Intervention Effects of Physical Activity on Type 2 Diabetic Patients Potentially Infected with COVID-19
Abstract
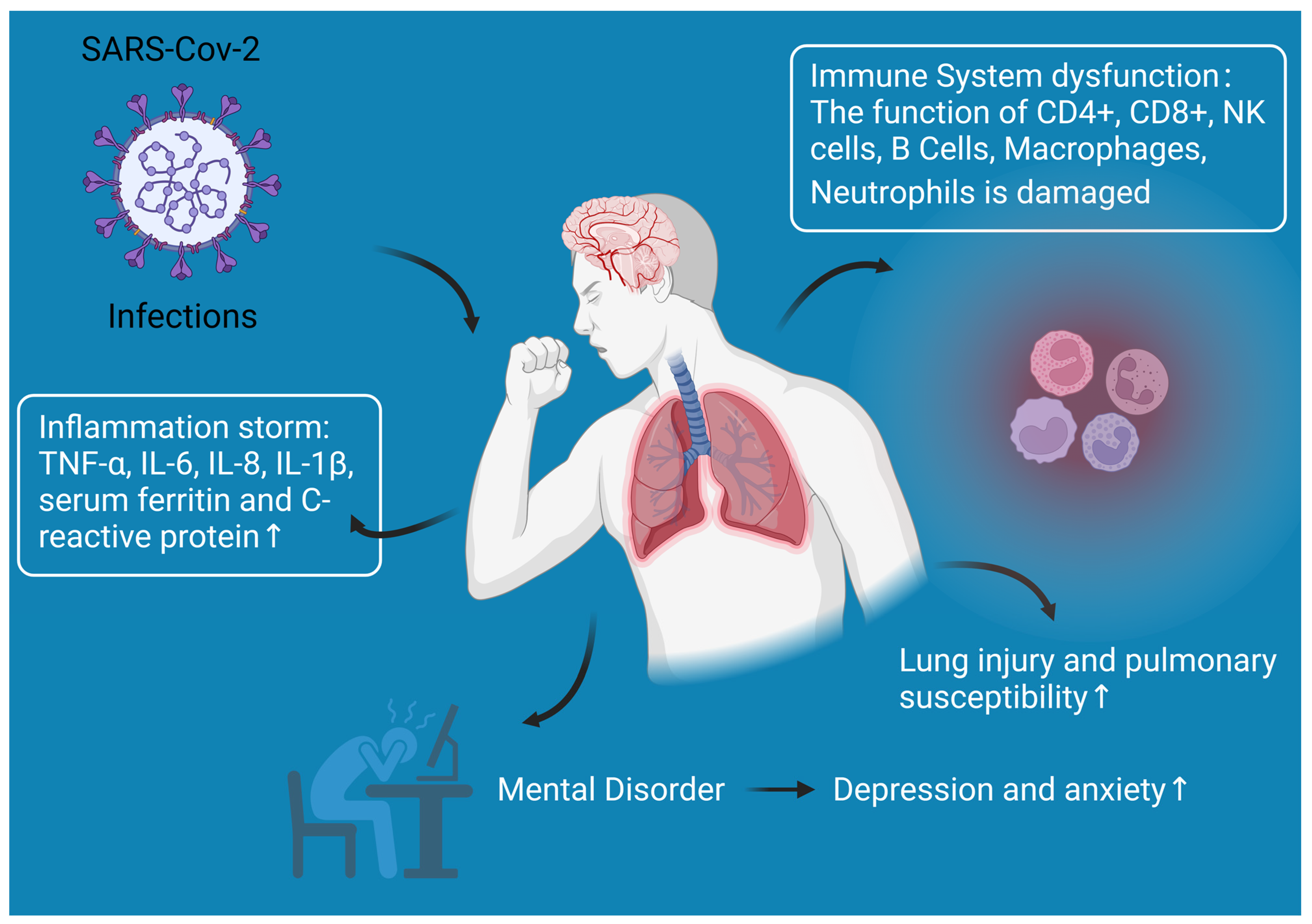
:1. Introduction
2. The Relationship between COVID-19 Infection and Diabetes
3. Reasons for the Increased Diabetic Susceptibility to COVID-19 Infection
3.1. Immune System Dysfunction
3.1.1. Abnormal T Cell Homeostasis
3.1.2. Dysregulation of Natural Killer (NK) Cells
3.1.3. Impaired B-Cell Function
3.1.4. Inhibition of Macrophage Phagocytosis
3.1.5. Dysfunction of Neutrophils
3.2. Lung Injury and Pulmonary Susceptibility
3.3. Inflammation Storm
3.4. Other Responses
4. Physical Activity Improves the Resistance of Diabetics to COVID-19 Infection
4.1. High-Intensity Training
4.2. Moderate-Intensity Exercise
4.3. Low-Intensity Exercise
4.4. Exercise Recommendations for Patients Infected with COVID-19
5. Limitations and Future Trends
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fischer, W.; Giorgi, E.E.; Chakraborty, S.; Nguyen, K.; Bhattacharya, T.; Theiler, J.; Goloboff, P.A.; Yoon, H.; Abfalterer, W.; Foley, B.T.; et al. HIV-1 and SARS-CoV-2: Patterns in the evolution of two pandemic pathogens. Cell Host Microbe 2021, 29, 1093–1110. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Bundgaard, J.S.; Raaschou-Pedersen, D.E.T.; von Buchwald, C.; Todsen, T.; Norsk, J.B.; Pries-Heje, M.M.; Vissing, C.R.; Nielsen, P.B.; Winslow, U.C.; et al. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers a Randomized Controlled Trial. Ann. Intern. Med. 2021, 174, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, X.; Li, Y.Q.; Cheng, L.; Zhou, X.D.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia A systematic review, meta analysis, and meta-regression. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 395–403. [Google Scholar] [CrossRef]
- Umakanthan, S.; Senthil, S.; John, S.; Madhavan, M.K.; Das, J.; Patil, S.; Rameshwaram, R.; Cintham, A.; Subramaniam, V.; Yogi, M.; et al. The Effect of Statins on Clinical Outcome Among Hospitalized Patients with COVID-19: A Multi-Centric Cohort Study. Front. Pharmacol. 2022, 13, 742273. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.W.; Lv, W.S.; Wang, Y.G. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes-Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Dantzer, C.; Swendsen, J.; Maurice-Tison, S.; Salamon, R. Anxiety and depression in juvenile diabetes: A critical review. Clin. Psychol. Rev. 2003, 23, 787–800. [Google Scholar] [CrossRef]
- Corona, G.; Pizzocaro, A.; Vena, W.; Rastrelli, G.; Semeraro, F.; Isidori, A.M.; Pivonello, R.; Salonia, A.; Sforza, A.; Maggi, M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 275–296. [Google Scholar] [CrossRef]
- Story, M.J. Essential sufficiency of zinc, omega-3 polyunsaturated fatty acids, vitamin D and magnesium for prevention and treatment of COVID-19, diabetes, cardiovascular diseases, lung diseases and cancer. Biochimie 2021, 187, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Scartoni, F.R.; Sant’Ana, L.D.; Murillo-Rodriguez, E.; Yamamoto, T.; Imperatori, C.; Budde, H.; Vianna, J.M.; Machado, S. Physical Exercise and Immune System in the Elderly: Implications and Importance in COVID-19 Pandemic Period. Front. Psychol. 2020, 11, 593903. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Kruger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Golbidi, S.; Badran, M.; Laher, I. Antioxidant and Anti-Inflammatory Effects of Exercise in Diabetic Patients. Exp. Diabetes Res. 2011, 2012, 941868. [Google Scholar] [CrossRef]
- Salmon, P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clin. Psychol. Rev. 2001, 21, 33–61. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065. [Google Scholar] [CrossRef]
- Laddu, R.; Lavie, C.J.; Phillips, S.A.; Arena, R. Physical activity for immunity protection: Inoculating populations with healthy living medicine in preparation for the next pandemic. Prog. Cardiovasc. Dis. 2021, 64, 102–104. [Google Scholar] [CrossRef]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of Acute and Chronic Exercise on Immunological Parameters in the Elderly Aged: Can Physical Activity Counteract the Effects of Aging? Front. Immunol. 2018, 9, 2187. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, J.; Xu, Z.; Zhang, D.J.; Xia, P.P.; Ling, J.T.; Tang, X.Y.; Liu, X.; Xuan, R.; Zhang, M.Y.; et al. Regulation of NcRNA-protein binding in diabetic foot. Biomed. Pharmacother. 2023, 160, 114361. [Google Scholar] [CrossRef]
