The Effects of Prenatal and Postnatal Exposure to 50-Hz and 3 mT Electromagnetic Field on Rat Testicular Development
Abstract
1. Introduction
2. Materials and Methods
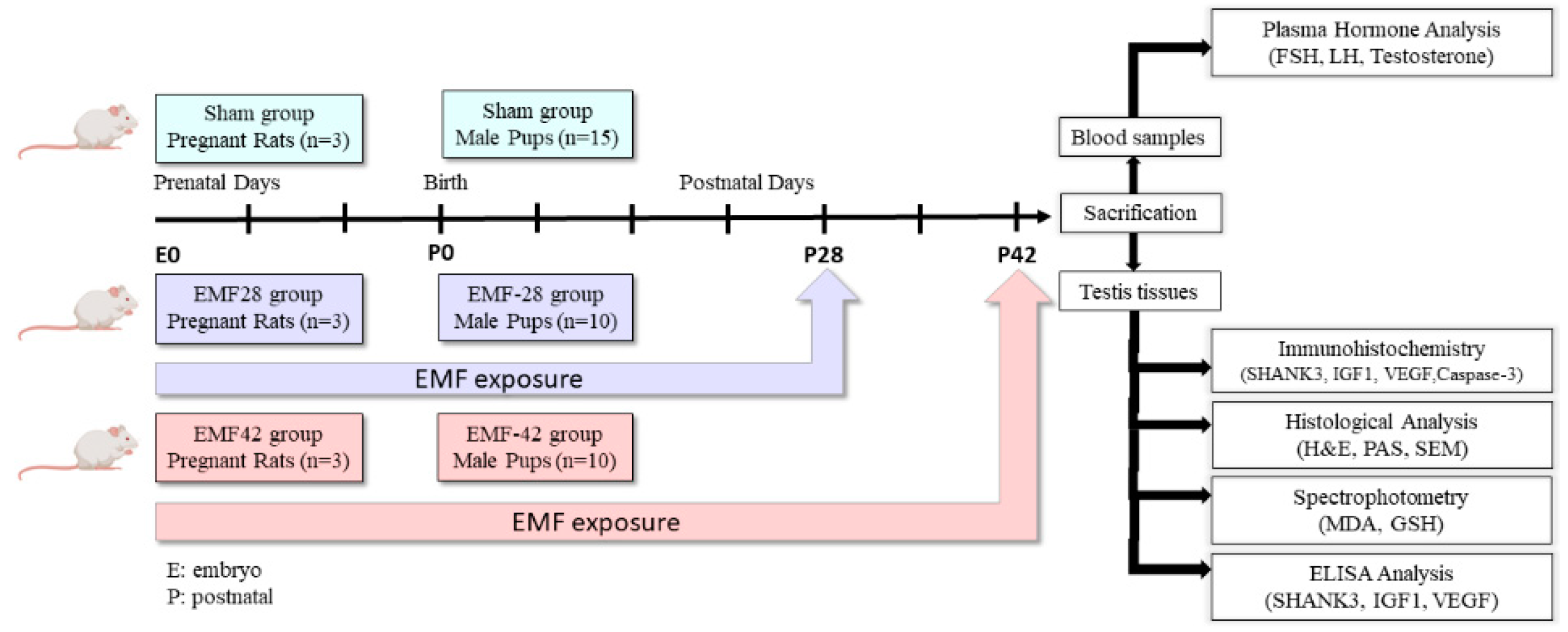
2.1. Animals
2.2. EMF Exposure System
2.3. Biochemical Analyses
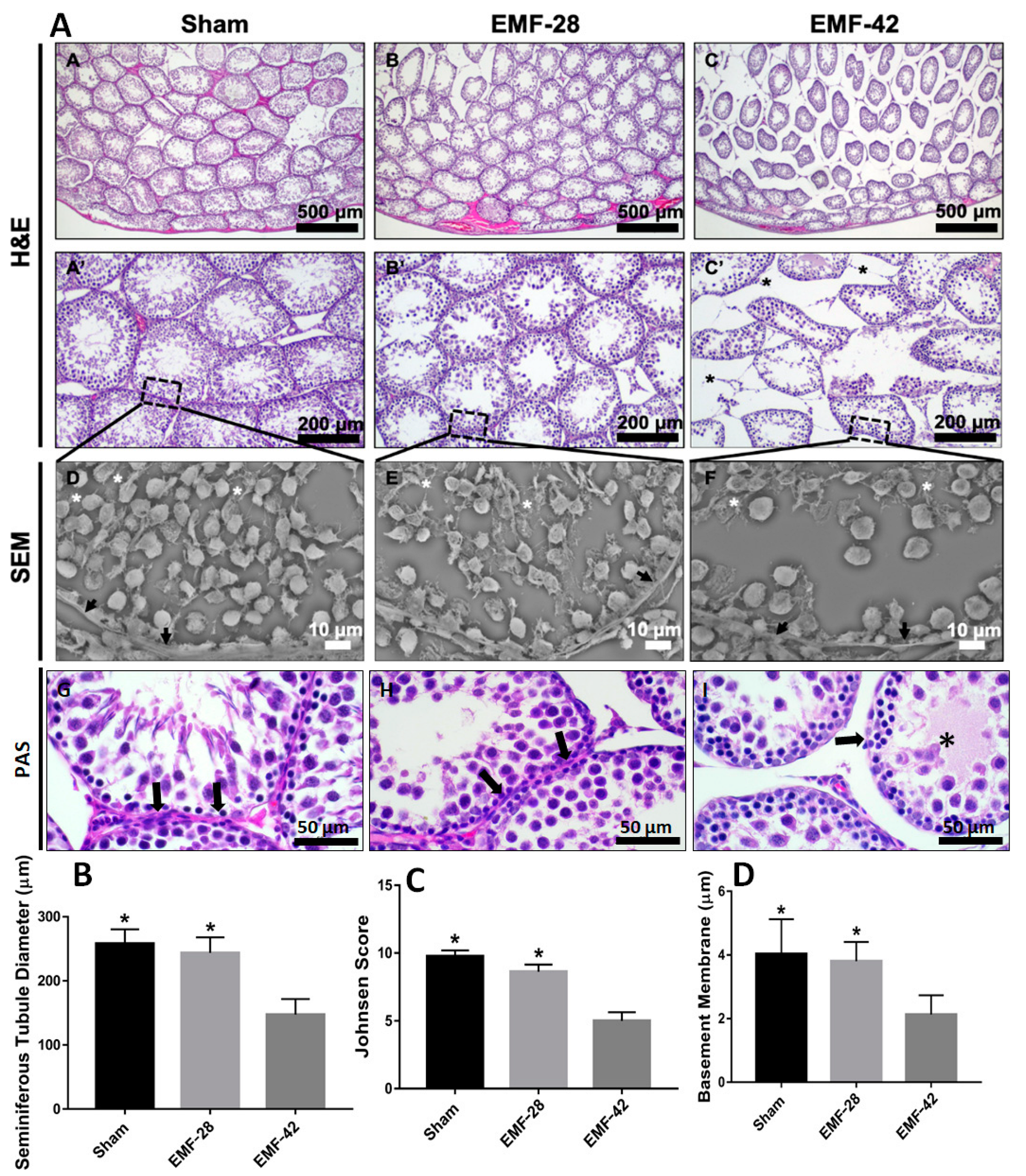
2.4. Histological Analysis
2.5. Examination of Spermatogenesis
2.6. Measurement of Seminiferous Tubule Diameter (STD) and Basement Membrane Thickness (STBM)
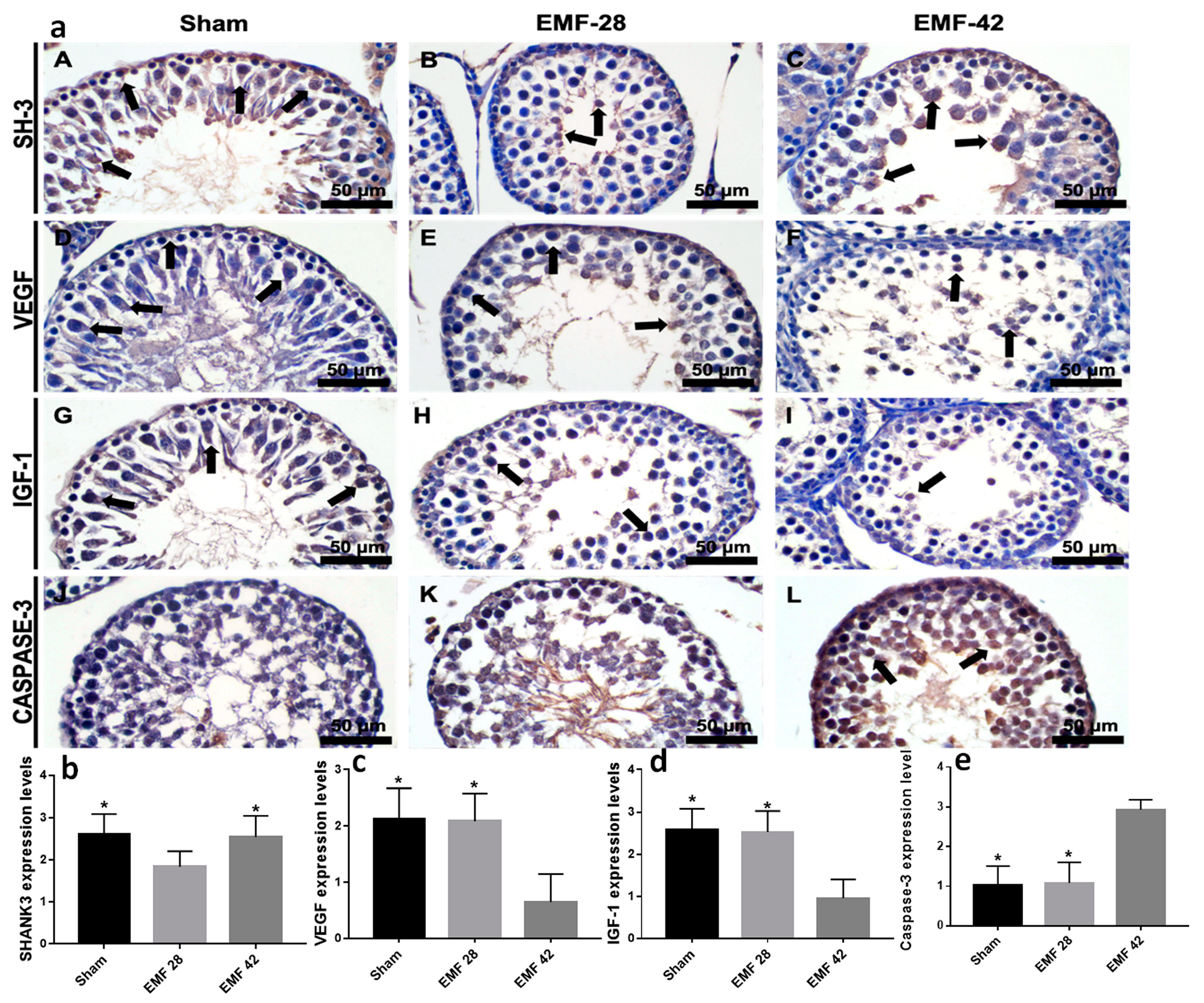
2.7. Immunohistochemistry
2.8. Scanning Electron Microscopy (SEM) Evaluation
2.9. Statistical Analysis
3. Results
3.1. Body and Testis Weights
