Tapentadol Immediate Release (IR) versus Morphine Hydrochloride for Postoperative Analgesia of Patients Undergoing Total Abdominal Hysterectomy—A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schug, S.A.; Lavand’homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D.; The IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Imani, F.; Rahimzadeh, P. Gabapentinoids: Gabapentin and pregabalin for postoperative pain management. Anesth. Pain Med. 2012, 2, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Goudas, L.C. Acute pain. Lancet 1999, 353, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, P.E.; Schug, S.A.; Scott, D.A.; Visser, E.J.; Walker, S.M. Acute Pain Management: Scientific Evidence, 3rd ed.; Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine: Melbourne, Australia, 2010. Available online: http://www.nhmrc.gov.au/guidelines/publications/cp104 (accessed on 6 February 2023).
- Perkins, F.M.; Kehlet, H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000, 93, 1123–1133. [Google Scholar] [CrossRef]
- Gerbershagen, H.J.; Aduckathil, S.; van Wijck, A.J.M.; Peelen, L.M.; Kalkman, C.J.; Meissner, W. Pain intensity on the first day after surgery: A prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013, 118, 934–944. [Google Scholar] [CrossRef]
- Tighe, P.J.; Riley, J.L., 3rd; Fillingim, R.B. Sex differences in the incidence of severe pain events following surgery: A review of 333,000 pain scores. Pain Med. 2014, 15, 1390–1404. [Google Scholar] [CrossRef]
- Glare, P.; Aubrey, K.R.; Myles, P.S. Transition from acute to chronic pain after surgery. Lancet 2019, 393, 1537–1546. [Google Scholar]
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012, 116, 248–273. [Google Scholar] [CrossRef]
- Chevlen, E. Opioids: A review. Curr Pain Headache Rep. 2003, 7, 15–23. [Google Scholar] [CrossRef]
- Wheeler, M.; Oderda, G.M.; Ashburn, M.A.; Lipman, A.G. Adverse events associated with postoperative opioid analgesia: A systematic review. J. Pain 2002, 3, 159–180. [Google Scholar] [CrossRef]