- Lukmanto, R.B.; Suharjito; Nugroho, A.; Akbar, H. Early Detection of Diabetes Mellitus using Feature Selection and Fuzzy Support Vector Machine. In Proceedings of the 4th International Conference on Computer Science and Computational Intelligence (ICCSCI)—Enabling Collaboration to Escalate Impact of Research Results for Society, Yogyakarta, Indonesia, 12–13 September 2019. [Google Scholar]
- Márquez-Valadez, B.; Valle-Bautista, R.; García-López, G.; Díaz, N.F.; Molina-Hernández, A. Maternal Diabetes and Fetal Programming Toward Neurological Diseases: Beyond Neural Tube Defects. Front. Endocrinol. 2018, 9, 664. [Google Scholar] [CrossRef]
- Wu, Y.F.; Lan, H.X.; Zhang, D.J.; Hu, Z.Y.; Zhang, J.; Li, Z.W.; Xia, P.P.; Tang, X.Y.; Cai, X.; Yu, P. Research progress on ncRNAs regulation of mitochondrial dynamics in diabetes. J. Cell. Physiol. 2022, 237, 4112–4131. [Google Scholar] [CrossRef] [PubMed]
- Allard, R.; Leclerc, P.; Tremblay, C.; Tannenbaum, T.N. Diabetes and the Severity of Pandemic Influenza A (H1N1) Infection. Diabetes Care 2010, 33, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.N.; Li, M.Y.; Dong, Y.L.; Zhou, H.F.; Zhang, Z.L.; Tian, C.X.; Qin, R.J.; Wang, H.J.; Shen, Y.; Du, K.Y.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes-Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, R.; Misra, A. Diabetes and COVID19: A bidirectional relationship. Nutr. Diabetes 2021, 11, 21. [Google Scholar] [CrossRef]
- Framke, E.; Sorensen, J.K.; Andersen, P.K.; Svane-Petersen, A.C.; Alexanderson, K.; Bonde, J.P.; Farrants, K.; Flachs, E.M.; Hanson, L.L.M.; Nyberg, S.T.; et al. Contribution of income and job strain to the association between education and cardiovascular disease in 1.6 million Danish employees. Eur. Hear. J. 2020, 41, 1164–1178. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef]
- Sun, B.; Huang, S.Q.; Zhou, J.C. Perspectives of Antidiabetic Drugs in Diabetes with Coronavirus Infections. Front. Pharmacol. 2021, 11, 592439. [Google Scholar] [CrossRef]
- Feng, Z.J.; Li, Q.; Zhang, Y.P.; Wu, Z.Y.; Dong, X.P.; Ma, H.L.; Yin, D.P.; Lyu, K.; Wang, D.Y.; Zhou, L.; et al. Vital Surveillances: The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020. China CDC Wkly. 2020, 2, 113–122. [Google Scholar]
- Yang, X.B.; Yu, Y.; Xu, J.Q.; Shu, H.Q.; Xia, J.A.; Liu, H.; Wu, Y.R.; Zhang, L.; Yu, Z.; Fang, M.H.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Guan, W.-j.; Ni, Z.-y.; Hu, Y.; Liang, W.-h.; Ou, C.-q.; He, J.-x.; Liu, L.; Shan, H.; Lei, C.-l.; Hui, D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. J. Am. Med. Assoc. 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, R.; Misra, A. Comorbidities in COVID-19: Outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Shen, M.; Zhang, J.; Liu, B.; Dai, M.; Chen, L.; Han, D.; Fan, Y.; Zeng, Y.J.M. Comorbid diabetes mellitus was associated with poorer prognosis in patients with COVID-19: A retrospective cohort study. medRxiv 2020. [Google Scholar] [CrossRef]
- Roncon, L.; Zuin, M.; Rigatelli, G.; Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020, 127, 104354. [Google Scholar] [CrossRef]
- Abdi, A.; Jalilian, M.; Sarbarzeh, P.A.; Vlaisavljevic, Z. Diabetes and COVID-19: A systematic review on the current evidences. Diabetes Res. Clin. Pract. 2020, 166, 108347. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar]
- Frydrych, L.; Bian, G.W.; O’Lone, D.E.; Ward, P.A.; Delano, M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018, 104, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, D.P.; Dai, Z.J.; Li, X.K. Association Between Systemic Immune-Inflammation Index and Diabetic Depression. Clin. Interv. Aging 2021, 16, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, L.R.; Stephens, A.C.; Hurley, K.E.; Richardson, A.R. Lack of nutritional immunity in diabetic skin infections promotes Staphylococcus aureus virulence. Sci. Adv. 2020, 6, eabc5569. [Google Scholar] [CrossRef]
- Prentice, B.J.; Jaffe, A.; Hameed, S.; Verge, C.F.; Waters, S.; Widger, J. Cystic fibrosis-related diabetes and lung disease: An update. Eur. Respir. Rev. 2021, 30, 200293. [Google Scholar] [CrossRef]
- Dryden, M.; Baguneid, M.; Eckmann, C.; Corman, S.; Stephens, J.; Solem, C.; Li, J.; Charbonneau, C.; Baillon-Plot, N.; Haider, S. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: Focus on skin and soft-tissue infections. Clin. Microbiol. Infect. 2015, 21, S27–S32. [Google Scholar] [CrossRef]
- O’Toole, P.; Maltenfort, M.G.; Chen, A.F.; Parvizi, J. Projected Increase in Periprosthetic Joint Infections Secondary to Rise in Diabetes and Obesity. J. Arthroplast. 2016, 31, 7–10. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Navand, A.H.; Soltani, S.; Moghadami, M.; Hosseini, P.; Nasimzadeh, S.; Zandi, M. Diabetes and coronavirus infections (SARS-CoV, MERS-CoV, and SARS-CoV-2). J. Acute Dis. 2020, 9, 244–247. [Google Scholar]
