Prognostic Impact of Atrial Fibrillation in Patients with Heavily Calcified Coronary Artery Disease Receiving Rotational Atherectomy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. In-Hospital Events and Procedural Outcomes
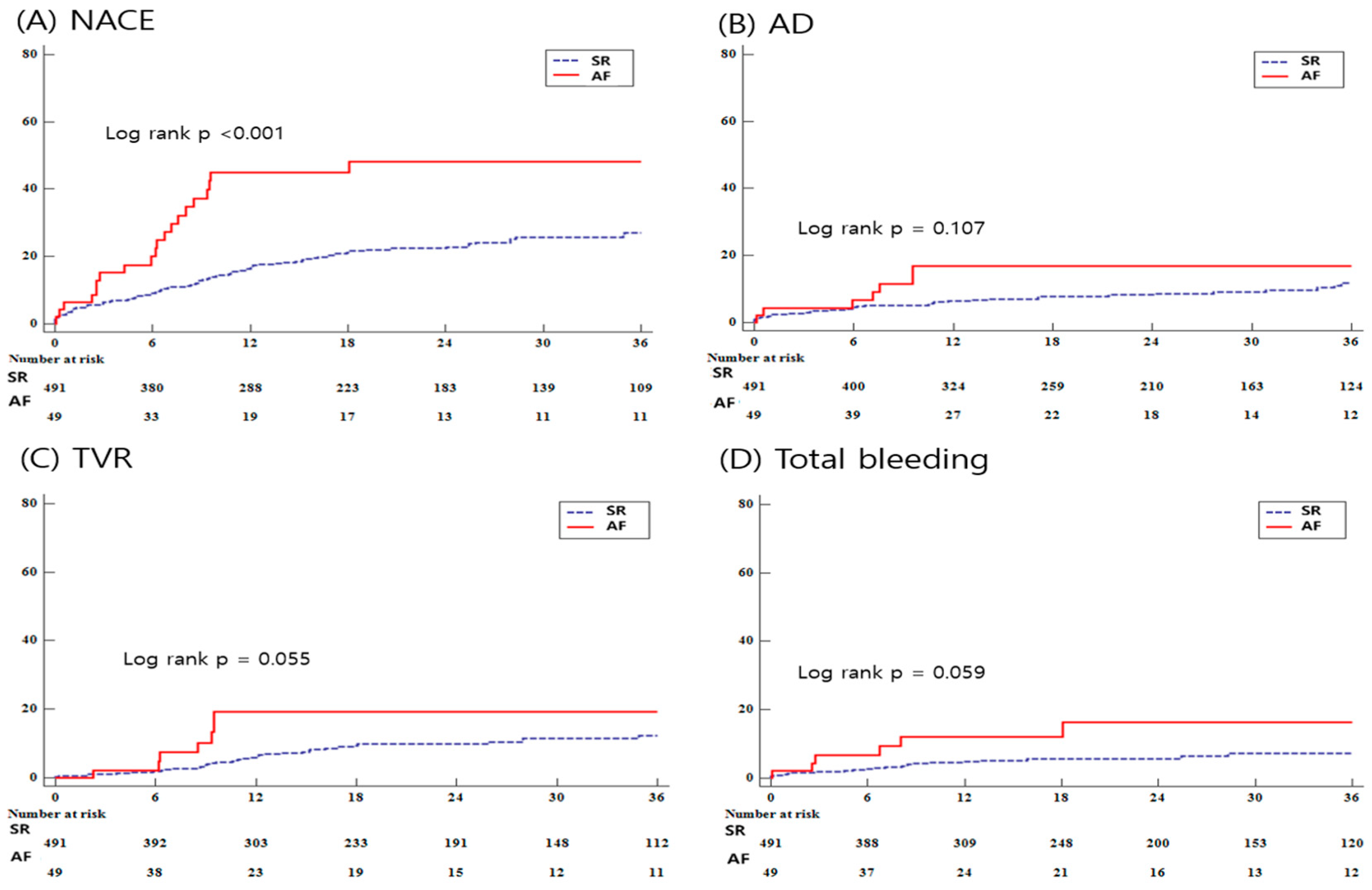
3.3. Clinical Outcomes
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Pizzetti, F.; Turazza, F.M.; Franzosi, M.G.; Barlera, S.; Ledda, A.; Maggioni, A.P.; Santoro, L.; Tognoni, G. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: The GISSI-3 data. Heart 2001, 86, 527–532. [Google Scholar] [PubMed]
- Crenshaw, B.S.; Ward, S.R.; Granger, C.B.; Stebbins, A.L.; Topol, E.J.; Califf, R.M. Atrial fibrillation in the setting of acute myocardial infarction: The GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J. Am. Coll. Cardiol. 1997, 30, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Lehto, M.; Snapinn, S.; Dickstein, K.; Swedberg, K.; Nieminen, M.S.; Optimaal Investigators. Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: The OPTIMAAL experience. Eur. Heart J. 2005, 26, 350–356. [Google Scholar] [CrossRef]
- Bauersachs, R.; Zeymer, U.; Briere, J.B.; Marre, C.; Bowrin, K.; Huelsebeck, M. Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc. Ther. 2019, 2019, 8295054. [Google Scholar] [CrossRef]
- Weijs, B.; Pisters, R.; Haest, R.J.; Kragten, J.A.; Joosen, I.A.; Versteylen, M.; Timmermans, C.C.; Pison, L.; Blaauw, Y.; Hofstra, L.; et al. Patients originally diagnosed with idiopathic atrial fibrillation more often suffer from insidious coronary artery disease compared to healthy sinus rhythm controls. Heart Rhythm. 2012, 9, 1923–1929. [Google Scholar] [CrossRef]
- Nucifora, G.; Schuijf, J.D.; Tops, L.F.; van Werkhoven, J.M.; Kajander, S.; Jukema, J.W.; Schreur, J.H.; Heijenbrok, M.W.; Trines, S.A.; Gaemperli, O.; et al. Prevalence of coronary artery disease assessed by multislice computed tomography coronary angiography in patients with paroxysmal or persistent atrial fibrillation. Circ. Cardiovasc. Imaging 2009, 2, 100–106. [Google Scholar] [CrossRef]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease—Double trouble. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef]
- Potpara, T.S.; Mujovic, N.; Proietti, M.; Dagres, N.; Hindricks, G.; Collet, J.-P.; Valgimigli, M.; Heidbuchel, H.; Lip, G.Y.H. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: Meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. Europace 2020, 22, 33–46. [Google Scholar]