- Scarth, E.; Smith, S. Drugs in Anesthesia and Intensive Care A-Z. In Drugs in Anesthesia and Intensive Care, 5th ed.; Scarth, E., Smith, S., Eds.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Tzschentke, T.M.; Christoph, T.; Kögel, B.; Schiene, K.; Hennies, H.H.; Englberger, W.; Haurand, M.; Jahnel, U.; Cremers, T.I.F.H.; Friderichs, E.; et al. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): A novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J. Pharmacol. Exp. Ther. 2007, 323, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, T.M.; Christoph, T.; Kögel, B.Y. The mu-opioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: The case of tapentadol. CNS Drugs 2014, 28, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, H.; Liu, C.H.; Alafris, A.; Cohen, H. Probable Tapentadol-Associated Serotonin Syndrome After Overdose. Hosp. Pharm. 2016, 51, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Barkin, R.L.; Barkin, S.J. Treating postoperative pain in the patient who is in recovery or remission from opioid abuse: Focus on tapentadol. J. Opioid Manag. 2017, 13, 133–134. [Google Scholar] [CrossRef]
- Daniels, S.; Casson, E.; Stegmann, J.U.; Oh, C.; Okamoto, A.; Rauschkolb, C.; Upmalis, D. A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr. Med. Res. Opin. 2009, 25, 1551–1561. [Google Scholar] [CrossRef]
- Daniels, S.E.; Upmalis, D.; Okamoto, A.; Lange, C.; Häeussler, J. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Curr. Med. Res. Opin. 2009, 25, 765–776. [Google Scholar] [CrossRef]
- Hale, M.; Upmalis, D.; Okamoto, A.; Lange, C.; Rauschkolb, C. Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: A randomized, double-blind study. Curr. Med. Res. Opin. 2009, 25, 1095–1104. [Google Scholar] [CrossRef]
- Frampton, J.E. Tapentadol immediate release. A review of its use in the treatment of moderate to severe acute pain. Drugs 2010, 70, 1719–1743. [Google Scholar] [CrossRef]
- Xiao, J.-P.; Li, A.-L.; Feng, B.-M.; Ye, Y.; Wang, G.J. Efficacy and safety of tapentadol immediate release assessment in the treatment of moderate to severe pain: A systematic review and meta-analysis. Pain Med. 2017, 18, 14–24. [Google Scholar] [CrossRef]
- Comelon, M.; Raeder, J.; Drægni, T.; Lieng, M.; Lenz, H. Tapentadol versus oxycodone analgesia and side effects after laparoscopic hysterectomy: A randomized controlled trial. Eur. J. Anaesthesiol. 2021, 38, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, D.; Szkutnik-Fiedler, D.; Bosacki, R.; Michalak, M.; Grzeskowiak, E.; Szalek, E. Analgesic efficacy and safety of tapentadol in comparison with oxycodone in patients after open abdominal hysterectomy. Acta Pol. Pharm. 2020, 77, 505–514. [Google Scholar] [CrossRef]