- Zhao, R.X.; Sun, Y.J.; Zhang, Y.Y.; Wang, W.L.; Wang, S.Y.; Wang, C.; Liu, J.B.; Gao, L.; Hu, Z.; Fei, J.C.; et al. Distinguishable Immunologic Characteristics of COVID-19 Patients with Comorbid Type 2 Diabetes Compared with Nondiabetic Individuals. Mediat. Inflamm. 2020, 2020, 6914878. [Google Scholar] [CrossRef]
- Helmi, N.; Alammari, D.; Mobashir, M. Role of Potential COVID-19 Immune System Associated Genes and the Potential Pathways Linkage with Type-2 Diabetes. Comb. Chem. High Throughput Screen 2022, 25, 2452–2462. [Google Scholar] [PubMed]
- Alguwaihes, A.M.; Al-Sofiani, M.E.; Megdad, M.; Albader, S.S.; Alsari, M.H.; Alelayan, A.; Alzahrani, S.H.; Sabico, S.; Al-Daghri, N.M.; Jammah, A.A. Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: A single-centre retrospective study. Cardiovasc. Diabetol. 2020, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; D’Onofrio, N.; Sardu, C.; Scisciola, L.; Maggi, P.; Coppola, N.; Romano, C.; Messina, V.; Turriziani, F.; Siniscalchi, M.; et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes Obes. Metab. 2022, 24, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ye, S.D.; Wang, W.; Li, S.M.; Hu, Q.G. Clinical Features of COVID-19 Patients with Diabetes and Secondary Hyperglycemia. J. Diabetes Res. 2020, 2020, 3918723. [Google Scholar] [CrossRef]
- Nasr El-Din, A.; Ata, K.A.E.-S.; Abdel-Gawad, A.R.; Fahmy, N.F.J.I.; Resistance, D. Impact of high serum levels of MMP-7, MMP-9, TGF-β and PDGF macrophage activation markers on severity of COVID-19 in obese-diabetic patients. Infect. Drug Resist. 2021, 14, 4015–4025. [Google Scholar] [CrossRef]
- Bhandari, S.; Rankawat, G.; Singh, A.; Gupta, V.; Kakkar, S. Impact of glycemic control in diabetes mellitus on management of COVID-19 infection. Int. J. Diabetes Dev. Ctries. 2020, 40, 340–345. [Google Scholar] [CrossRef]
- Yan, Y.L.; Yang, Y.; Wang, F.; Ren, H.H.; Zhang, S.J.; Shi, X.L.; Yu, X.F.; Dong, K. Clinical characteristics and outcomes of patients with severe COVID-19 with diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001343. [Google Scholar] [CrossRef]
- Al-Sofiani, M.E.; Albunyan, S.; Alguwaihes, A.M.; Kalyani, R.R.; Golden, S.H.; Alfadda, A. Determinants of mental health outcomes among people with and without diabetes during the COVID-19 outbreak in the Arab Gulf Region. J. Diabetes 2021, 13, 339–352. [Google Scholar] [CrossRef]
- Ajele, W.K.; Oladejo, T.A.; Akanni, A.A.; Babalola, O.B. Spiritual intelligence, mindfulness, emotional dysregulation, depression relationship with mental well-being among persons with diabetes during COVID-19 pandemic. J. Diabetes Metab. Disord. 2021, 20, 1705–1714. [Google Scholar] [CrossRef]
- Hodgson, K.; Morris, J.; Bridson, T.; Govan, B.; Rush, C.; Ketheesan, N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015, 144, 171–185. [Google Scholar] [CrossRef]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, J.J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.K. Upregulated NLRP3 Inflammasome Activation in Patients with Type 2 Diabetes. Diabetes 2013, 62, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, X.; Zhang, D.; Ling, J.; Zhu, Z.; Chen, Y.; Yang, P.; Yang, Y.; Liu, X.; Liu, J.J. Role of NLRP3 Inflammasome in Diabetes and COVID-19 Role of NLRP3 Inflammasome in the Pathogenesis and Treatment of COVID-19 and Diabetes NLRP3 Inflammasome in Diabetes and COVID-19 Intervention. Front. Immunol. 2023, 14, 1203389. [Google Scholar]
- Davis, J.S.; Ferreira, D.; Paige, E.; Gedye, C.; Boyle, M. Infectious Complications of Biological and Small Molecule Targeted Immunomodulatory Therapies. Clin. Microbiol. Rev. 2020, 33, 1–117. [Google Scholar] [CrossRef]
- Yu, W.L.; Li, C.X.; Zhang, D.J.; Li, Z.W.; Xia, P.P.; Liu, X.; Cai, X.; Yang, P.P.; Ling, J.T.; Zhang, J.; et al. Advances in T Cells Based on Inflammation in Metabolic Diseases. Cells 2022, 11, 3554. [Google Scholar] [CrossRef]
- Nekoua, M.P.; Fachinan, R.; Atchamou, A.K.; Nouatin, O.; Amoussou-Guenou, D.; Amoussou-Guenou, M.K.; Moutairou, K.; Yessoufou, A. Modulation of immune cells and Th1/Th2 cytokines in insulin-treated type 2 diabetes mellitus. Afr. Health Sci. 2016, 16, 712–724. [Google Scholar] [CrossRef]
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef]