- Al-Khatib, S.M.; Pieper, K.S.; Lee, K.L.; Mahaffey, K.W.; Hochman, J.S.; Pepine, C.J.; Kopecky, S.L.; Akkerhuis, M.; Stepinska, J.; Simoons, M.L.; et al. Atrial fibrillation and mortality among patients with acute coronary syndromes without ST-segment elevation: Results from the PURSUIT trial. Am. J. Cardiol. 2001, 88, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Almendro-Delia, M.; Valle-Caballero, M.J.; Garcia-Rubira, J.C.; Munoz-Calero, B.; Garcia-Alcantara, A.; Reina-Toral, A.; Benítez-Parejo, J.; Hidalgo-Urbano, R.; ARIAM Andalucia Study Group. Prognostic impact of atrial fibrillation in acute coronary syndromes: Results from the ARIAM registry. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 141–148. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.D.; Huang, W.; Domakonda, K.V.; Ward, J.; Saczysnki, J.S.; Gore, J.M.; Goldberg, R.J. Trends in atrial fibrillation in patients hospitalized with an acute coronary syndrome. Am. J. Med. 2012, 125, 1076–1084. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Stiermaier, T.; Eitel, C.; Fuernau, G.; Saad, M.; Poss, J.; de Waha, S.; Mende, M.; Desch, S.; Metzler, B.; et al. Impact of Atrial Fibrillation During ST-Segment-Elevation Myocardial Infarction on Infarct Characteristics and Prognosis. Circ. Cardiovasc. Imaging 2018, 11, e006955. [Google Scholar] [CrossRef] [PubMed]
- Sutton, N.R.; Seth, M.; Ruwende, C.; Gurm, H.S. Outcomes of Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2016, 68, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, T.; Kalesan, B.; Zanchin, T.; Pulver, C.; Jung, S.; Mattle, H.; Carrel, T.; Moschovitis, A.; Stortecky, S.; Wenaweser, P.; et al. Impact of atrial fibrillation on clinical outcomes among patients with coronary artery disease undergoing revascularisation with drug-eluting stents. EuroIntervention 2013, 8, 1061–1071. [Google Scholar] [CrossRef]
- Lee, K.; Jung, J.H.; Lee, M.; Kim, D.W.; Park, M.W.; Choi, I.J.; Lee, J.-H.; Lee, J.-H.; Lee, S.-R.; Lee, P.-H.; et al. Clinical Outcome of Rotational Atherectomy in Calcified Lesions in Korea-ROCK Registry. Medicina 2021, 57, 694. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2016, 152, 1243–1275. [Google Scholar] [CrossRef]
- Mintz, G.S.; Popma, J.J.; Pichard, A.D.; Kent, K.M.; Satler, L.F.; Chuang, Y.C.; Ditrano, C.J.; Leon, M.B. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation 1995, 91, 1959–1965. [Google Scholar] [CrossRef]
- Dewland, T.A.; Olgin, J.E.; Vittinghoff, E.; Marcus, G.M. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 2013, 128, 2470–2477. [Google Scholar] [CrossRef] [PubMed]
- Marte, T.; Saely, C.H.; Schmid, F.; Koch, L.; Drexel, H. Effectiveness of atrial fibrillation as an independent predictor of death and coronary events in patients having coronary angiography. Am. J. Cardiol. 2009, 103, 36–40. [Google Scholar] [CrossRef]