- Stegmann, J.U.; Weber, H.; Steup, A.; Okamoto, A.; Upmalis, D.; Daniels, S. The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery. Curr. Med. Res. Opin. 2008, 24, 3185–3196. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, R.; Lange, C.; Steup, A.; Black, P.; Goldberg, J.; Desjardins, P. Single-dose analgesic efficacy of tapentadol in postsurgical dental pain: The results of a randomized, double-blind, placebo-controlled study. Anesth. Analg. 2008, 107, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Mubita, W.M.; Richardson, C.; Briggs, M. Patient satisfaction with pain relief following major abdominal surgery is influenced by good communication, pain relief and empathic caring: A qualitative interview study. Br. J. Pain 2020, 14, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Biondi, D.; Xiang, J.; Benson, C.; Etropolski, M.; Moskovitz, B.; Rauschkolb, C. Tapentadol immediate release versus oxycodone immediate release for treatment of acute low back pain. Pain Physician 2013, 16, E237–E246. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Tapentadol IR (n = 50) | Morphine Hydrochloride (n = 50) | Test | p |
|---|---|---|---|---|
| Mean age (years ± SD) | 58.95 ± 10.98 | 60.48 ± 9.23 | t = 0.75 | 0.45 |
| 18–34 years n (%) | 0 (0) | 2 (4) | ||
| 35–48 years n (%) | 12 (24) | 7 (14) | ||
| 49–64 years n (%) | 19 (38) | 19 (38) | ||
| 65–79 years n (%) | 18 (36) | 19 (38) | ||
| >80 years n (%) | 1 (2) | 3 (6) | ||
| BMI (kg/m2 ± SD) | 27.34 ± 5.98 | 28.99 ± 7.04 | t = −1.26 | 0.21 |
| Smoking—yes n (%) | 19 (38) | 16 (32) | χ2 = 0.65 | 0.53 |
| Kinetosis—yes n (%) | 2 (4) | 0 (0) | χ2 = 0.59 | 0.56 |
| ASA I n (%) | 4 (8) | 1 (2) | ||
| ASA II n (%) | 37 (74) | 35 (70) | ||
| ASA III n (%) | 9 (18) | 14 (28) | ||
| Duration of surgery (min ± SD) | 120.00 ± 49.59 | 138.30 ± 48.69 | t = 1.92 | 0.07 |
| Parameters | Tapentadol IR | Morphine Hydrochloride | t | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | |||
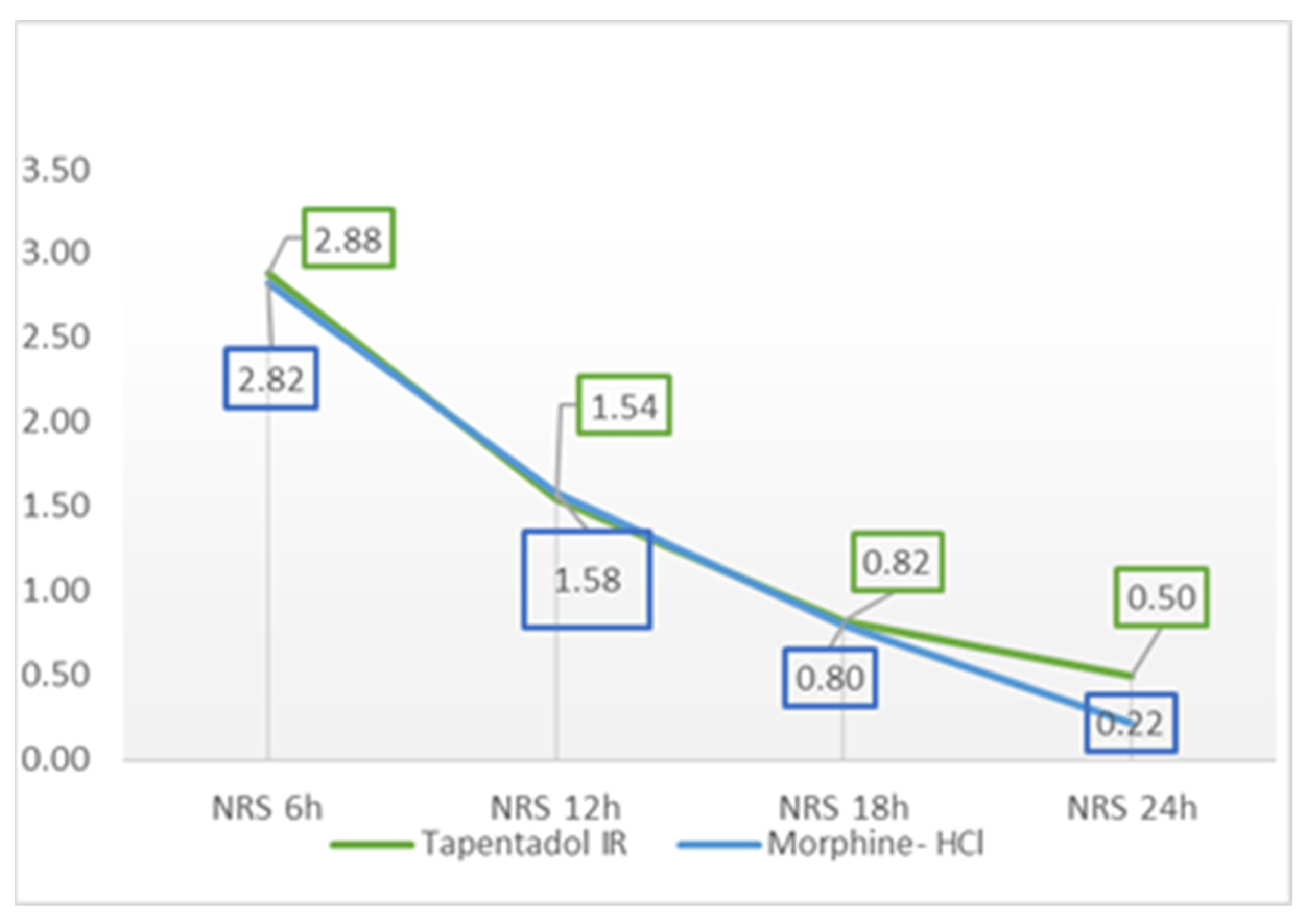