- Asadikaram, G.; Ram, M.; Izadi, A.; Fathollahi, M.S.; Nematollahi, M.H.; Najafipour, H.; Shahoozehi, B.; Mirhoseini, M.; Masoumi, M.; Shahrokhi, N.; et al. The study of the serum level of IL-4, TGF-beta, IFN-gamma, and IL-6 in overweight patients with and without diabetes mellitus and hypertension. J. Cell. Biochem. 2019, 120, 4147–4157. [Google Scholar] [CrossRef]
- Sireesh, D.; Dhamodharan, U.; Ezhilarasi, K.; Vijay, V.; Ramkumar, K.M. Association of NF-E2 Related Factor 2 (Nrf2) and inflammatory cytokines in recent onset Type 2 Diabetes Mellitus. Sci. Rep. 2018, 8, 5126. [Google Scholar] [CrossRef]
- Zheng, Y.; Rudensky, A.Y. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 2007, 8, 457–462. [Google Scholar] [CrossRef]
- Li, X.Y.; Zheng, Z.H.; Li, X.Y.; Guo, J.; Zhang, Y.; Li, H.; Wang, Y.W.; Ren, J.; Wu, Z.B. Treatment of Foot Disease in Patients with Type 2 Diabetes Mellitus using Human Umbilical Cord Blood Mesenchymal Stem Cells: Response and Correction of Immunological Anomalies. Curr. Pharm. Des. 2013, 19, 4893–4899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Ruan, S.H.; Yin, L.L.; Zhao, D.M.; Chen, C.; Pan, B.; Zeng, L.Y.; Li, Z.Y.; Xu, K.L. Dynamic regulation of effector IFN-gamma-producing and IL-17-producing T cell subsets in the development of acute graft-versus-host disease. Mol. Med. Rep. 2016, 13, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Kavazović, I.; Krapić, M.; Beumer-Chuwonpad, A.; Polić, B.; Turk Wensveen, T.; Lemmermann, N.A.; van Gisbergen, K.P.; Wensveen, F.M. Hyperglycemia and not hyperinsulinemia mediates diabetes-induced memory CD8 T-cell dysfunction. Diabetes 2022, 71, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Salvati, L.; Maggi, L.; Capone, M.; Vanni, A.; Spinicci, M.; Mencarini, J.; Caporale, R.; Peruzzi, B.; Antonelli, A.; et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020, 130, 4694–4703. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qin, C.J.; Wu, M.Z.; Liu, F.F.; Liu, S.S.; Liu, L. The Frequency of Natural Killer Cell Subsets in Patients with Acquired Immune Deficiency Syndrome with Deep Fungal Infections. Infect. Drug Resist. 2021, 14, 467–473. [Google Scholar] [CrossRef]
- Colucci, F.; Caligiuri, M.A.; Di Santo, J.P. What does it take to make a natural killer? Nat. Rev. Immunol. 2003, 3, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Berrou, J.; Fougeray, S.; Venot, M.; Chardiny, V.; Gautier, J.F.; Dulphy, N.; Toubert, A.; Peraldi, M.N. Natural Killer Cell Function, an Important Target for Infection and Tumor Protection, Is Impaired in Type 2 Diabetes. PLoS ONE 2013, 8, e62418. [Google Scholar] [CrossRef]
- Han, M.F.; Ma, K.; Wang, X.J.; Yan, W.M.; Wang, H.W.; You, J.; Wang, Q.X.; Chen, H.L.; Guo, W.; Chen, T.; et al. Immunological Characteristics in Type 2 Diabetes Mellitus Among COVID-19 Patients. Front. Endocrinol. 2021, 12, 596518. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Haas, K.M.; Beck, M.A.; Teague, H. The effects of diet-induced obesity on B cell function. Clin. Exp. Immunol. 2015, 179, 90–99. [Google Scholar] [CrossRef]
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.; Souwer, Y.; Jorritsma, T.; Bos, H.K.; ten Brinke, A.; Neefjes, J.; van Ham, S.M. Antigen-Specific B Cells Reactivate an Effective Cytotoxic T Cell Response against Phagocytosed Salmonella through Cross-Presentation. PLoS ONE 2010, 5, e13016. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M. Are people with uncontrolled diabetes mellitus at high risk of reinfections with COVID-19? Prim. Care Diabetes 2021, 15, 18–20. [Google Scholar] [CrossRef]
- Chiu, C.; Ellebedy, A.H.; Wrammert, J.; Ahmed, R. B Cell Responses to Influenza Infection and Vaccination. Curr. Top. Microbiol. Immunol. 2015, 386, 381–398. [Google Scholar]
- Jagannathan, M.; McDonnell, M.; Liang, Y.; Hasturk, H.; Hetzel, J.; Rubin, D.; Kantarci, A.; Van Dyke, T.E.; Ganley-Leal, L.M.; Nikolajczyk, B.S. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia 2010, 53, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Huang, X.X.; Sun, J.X.; Xie, T.; Lei, Y.F.; Muhammad, J.; Li, X.R.; Zeng, X.R.; Zhou, F.L.; Qin, H.; et al. Clinical Characteristics and Immune Injury Mechanisms in 71 Patients with COVID-19. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Chen, X.; Hu, W.; Ling, J.; Mo, P.; Zhang, Y.; Jiang, Q.; Ma, Z.; Cao, Q.; Deng, L.; Song, S.J.M. Hypertension and diabetes delay the viral clearance in COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 41–51. [Google Scholar] [CrossRef]
- Ravi, J.; Elbaz, M.; Wani, N.A.; Nasser, M.W.; Ganju, R.K. Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol. Carcinog. 2016, 55, 2063–2076. [Google Scholar] [CrossRef]