- Choi, H.I.; Ahn, J.M.; Kang, S.H.; Lee, P.H.; Kang, S.J.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W.; Park, S.-W.; Park, D.-W.; et al. Prevalence, Management, and Long-Term (6-Year) Outcomes of Atrial Fibrillation among Patients Receiving Drug-Eluting Coronary Stents. JACC Cardiovasc. Interv. 2017, 10, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Miyashita, Y.; Motoki, H.; Kobayashi, H.; Kobayashi, M.; Nakajima, H.; Kimura, H.; Akanuma, H.; Mawatari, E.; Sato, T.; et al. Comparison of mid-term outcomes between patients with and without atrial fibrillation undergoing coronary stenting in the second-generation drug-eluting stent era: From the SHINANO registry. Cardiovasc. Interv. Ther. 2017, 32, 206–215. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Efird, J.T.; Dawood, F.Z.; Yeboah, J.; Alonso, A.; Heckbert, S.R.; Soliman, E.Z. Coronary artery calcium and risk of atrial fibrillation (from the multi-ethnic study of atherosclerosis). Am. J. Cardiol. 2014, 114, 1707–1712. [Google Scholar] [CrossRef]
- Pan, N.H.; Tsao, H.M.; Chang, N.C.; Lee, C.M.; Chen, Y.J.; Chen, S.A. Dilated left atrium and pulmonary veins in patients with calcified coronary artery: A potential contributor to the genesis of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009, 20, 153–158. [Google Scholar] [CrossRef]
- Barbato, E.; Carrie, D.; Dardas, P.; Fajadet, J.; Gaul, G.; Haude, M.; Khashaba, A.; Koch, K.; Meyer-Gessner, M.; Palazuelos, J.; et al. European expert consensus on rotational atherectomy. EuroIntervention 2015, 11, 30–36. [Google Scholar] [CrossRef]
- Kuriyama, N.; Kobayashi, Y.; Yamaguchi, M.; Shibata, Y. Usefulness of rotational atherectomy in preventing polymer damage of everolimus-eluting stent in calcified coronary artery. JACC Cardiovasc. Interv. 2011, 4, 588–589. [Google Scholar] [CrossRef]
- Fujii, K.; Mintz, G.S.; Kobayashi, Y.; Carlier, S.G.; Takebayashi, H.; Yasuda, T.; Moussa, I.; Dangas, G.; Mehran, R.; Lansky, A.J.; et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation 2004, 109, 1085–1088. [Google Scholar] [CrossRef]
- Genereux, P.; Madhavan, M.V.; Mintz, G.S.; Maehara, A.; Palmerini, T.; Lasalle, L.; Xu, K.; McAndrew, T.; Kirtane, A.; Lansky, A.J.; et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J. Am. Coll. Cardiol. 2014, 63, 1845–1854. [Google Scholar] [PubMed]
- Abdel-Wahab, M.; Richardt, G.; Joachim Buttner, H.; Toelg, R.; Geist, V.; Meinertz, T.; Schofer, J.; King, L.; Neumann, F.-J.; Khattab, A.A. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: The randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc. Interv. 2013, 6, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.F.; Ma, Y.L.; Li, Q.; Liu, J.; Zhao, H.; Lu, M.-Y.; Wang, W.-M. White thrombi on optical coherence tomography after rotational atherectomy of severely calcified coronary lesions. Herz 2022, 47, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J. Peri-procedural myocardial injury: 2005 update. Eur. Heart J. 2005, 26, 2493–2519. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, M.Y.; Shin, S.Y.; Lee, W.S.; Kim, S.W.; Park, S.J.; Kim, J.S.; Sung, K.-C. Relationship between Cardiovascular Calcium and Atrial Fibrillation. J. Clin. Med. 2022, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Kang, Y.; Jang, H.G.; Kim, K.; Koh, J.S.; Park, J.R.; Hwang, S.-J.; Hwang, J.-Y.; Bae, J.S.; Ahn, J.-H.; et al. Coronary artery calcium score in predicting periprocedural myocardial infarction in patients undergoing an elective percutaneous coronary intervention. Coron. Artery Dis. 2018, 29, 589–596. [Google Scholar] [CrossRef]
- Komaki, S.; Ishii, M.; Kaichi, R.; Takae, M.; Mori, T.; Toida, R.; Kurogi, K.; Matsuura, Y.; Yamamoto, N.; Tsujita, K.; et al. Relationship between coronary artery calcium score and bleeding events after percutaneous coronary intervention in chronic coronary syndrome. Heart Vessel. 2023, 38, 919–928. [Google Scholar] [CrossRef]
- Kaya, A.; Keskin, M.; Tatlisu, M.A.; Uzman, O.; Borklu, E.; Cinier, G.; Yildirim, E.; Kayapinar, O. Atrial Fibrillation: A Novel Risk Factor for No-Reflow Following Primary Percutaneous Coronary Intervention. Angiology 2020, 71, 175–182. [Google Scholar] [CrossRef]
- Mitar, M.D.; Ratner, S.; Lavi, S. Heart block and temporary pacing during rotational atherectomy. Can. J. Cardiol. 2015, 31, 335–340. [Google Scholar] [CrossRef]
- Kosmidou, I.; Redfors, B.; Dordi, R.; Dizon, J.M.; McAndrew, T.; Mehran, R.; Ben-Yehuda, O.; Mintz, G.S.; Stone, G.W. Incidence, Predictors, and Outcomes of High-Grade Atrioventricular Block in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention (from the HORIZONS-AMI Trial). Am. J. Cardiol. 2017, 119, 1295–1301. [Google Scholar] [CrossRef]
- Goette, A.; Bukowska, A.; Dobrev, D.; Pfeiffenberger, J.; Morawietz, H.; Strugala, D.; Wiswedel, I.; Röhl, F.-W.; Wolke, C.; Bergmann, S.; et al. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur. Heart J. 2009, 30, 1411–1420. [Google Scholar] [CrossRef]
- Pinho-Gomes, A.C.; Reilly, S.; Brandes, R.P.; Casadei, B. Targeting inflammation and oxidative stress in atrial fibrillation: Role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statins. Antioxid. Redox Signal. 2014, 20, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, B.J.; Copeland-Halperin, R.S.; Halperin, J.L. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: Mechanistic links and clinical inferences. J. Am. Coll. Cardiol. 2015, 65, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Raaz-Schrauder, D.; Klinghammer, L.; Baum, C.; Frank, T.; Lewczuk, P.; Achenbach, S.; Cicha, I.; Stumpf, C.; Wiltfang, J.; Kornhuber, J.; et al. Association of systemic inflammation markers with the presence and extent of coronary artery calcification. Cytokine 2012, 57, 251–257. [Google Scholar] [CrossRef]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: A collaborative analysis of three randomised trials. Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, Y.; Kawaguchi, A.; Kajiwara, I.; Ohmori, R.; Okada, K.; Saito, I.; Konishi, M.; Nakamura, M.; Sato, S.; Kokubo, Y.; et al. Prognostic value of plasma high-sensitivity C-reactive protein levels in Japanese patients with stable coronary artery disease: The Japan NCVC-Collaborative Inflammation Cohort (JNIC) Study. Atherosclerosis 2009, 207, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
| SR (n = 491) | AF (n = 49) | p-Value | |
|---|---|---|---|
| Age, years | 71.1 ± 10.2 | 75.2 ± 8.6 | 0.006 |
| Sex | 0.925 | ||
| Male | 294 (59.9) | 29 (59.2) | |
| Female | 197 (40.1) | 20 (40.8) | |
| BMI | 24.2 ± 3.9 | 24.3 ± 4.0 | 0.952 |
| Smoking | 96 (19.6) | 7 (14.3) | 0.371 |
| HTN | 375 (76.4) | 40 (81.6) | 0.405 |
| DM | 278 (56.6) | 27 (55.1) | 0.838 |
| Hyperlipidemia | 218 (44.4) | 17 (34.7) | 0.191 |
| CKD | 82 (16.7) | 14 (28.6) | 0.038 |
| Dialysis | 44 (9.0) | 5 (10.2) | 0.793 |
| Previous PCI | 126 (25.7) | 13 (26.5) | 0.895 |
| Previous CABG | 22 (4.5) | 2 (4.1) | >0.999 |
| Previous MI | 61 (12.4) | 5 (10.2) | 0.651 |
| CVA | 64 (13.0) | 11 (22.5) | 0.069 |
| Clinical diagnosis | 0.510 | ||
| Stable angina | 297 (60.5) | 32 (65.3) | |
| ACS | 194 (39.5) | 17 (34.7) | |
| LVEF, % | 53.4 ± 13.1 | 48.7 ± 15.5 | 0.018 |
| NOAC | 2 (0.4) | 14 (28.6) | <0.001 |
| DAPT | 473 (96.3) | 46 (93.9) | 0.426 |
| Beta blocker | 347 (70.7) | 33 (67.4) | 0.627 |
| ACEi/ARB | 316 (64.4) | 25 (51.0) | 0.065 |
| Statin | 457 (93.1) | 45 (91.8) | 0.767 |
| HbA1C | 6.7 ± 1.4 | 6.7 ± 1.6 | 0.973 |
| Total cholesterol | 144.0 ± 39.1 | 140.2 ± 34.3 | 0.538 |
| LDL cholesterol | 84.8 ± 40.3 | 84.0 ± 29.8 | 0.897 |
| HDL cholesterol | 46.0 ± 14.3 | 47.0 ± 16.5 | 0.660 |
| Triglyceride | 119.7 ± 75.8 | 119.7 ± 54.9 | 1.000 |
| SR (n = 491) | AF (n = 49) | p-Value | |
|---|---|---|---|
| ACC/AHA classification | 0.228 | ||
| A | 2 (0.4) | 1 (2.0) | |
| B1 | 39 (7.9) | 1 (2.0) | |
| B2 | 47 (9.6) | 5 (10.2) | |
| C | 403 (82.1) | 42 (85.7) | |
| Left main disease | 68 (13.9) | 6 (12.2) | 0.756 |
| MVD | 385 (78.4) | 39 (79.6) | 0.848 |
| IVUS | 220 (44.8) | 29 (59.2) | 0.054 |
| Arc of calcification > 270° | 83/143 (56.9) | 18/22 (81.8) | 0.026 |
| Procedure time, min | 79.5 ± 51.1 | 76.8 ± 45.9 | 0.725 |
| SR (n = 491) | AF (n = 49) | p-Value | |
|---|---|---|---|
| In-hospital events | 54 (11.0) | 6 (12.2) | 0.791 |
| In-hospital death | 10 (2.0) | 1 (2.0) | >0.999 |
| Urgent CABG/PCI | 9 (1.8) | 0 (0.0) | >0.999 |
| Periprocedural MI | 39 (7.9) | 6 (12.2) | 0.281 |
| In-hospital CVA | 1 (0.2) | 1 (2.0) | 0.173 |