| NRS 6 h | 2.88 | 1.94 | 0.00 | 7.00 | 2.82 | 1.38 | 0.00 | 7.00 | 0.18 | 0.86 |
| NRS 12 h | 1.54 | 1.54 | 0.00 | 6.00 | 1.58 | 0.99 | 0.00 | 4.00 | 0.16 | 0.88 |
| NRS 18 h | 0.82 | 1.18 | 0.00 | 4.00 | 0.80 | 0.98 | 0.00 | 4.00 | 0.09 | 0.93 |
| NRS 24 h | 0.50 | 0.97 | 0.00 | 4.00 | 0.22 | 0.50 | 0.00 | 2.00 | 1.81 | 0.07 |
| Parameters | Tapentadol IR | Morphine Hydrochloride | t | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | |||
| NRS 6 h | 4.30 | 2.14 | 0.00 | 9.00 | 3.74 | 1.32 | 1.00 | 8.00 | 1.58 | 0.12 |
| NRS 12 h | 2.90 | 1.82 | 0.00 | 6.00 | 2.42 | 0.92 | 1.00 | 5.00 | 1.67 | 0.10 |
| NRS 18 h | 2.08 | 1.57 | 0.00 | 6.00 | 1.56 | 1.07 | 0.00 | 5.00 | 1.94 | 0.06 |
| NRS 24 h | 1.60 | 1.59 | 0.00 | 7.00 | 0.56 | 0.70 | 0.00 | 2.00 | 4.23 | <0.01 |
| Parameters | Group | χ2 | p | |||
|---|---|---|---|---|---|---|
| Tapentadol IR | Morphine Hydrochloride | |||||
| Contentment with analgesia | 2—not that good | n | 2 | 7 | ||
| % | 4 | 14 | ||||
| 3—good | n | 35 | 19 | 10.79 | 0.005 | |
| % | 70 | 38 | ||||
| 4—very good | n | 13 | 24 | |||
| % | 26 | 48 | ||||
| Total | n | 50 | 50 | |||
| % | 100 | 100 | ||||
| Parameter | Tapentadol IR | Morphine Hydrochloride | Test | p |
|---|---|---|---|---|
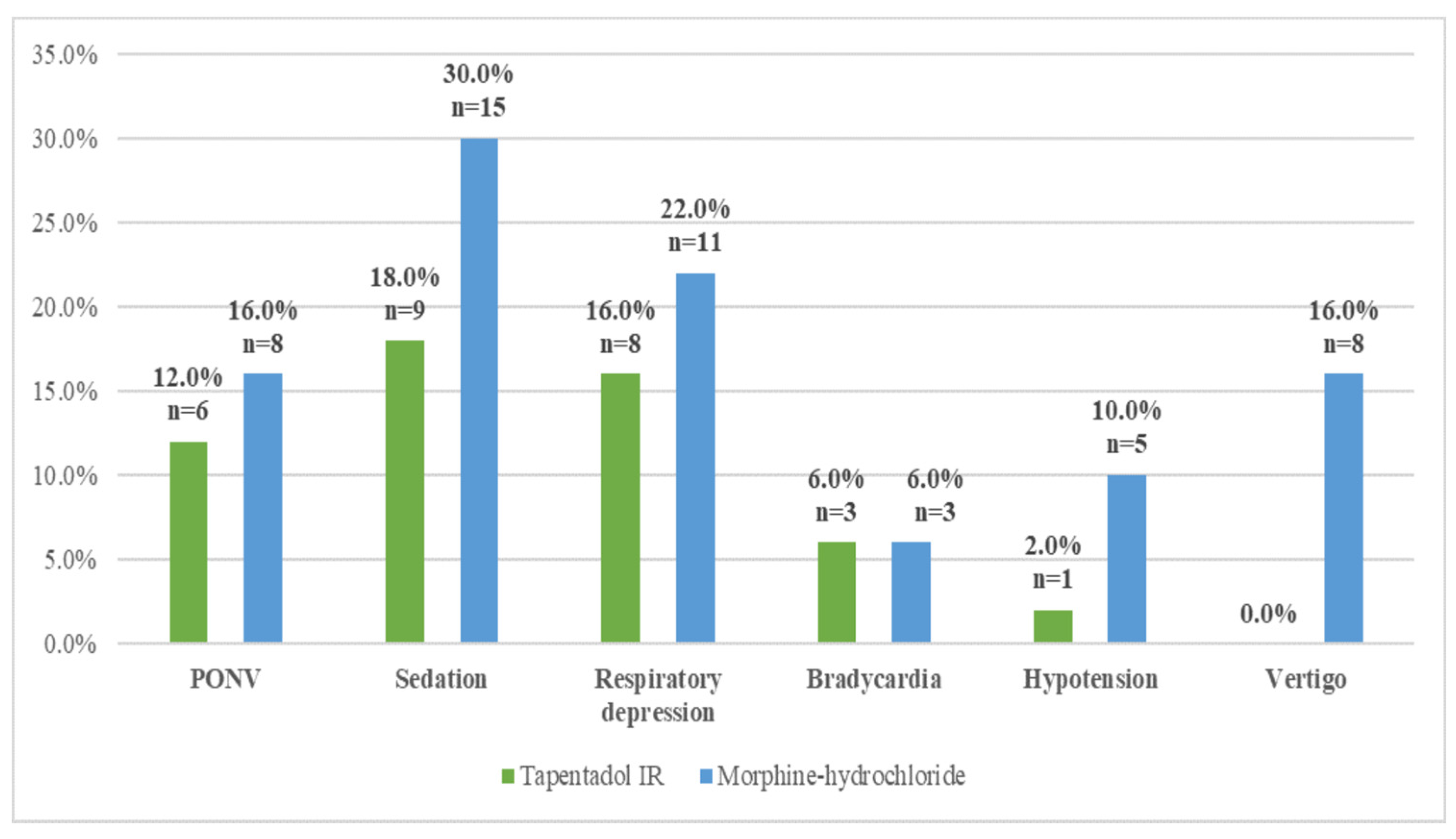
| Opioid-related side effects—yes n (%) | 17 (34%) | 23 (46%) | χ2 = 1.50 | 0.22 |
| Contentment with analgesia | 3.22 ± 0.51 | 3.34 ± 0.71 | t = −0.90 | 0.37 |
| Pain grade at rest | 1.44 ± 1.11 | 1.36 ± 0.73 | t = 0.41 | 0.69 |
| Pain grade on exertion | 2.72 ± 1.43 | 2.07 ± 0.80 | t = 2.85 | <0.01 |
| Total pain grade | 2.08 ± 1.19 | 1.71 ± 0.75 | t = 1.82 | 0.08 |
| Doses of medication | 5.22 ± 0.68 (min 4 to max 6 tablets) 261 ± 33.94 (min 200 mg to max 300 mg) | 20.20 ± 4.60 (min 10 to max 32.5 mg) |
| Contentment with Analgesia—All (n = 100) | Contentment with Analgesia— Morphine Hydrochloride (n = 50) | Contentment with Analgesia—Tapentadol IR (n = 50) | ||
|---|---|---|---|---|
| Contentment with analgesia/NRS | Pearson Correlation | 1 | 1 | 1 |
| p | ||||
| NRS_rest_6h | Pearson Correlation | −0.560 | −0.679 | −0.511 |
| p | 0.000 | 0.000 | 0.000 | |
| NRS_rest_12h | Pearson Correlation | −0.438 | −0.684 | −0.286 |
| p | 0.000 | 0.000 | 0.044 | |