- Restrepo, B.I.; Twahirwa, M.; Rahbar, M.H.; Schlesinger, L.S. Phagocytosis via Complement or Fc-Gamma Receptors Is Compromised in Monocytes from Type 2 Diabetes Patients with Chronic Hyperglycemia. PLoS ONE 2014, 9, e92977. [Google Scholar] [CrossRef]
- Pavlou, S.; Lindsay, J.; Ingram, R.; Xu, H.P.; Chen, M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef]
- Baggiolini, M.; Dewald, B.; Moser, B. Human chemokines: An update. Annu. Rev. Immunol. 1997, 15, 675–705. [Google Scholar] [CrossRef]
- Kida, K.; Utsuyama, M.; Takizawa, T.; Thurlbeck, W.M. Changes in Lung Morphologic Features and Elasticity Caused by Streptozotocin-Induced Diabetes-Mellitus In Growing-RatS. Am. Rev. Respir. Dis. 1983, 128, 125–131. [Google Scholar] [CrossRef]
- Zhao, H.M.; Shi, L.; Wang, X.H.; Yu, X.L.; Wang, D.F. Sp1 transcription factor represses transcription of phosphatase and tensin homolog to aggravate lung injury in mice with type 2 diabetes mellitus-pulmonary tuberculosis. Bioengineered 2022, 13, 9928–9944. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Shah, M.; Scherer, P.E. Obesity and diabetes as comorbidities for COVID-19: Underlying mechanisms and the role of viral-bacterial interactions. eLife 2020, 9, 61330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wu, J.Z.; Jin, Z.; Yan, L.J. Potential Biochemical Mechanisms of Lung Injury in Diabetes. Aging Dis. 2017, 8, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Alblihed, M.; Cruz-Martins, N.; Batiha, G.E. COVID-19 and Risk of Acute Ischemic Stroke and Acute Lung Injury in Patients with Type II Diabetes Mellitus: The Anti-inflammatory Role of Metformin. Front. Med. 2021, 8, 644295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Du, Z.; Zhu, F.X. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res. Clin. Pract. 2020, 164, 108214. [Google Scholar] [CrossRef]
- Fedullo, A.L.; Schiattarella, A.; Morlando, M.; Raguzzini, A.; Toti, E.; De Franciscis, P.; Peluso, I. Mediterranean Diet for the Prevention of Gestational Diabetes in the Covid-19 Era: Implications of Il-6 In Diabesity. Int. J. Mol. Sci. 2021, 22, 1213. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Wang, X.B.; Yan, H.D.; Sun, Q.F.; Pan, K.H.; Byrne, C.D.; Zheng, K.I.; Chen, Y.P.; Eslam, M.; et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020, 46, 335–337. [Google Scholar] [CrossRef]
- Owens-Gary, M.D.; Zhang, X.P.; Jawanda, S.; Bullard, K.M.; Allweiss, P.; Smith, B.D. The Importance of Addressing Depression and Diabetes Distress in Adults with Type 2 Diabetes. J. Gen. Intern. Med. 2019, 34, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Meng, H.; Liu, T.Y.; Feng, Y.L.; Qi, Y.; Zhang, D.H.; Wang, H.L. Blue berry Phenolics Reduce Gastrointestinal Infection of Patients with Cerebral Venous Thrombosis by Improving Depressant-Induced Autoimmune Disorder via miR-155-Mediated Brain-Derived Neurotrophic Factor. Front. Pharmacol. 2017, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Jaremka, L.M.; Fagundes, C.P.; Glaser, R.; Bennett, J.M.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Loneliness predicts pain, depression, and fatigue: Understanding the role of immune dysregulation. Psychoneuroendocrinology 2013, 38, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Rudenstine, S.; McNeal, K.; Schulder, T.; Ettman, C.K.; Hernandez, M.; Gvozdieva, K.; Galea, S. Depression and Anxiety During the COVID-19 Pandemic in an Urban, Low-Income Public University Sample. J. Trauma. Stress 2021, 34, 12–22. [Google Scholar] [CrossRef]
- Gao, N.; Yan, C.X.; Lee, P.; Sun, H.J.; Yu, F.S. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J. Clin. Investig. 2016, 126, 1998–2011. [Google Scholar] [CrossRef]
- Lu, H.X.; Liu, P.; Zhang, X.X.; Bao, T.; Wang, T.; Guo, L.; Li, Y.W.; Dong, X.Y.; Li, X.R.; Dong, Y.P.; et al. Inulin and Lycium barbarum polysaccharides ameliorate diabetes by enhancing gut barrier via modulating gut microbiota and activating gut mucosal TLR2(+) intraepithelial gamma delta T cells in rats. J. Funct. Foods 2021, 79, 104407. [Google Scholar] [CrossRef]
- Lukas, H.; Xu, C.H.; Yu, Y.; Gao, W. Emerging Telemedicine Tools for Remote COVID-19 Diagnosis, Monitoring, and Management. ACS Nano 2020, 14, 16180–16193. [Google Scholar] [CrossRef]
- Dixit, S. Can moderate intensity aerobic exercise be an effective and valuable therapy in preventing and controlling the pandemic of COVID-19? Med. Hypotheses 2020, 143, 109854. [Google Scholar] [CrossRef]
- Halle, M.; Bloch, W.; Niess, A.M.; Predel, H.G.; Reinsberger, C.; Scharhag, J.; Steinacker, J.; Wolfarth, B.; Scherr, J.; Niebauer, J. Exercise and sports after COVID-19-Guidance from a clinical perspective. Transl. Sports Med. 2021, 4, 310–318. [Google Scholar] [CrossRef]