| Procedural outcomes | |||
| Coronary dissection * | 69 (14.1) | 9 (18.4) | 0.413 |
| Temporary pacemaker during procedure | 11 (2.2) | 5 (10.2) | 0.010 |
| Coronary perforation | 10 (2.0) | 0 (0.0) | 0.611 |
| In-hospital bleeding | 23 (4.7) | 4 (8.2) | 0.294 |
| Procedure success | 472 (96.1) | 48 (98.0) | >0.999 |
| SR (n = 491) | AF (n = 49) | p-Value | Univariate HR (95% CI) | p-Value | Multivariate HR ** (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|
| NACE | 100 (20.4) | 21 (42.9) | <0.001 | 2.219 (1.404–3.507) | <0.001 | 1.880 (1.096–3.227) | 0.022 |
| All-cause death | 37 (7.9) | 7 (14.3) | 0.107 | 1.636 (0.737–3.633) | 0.226 | 1.396 (0.584–3.339) | 0.453 |
| Cardiac death | 28 (5.7) | 2 (4.1) | 0.712 | 0.627 (0.150–2.612) | 0.521 | 0.645 (0.150–2.784) | 0.557 |
| Myocardial infarction | 17 (3.5) | 3 (6.1) | 0.273 | 1.779 (0.524–6.042) | 0.356 | 1.335 (0.316–5.644) | 0.694 |
| Any repeat revascularization | 46 (9.4) | 8 (16.3) | 0.062 | 2.268 (1.151–4.470) | 0.018 | 1.971 (0.843–4.609) | 0.118 |
| TVR | 38 (7.7) | 7 (14.3) | 0.055 | 2.268 (1.061–4.849) | 0.035 | 2.259 (0.891–5.728) | 0.086 |
| CVA | 8 (1.6) | 2 (4.1) | 0.228 | 2.273 (0.491–10.521) | 0.294 | 0.320 (0.012–8.291) | 0.492 |
| Total bleeding | 26 (5.3) | 6 (12.2) | 0.059 | 2.125 (0.884–5.108) | 0.092 | 1.950 (0.774–5.111) | 0.175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Seo, Y.; Her, S.-H.; Lee, J.-H.; Lee, K.; Yoo, K.-D.; Moon, K.-W.; Moon, D.; Lee, S.-N.; Jang, W.-Y.; et al. Prognostic Impact of Atrial Fibrillation in Patients with Heavily Calcified Coronary Artery Disease Receiving Rotational Atherectomy. Medicina 2023, 59, 1808. https://doi.org/10.3390/medicina59101808
Jung J, Seo Y, Her S-H, Lee J-H, Lee K, Yoo K-D, Moon K-W, Moon D, Lee S-N, Jang W-Y, et al. Prognostic Impact of Atrial Fibrillation in Patients with Heavily Calcified Coronary Artery Disease Receiving Rotational Atherectomy. Medicina. 2023; 59(10):1808. https://doi.org/10.3390/medicina59101808
Chicago/Turabian StyleJung, Jin, Yeonjoo Seo, Sung-Ho Her, Jae-Hwan Lee, Kyusup Lee, Ki-Dong Yoo, Keon-Woong Moon, Donggyu Moon, Su-Nam Lee, Won-Young Jang, and et al. 2023. "Prognostic Impact of Atrial Fibrillation in Patients with Heavily Calcified Coronary Artery Disease Receiving Rotational Atherectomy" Medicina 59, no. 10: 1808. https://doi.org/10.3390/medicina59101808
APA StyleJung, J., Seo, Y., Her, S.-H., Lee, J.-H., Lee, K., Yoo, K.-D., Moon, K.-W., Moon, D., Lee, S.-N., Jang, W.-Y., Choi, I.-J., Lee, J.-H., Lee, S.-R., Lee, S.-W., Yun, K.-H., & Lee, H.-J. (2023). Prognostic Impact of Atrial Fibrillation in Patients with Heavily Calcified Coronary Artery Disease Receiving Rotational Atherectomy. Medicina, 59(10), 1808. https://doi.org/10.3390/medicina59101808