3.2. Biochemical Analyses
3.3. Histological Analysis
3.4. Immunohistochemistry
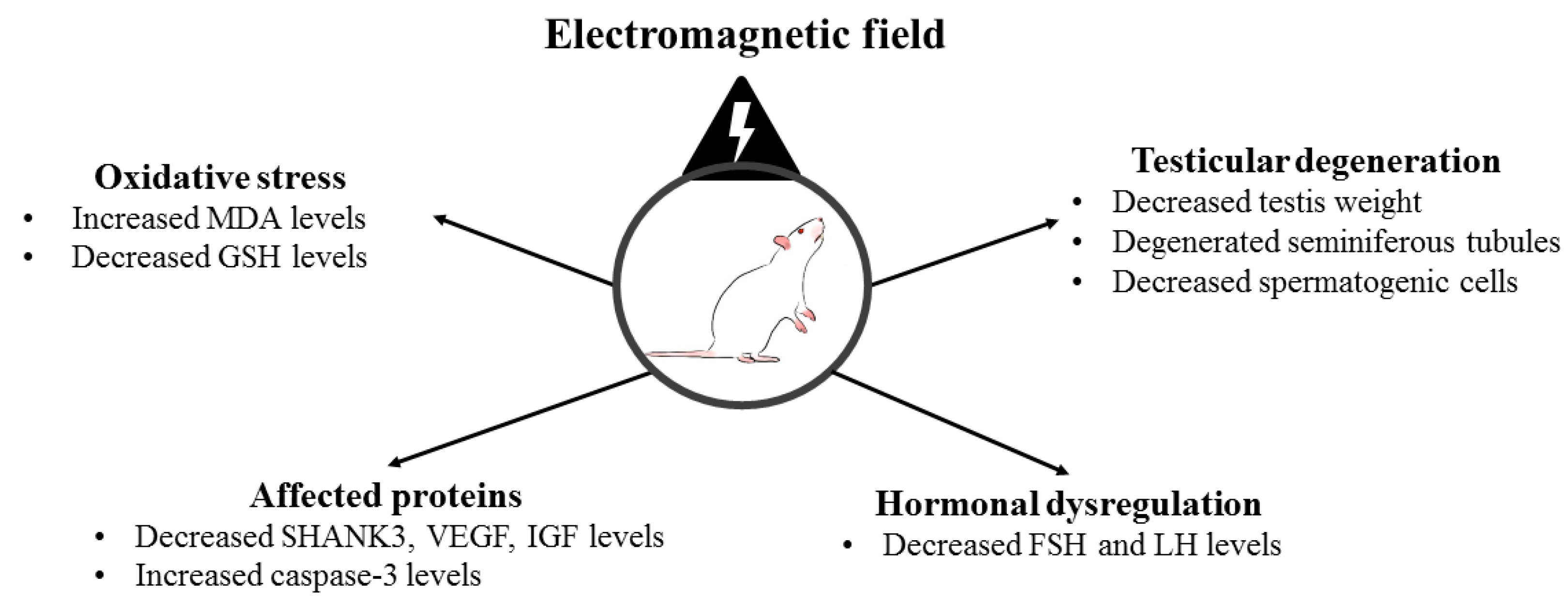
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belyaev, I.; Dean, A.; Eger, H.; Hubmann, G.; Jandrisovits, R.; Kern, M.; Kundi, M.; Moshammer, H.; Lercher, P.; Müller, K.; et al. EUROPAEM EMF Guideline 2016 for the prevention, diagnosis and treatment of EMF-related health problems and illnesses. Rev. Environ. Health 2016, 31, 363–397. [Google Scholar] [CrossRef] [PubMed]
- Seifirad, S.; Farzampour, S.; Nourbakhsh, M.; Amoli, M.M.; Razzaghy-Azar, M.; Larijani, B. Effects of extremely low frequency electromagnetic fields on paraoxonase serum activity and lipid peroxidation metabolites in rat. J. Diabetes Metab. Disord. 