001 compared with ACD group. Two-way analysis of variance, post hoc Bonferroni test. Data are expressed as mean ± SD (n = 16 ears, n = 8 mice).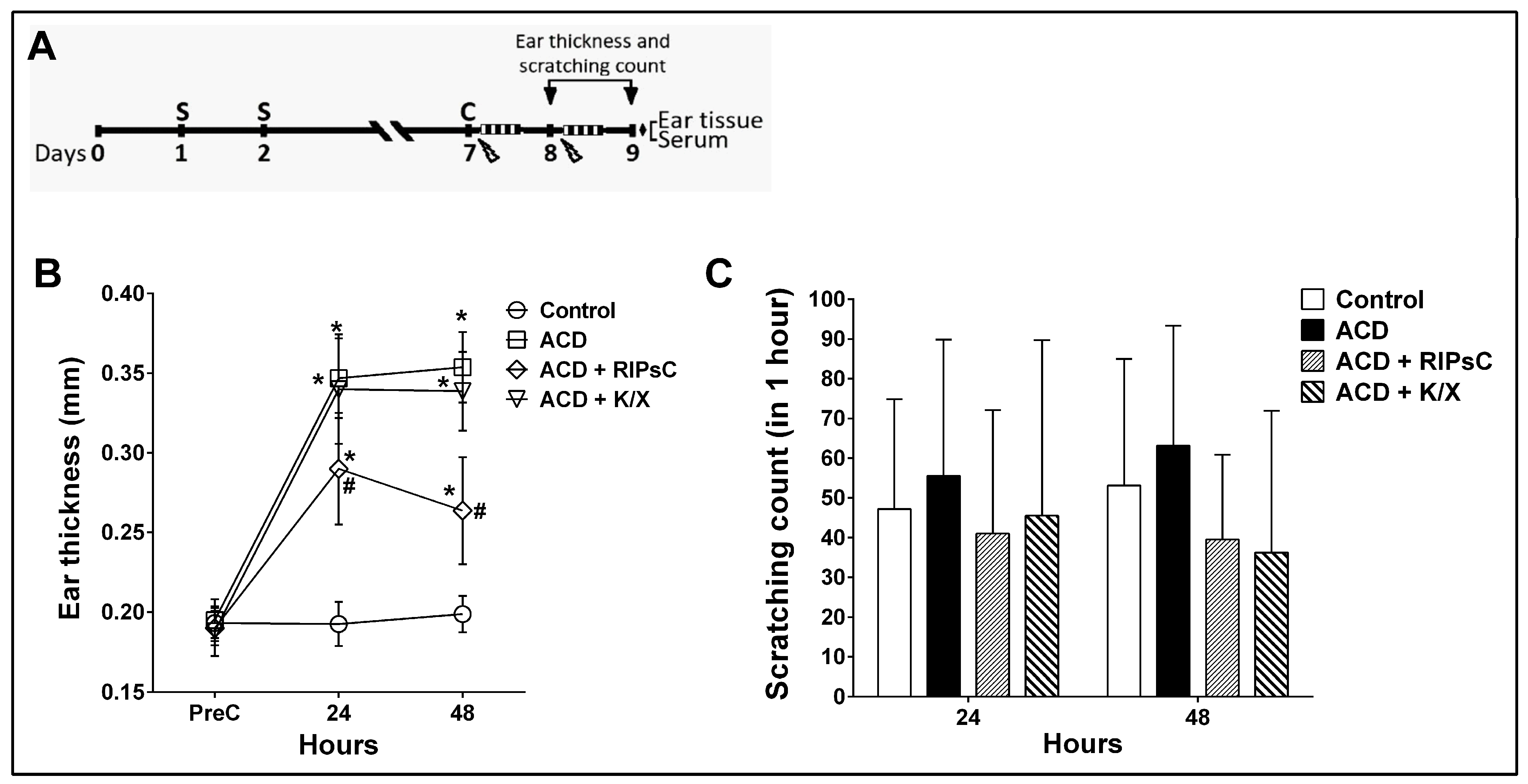
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunduz, O.; Sapmaz-Metin, M.; Topuz, R.D.; Kaya, O.; Karadag, C.H.; Ulugol, A. Anti-Inflammatory and Antipruritic Effects of Remote Ischaemic Postconditioning in a Mouse Model of Experimental Allergic Contact Dermatitis. Medicina 2023, 59, 1816. https://doi.org/10.3390/medicina59101816
Gunduz O, Sapmaz-Metin M, Topuz RD, Kaya O, Karadag CH, Ulugol A. Anti-Inflammatory and Antipruritic Effects of Remote Ischaemic Postconditioning in a Mouse Model of Experimental Allergic Contact Dermatitis. Medicina. 2023; 59(10):1816. https://doi.org/10.3390/medicina59101816
Chicago/Turabian StyleGunduz, Ozgur, Melike Sapmaz-Metin, Ruhan Deniz Topuz, Oktay Kaya, Cetin Hakan Karadag, and Ahmet Ulugol. 2023. "Anti-Inflammatory and Antipruritic Effects of Remote Ischaemic Postconditioning in a Mouse Model of Experimental Allergic Contact Dermatitis" Medicina 59, no. 10: 1816. https://doi.org/10.3390/medicina59101816
APA StyleGunduz, O., Sapmaz-Metin, M., Topuz, R. D., Kaya, O., Karadag, C. H., & Ulugol, A. (2023). Anti-Inflammatory and Antipruritic Effects of Remote Ischaemic Postconditioning in a Mouse Model of Experimental Allergic Contact Dermatitis. Medicina, 59(10), 1816. https://doi.org/10.3390/medicina59101816