- Hopps, E.; Canino, B.; Caimi, G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol. 2011, 48, 183–189. [Google Scholar] [CrossRef]
- Denou, E.; Marcinko, K.; Surette, M.G.; Steinberg, G.R.; Schertzer, J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E982–E993. [Google Scholar] [CrossRef] [PubMed]
- Borrega-Mouquinho, Y.; Sanchez-Gomez, J.; Fuentes-Garcia, J.P.; Collado-Mateo, D.; Villafaina, S. Effects of High-Intensity Interval Training and Moderate-Intensity Training on Stress, Depression, Anxiety, and Resilience in Healthy Adults During Coronavirus Disease 2019 Confinement: A Randomized Controlled Trial. Front. Psychol. 2021, 12, 643069. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Goodyear, L.J. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 2014, 38, 308–314. [Google Scholar] [CrossRef]
- Seidu, S.; Khunti, K.; Yates, T.; Almaqhawi, A.; Davies, M.J.; Sargeant, J. The importance of physical activity in management of type 2 diabetes and COVID-19. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211054686. [Google Scholar] [CrossRef]
- Amini, H.; Habibi, S.; Islamoglu, A.H.; Isanejad, E.; Uz, C.; Daniyari, H. COVID-19 pandemic-induced physical inactivity: The necessity of updating the Global Action Plan on Physical Activity 2018–2030. Environ. Health Prev. Med. 2021, 26, 32. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Isanejad, A.; Chamani, N.; Movahedi-Fard, F.; Salimi, F.; Moezi, M.; Habibi, S. Physical activity during COVID-19 pandemic in the Iranian population: A brief report. Heliyon 2020, 6, e05411. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, E1077–E1086. [Google Scholar] [CrossRef]
- Lakhan, R.; Agrawal, A.; Sharma, M. Prevalence of Depression, Anxiety, and Stress during COVID-19 Pandemic. J. Neurosci. Rural. Pract. 2020, 11, 519–525. [Google Scholar] [CrossRef]
- Pappa, S.; Ntella, V.; Giannakas, T.; Giannakoulis, V.G.; Papoutsi, E.; Katsaounou, P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 901–907. [Google Scholar] [CrossRef]
- Elbay, R.Y.; Kurtulmus, A.; Arpacioglu, S.; Karadere, E. Depression, anxiety, stress levels of physicians and associated factors in Covid-19 pandemics. Psychiatry Res. 2020, 290, 113130. [Google Scholar]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [PubMed]
- Gupta, P.; Gupta, M.; Katoch, N.; Garg, K.; Garg, B. A Systematic Review and Meta-analysis of Diabetes Associated Mortality in Patients with COVID-19. Int. J. Endocrinol. Metab. 2021, 19, e113220. [Google Scholar] [PubMed]
- da Silva, D.E.; Grande, A.J.; Roever, L.; Tse, G.; Liu, T.; Biondi-Zoccai, G.; de Farias, J.M. High-Intensity Interval Training in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Curr. Atheroscler. Rep. 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, T.; Nigam, A.; Gremeaux, V.; Meyer, P.; Juneau, M.; Bosquet, L. High-Intensity Interval Training in Cardiac Rehabilitation. Sports Med. 2012, 42, 587–605. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhu, L.; Su, V. Comparative Effectiveness of High-Intensity Interval Training and Moderate-Intensity Continuous Training for Cardiometabolic Risk Factors and Cardiorespiratory Fitness in Childhood Obesity: A Meta-Analysis of Randomized Controlled Trials. Front. Physiol. 2020, 11. [Google Scholar]
- Tuan, T.C.; Hsu, T.G.; Fong, M.C.; Hsu, C.F.; Tsai, K.K.C.; Lee, C.Y.; Kong, C.W. Deleterious effects of short-term, high-intensity exercise on immune function: Evidence from leucocyte mitochondrial alterations and apoptosis. Br. J. Sports Med. 2008, 42, 11–15. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Khan, Q.; Drysdale, P.T.; Wallace, F.; Jeukendrup, A.E.; Drayson, M.T.; Gleeson, M. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J. Appl. Physiol. 2005, 98, 565–571. [Google Scholar] [CrossRef]
- Sabouri, M.; Hatami, E.; Pournemati, P.; Shabkhiz, F. Inflammatory, antioxidant and glycemic status to different mode of high-intensity training in type 2 diabetes mellitus. Mol. Biol. Rep. 2021, 48, 5291–5304. [Google Scholar] [CrossRef]
- Shahouzehi, B.; Nasri, H.; Aminizadeh, S.; Masoumi-Ardakani, Y.J. The Effect of High-intensity Interval Training and L-carnitine on the Expression of Some Pro-inflammatory Genes in the Liver and Cardiac Tissues of Rats. J. Kerman Univ. Med. Sci. 2021, 28, 56–68. [Google Scholar]