| NRS_rest_18h | Pearson Correlation | −0.473 | −0.535 | −0.441 |
| p | 0.000 | 0.000 | 0.001 | |
| NRS_rest_24h | Pearson Correlation | −0.250 | −0.154 | −0.351 |
| p | 0.012 | 0.286 | 0.012 | |
| NRS_exertion_6h | Pearson Correlation | −0.476 | −0.680 | −0.362 |
| p | 0.000 | 0.000 | 0.010 | |
| NRS_exertion_12h | Pearson Correlation | −0.418 | −0.679 | −0.307 |
| p | 0.000 | 0.000 | 0.030 | |
| NRS_exertion_18h | Pearson Correlation | −0.452 | −0.571 | −0.380 |
| p | 0.000 | 0.000 | 0.006 | |
| NRS_exertion_24h | Pearson Correlation | −0.345 | −0.425 | −0.370 |
| p | 0.000 | 0.002 | 0.008 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starčević, S.; Radovanović, D.; Škorić-Jokić, S.; Bojanić-Popovicki, M.; El Farra, S.; Mihalek, N.; Golijanin, D.; Dugandžija, T.; Tomas Petrović, A. Tapentadol Immediate Release (IR) versus Morphine Hydrochloride for Postoperative Analgesia of Patients Undergoing Total Abdominal Hysterectomy—A Prospective Cohort Study. Medicina 2023, 59, 1800. https://doi.org/10.3390/medicina59101800
Starčević S, Radovanović D, Škorić-Jokić S, Bojanić-Popovicki M, El Farra S, Mihalek N, Golijanin D, Dugandžija T, Tomas Petrović A. Tapentadol Immediate Release (IR) versus Morphine Hydrochloride for Postoperative Analgesia of Patients Undergoing Total Abdominal Hysterectomy—A Prospective Cohort Study. Medicina. 2023; 59(10):1800. https://doi.org/10.3390/medicina59101800
Chicago/Turabian StyleStarčević, Sanja, Dragana Radovanović, Svetlana Škorić-Jokić, Milica Bojanić-Popovicki, Suzana El Farra, Nora Mihalek, Danica Golijanin, Tihomir Dugandžija, and Ana Tomas Petrović. 2023. "Tapentadol Immediate Release (IR) versus Morphine Hydrochloride for Postoperative Analgesia of Patients Undergoing Total Abdominal Hysterectomy—A Prospective Cohort Study" Medicina 59, no. 10: 1800. https://doi.org/10.3390/medicina59101800
APA StyleStarčević, S., Radovanović, D., Škorić-Jokić, S., Bojanić-Popovicki, M., El Farra, S., Mihalek, N., Golijanin, D., Dugandžija, T., & Tomas Petrović, A. (2023). Tapentadol Immediate Release (IR) versus Morphine Hydrochloride for Postoperative Analgesia of Patients Undergoing Total Abdominal Hysterectomy—A Prospective Cohort Study. Medicina, 59(10), 1800. https://doi.org/10.3390/medicina59101800