- Robinson, E.; Durrer, C.; Simtchouk, S.; Jung, M.E.; Bourne, J.E.; Voth, E.; Little, J.P. Short-term high-intensity interval and moderate-intensity continuous training reduce leukocyte TLR4 in inactive adults at elevated risk of type 2 diabetes. J. Appl. Physiol. 2015, 119, 508–516. [Google Scholar] [CrossRef]
- Bartlett, D.B.; Slentz, C.A.; Willis, L.H.; Hoselton, A.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; Muoio, D.M.; et al. Rejuvenation of Neutrophil Functions in Association with Reduced Diabetes Risk Following Ten Weeks of Low-Volume High Intensity Interval Walking in Older Adults with Prediabetes—A Pilot Study. Front. Immunol. 2020, 11, 729. [Google Scholar] [PubMed]
- Huang, C.C.; Hsu, C.C.; Chiu, C.C.; Lin, H.J.; Wang, J.J.; Weng, S.F. Association between exercise and health-related quality of life and medical resource use in elderly people with diabetes: A cross-sectional population-based study. BMC Geriatr. 2020, 20, 331. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Abdelhafiz, A.H. Age, frailty and diabetes—Triple jeopardy for vulnerability to COVID-19 infection. Eclinicalmedicine 2020, 22, 100343. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Z. Physical activity and health in the presence of China’s economic growth: Meeting the public health challenges of the aging population. J. Sport Health Sci. 2016, 5, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, E.; Marinho, D.A.; Neiva, H.P.; Lourenco, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef]
- Cobianchi, S.; Arbat-Plana, A.; Lopez-Alvarez, V.M.; Navarro, X. Neuroprotective Effects of Exercise Treatments After Injury: The Dual Role of Neurotrophic Factors. Curr. Neuropharmacol. 2017, 15, 495–518. [Google Scholar] [CrossRef]
- Zar, A.; Ahmadi, F.; Miri, M.; Abedi, H.A.; Salesi, M. Cytokine Pattern is Affected by Training Intensity in Women Futsal Players. Immune Netw. 2016, 16, 109–115. [Google Scholar]
- Wing, R.; Epstein, L.; Paternostro-Bayles, M.; Kriska, A.; Nowalk, M.; Gooding, W.J.D. Exercise in a behavioural weight control programme for obese patients with type 2 (non-insulin-dependent) diabetes. Diabetologia 1988, 31, 902–909. [Google Scholar] [CrossRef]
- Li, S.N.; Liu, J.; Yan, H.M. Medium-intensity acute exhaustive exercise induces neural cell apoptosis in the rat hippocampus. Neural Regen. Res. 2013, 8, 127–132. [Google Scholar]
- Parker, L.; Shaw, C.S.; Banting, L.; Levinger, I.; Hill, K.M.; McAinch, A.J.; Stepto, N.K. Acute low-volume high-intensity interval exercise and continuous moderate-intensity exercise elicit a similar improvement in 24-h glycemic control in overweight and obese adults. Front. Physiol. 2017, 7, 661. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.S.; Patten, C.A.; Lewis, B.A.; Clark, M.M.; Ussher, M.; Ebbert, J.O.; Croghan, I.T.; Decker, P.A.; Hathaway, J.; Marcus, B.H.; et al. Feasibility of an exercise counseling intervention for depressed women smokers. Nicotine Tob. Res. 2009, 11, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Abraha, B.; Chaves, A.R.; Kelly, L.P.; Wallack, E.M.; Wadden, K.P.; McCarthy, J.; Ploughman, M. A Bout of High Intensity Interval Training Lengthened Nerve Conduction Latency to the Non-exercised Affected Limb in Chronic Stroke. Front. Physiol. 2018, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Hayney, M.S.; Muller, D.; Rakel, D.; Brown, R.; Zgierska, A.E.; Barlow, S.; Hayer, S.; Barnet, J.H.; Torres, E.R.; et al. Meditation or exercise for preventing acute respiratory infection (MEPARI-2): A randomized controlled trial. PLoS ONE 2018, 13, e0197778. [Google Scholar] [CrossRef]
- Chamorro-Vina, C.; Valentin, J.; Fernandez, L.; Gonzalez-Vicent, M.; Perez-Ruiz, M.; Lucia, A.; Culos-Reed, S.N.; Diaz, M.A.; Perez-Martinez, A. Influence of a Moderate-Intensity Exercise Program on Early NK Cell Immune Recovery in Pediatric Patients After Reduced-Intensity Hematopoietic Stem Cell Transplantation. Integr. Cancer Ther. 2017, 16, 464–472. [Google Scholar] [CrossRef]
- Khammassi, M.; Ouerghi, N.; Said, M.; Feki, M.; Khammassi, Y.; Pereira, B.; Thivel, D.; Bouassida, A.J.T.J.o.S.; Research, C. Continuous moderate-intensity but not high-intensity interval training improves immune function biomarkers in healthy young men. J. Strength Cond. Res. 2020, 34, 249–256. [Google Scholar] [CrossRef]
- Hekmatikar, A.H.A.; Shamsi, M.M.; Ashkazari, Z.S.Z.; Suzuki, K. Exercise in an Overweight Patient with Covid-19: A Case Study. Int. J. Environ. Res. Public Health 2021, 18, 5882. [Google Scholar] [CrossRef]
- Piquet, V.; Luczak, C.; Seiler, F.; Monaury, J.; Martini, A.; Ward, A.B.; Gracies, J.M.; Motavasseli, D.; Covid Rehabilitation Study Group. Do Patients with COVID-19 Benefit from Rehabilitation? Functional Outcomes of the First 100 Patients in a COVID-19 Rehabilitation Unit. Arch. Phys. Med. Rehabil. 2021, 102, 1067–1074. [Google Scholar] [CrossRef]
- Jesus, I.; Vanhee, V.; Deramaudt, T.B.; Bonay, M. Promising effects of exercise on the cardiovascular, metabolic and immune system during COVID-19 period. J. Hum. Hypertens. 2021, 35, 1–3. [Google Scholar] [CrossRef]
- Rykova, M.; Antropova, E.; Popov, D.; Vinogradova, O.; Larina, I.J. The activation processes of the immune system during low intensity exercise without relaxation. Ross. Fiziol. Zhurnal Im. IM Sechenova 2008, 94, 212–219. [Google Scholar]
- Kimura, A. The usefulness of low-intensity physical activity management for malaise in type 2 diabetic patients after ablation. Diabetes Res. Clin. Pract. 2022, 186, 109717. [Google Scholar] [CrossRef]
- Ji, C.X.; Yang, J.; Lin, L.; Chen, S. Physical Exercise Ameliorates Anxiety, Depression and Sleep Quality in College Students: Experimental Evidence from Exercise Intensity and Frequency. Behav. Sci. 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Tunkamnerdthai, O.; Auvichayapat, P.; Donsom, M.; Leelayuwat, N. Improvement of pulmonary function with arm swing exercise in patients with type 2 diabetes. J. Phys. Ther. Sci. 2015, 27, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Marcal, I.R.; Fernandes, B.; Viana, A.A.; Ciolac, E.G. The Urgent Need for Recommending Physical Activity for the Management of Diabetes During and Beyond COVID-19 Outbreak. Front. Endocrinol. 2020, 11, 584642. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Tantawy, S.A.; Kamel, D.M.; Alqahtani, B.A.; Elnegamy, T.E.; Soliman, G.S.; Ibrahim, A.A. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Medicine 2020, 99, e19471. [Google Scholar] [CrossRef]
- Polero, P.; Rebollo-Seco, C.; Adsuar, J.C.; Pérez-Gómez, J.; Rojo-Ramos, J.; Manzano-Redondo, F.; Garcia-Gordillo, M.Á.; Carlos-Vivas, J.J. Physical activity recommendations during COVID-19: Narrative review. Int. J. Environ. Res. Public Health 2020, 18, 65. [Google Scholar] [CrossRef]
| Model | Age | Research Findings | Reference |
|---|---|---|---|
| HWD | NDM: 62.1 T2DP: 63.4 | Neutrophil, LS, IgE, IFN-γ, TNF-α, IL-6 ↑ T, Tc, Th, and NK cells ↓ | [51] |
| Diabetic patients | SMARCD3, PARL, GLIPR1, STAT2, PMAIP1, GP1BA, and TOX genes and PI3K-Akt, focal adhesion, Foxo, phagosome, adrenergic, osteoclast differentiation, platelet activation, insulin, cytokine- cytokine interaction, apoptosis, ECM, JAK-STAT, and oxytocin signaling were the linkage between COVID-19 and Type-2 diabetes | [52] | |
| Diabetic patients | Median age: 55 | Creatinine, neutrophil, creatinine, alanine aminotransferase ↑ | [53] |
| HWD | Range from 18 to 60 | CD4+ T cells, CD4+/TNF-α, CD4+/IL-2, CD4+/IFN-γ ↓ | [54] |
| HWD | Median age: 47 | CD3+, CD4+, CD4+/CD8+ ↑ hs-CRP, LDH, IL-6, CD8+ ↓ | [55] |
| HWD | MMP-7, MMP-9, PDGF and TGF-β ↑ LBP↓ | [56] | |
| PUCD | Median age: 61.45 | Uncontrolled inflammatory responses, leukocyte, neutrophil-lymphocyte ratio, IL-6, FDP, D-dimer ↑ Pulmonary invasion | [57] |
| Patients with severe COVID-19 | Median age: 64 | Susceptible to receiving mechanical ventilation and admission to ICU Mortality, leukocyte, neutrophil, high-sensitivity C reaction protein, procalcitonin, ferritin, IL-2 receptor, IL-6, IL-8, TNF-α, D-dimer, fibrinogen, lactic dehydrogenase, and N-terminal pro-brain natriuretic peptide ↑ | [58] |
| HWD | Depression and anxiety ↑ | [59] | |
| HWD | Median age: 40.31 | Emotional dysregulation Depression ↑ | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Guo, S.; Ji, W.; Sun, H.; Lee, S.; Zhang, D. Intervention Effects of Physical Activity on Type 2 Diabetic Patients Potentially Infected with COVID-19. Medicina 2023, 59, 1772. https://doi.org/10.3390/medicina59101772
Yu L, Guo S, Ji W, Sun H, Lee S, Zhang D. Intervention Effects of Physical Activity on Type 2 Diabetic Patients Potentially Infected with COVID-19. Medicina. 2023; 59(10):1772. https://doi.org/10.3390/medicina59101772
Chicago/Turabian StyleYu, Lihua, Sainyu Guo, Wen Ji, Hailian Sun, Seongno Lee, and Deju Zhang. 2023. "Intervention Effects of Physical Activity on Type 2 Diabetic Patients Potentially Infected with COVID-19" Medicina 59, no. 10: 1772. https://doi.org/10.3390/medicina59101772
APA StyleYu, L., Guo, S., Ji, W., Sun, H., Lee, S., & Zhang, D. (2023). Intervention Effects of Physical Activity on Type 2 Diabetic Patients Potentially Infected with COVID-19. Medicina, 59(10), 1772. https://doi.org/10.3390/medicina59101772