2014, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Odacı, E.; Ünal, D.; Mercantepe, T.; Topal, Z.; Hancı, H.; Türedi, S.; Erol, H.S.; Mungan, S.; Kaya, H.; Çolakoğlu, S. Pathological effects of prenatal exposure to a 900 MHz electromagnetic field on the 21-day-old male rat kidney. Biotech. Histochem. 2014, 90, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Akdag, M.Z.; Dasdag, S.; Ulukaya, E.; Uzunlar, A.K.; Kurt, M.A.; Taşkın, A. Effects of Extremely Low-Frequency Magnetic Field on Caspase Activities and Oxidative Stress Values in Rat Brain. Biol. Trace Element Res. 2010, 138, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Hancı, H.; Kerimoğlu, G.; Mercantepe, T.; Odacı, E. Changes in testicular morphology and oxidative stress biomarkers in 60-day-old Sprague Dawley rats following exposure to continuous 900-MHz electromagnetic field for 1 h a day throughout adolescence. Reprod. Toxicol. 2018, 81, 71–78. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Salbacak, A.; Celik, I.; Sivrikaya, A. The role of zinc supplementation in the inhibition of tissue damage caused by exposure to electromagnetic field in rat lung and liver tissues. Bratisl Lek List. 2012, 113, 400–403. [Google Scholar] [CrossRef]
- Kiray, A.; Tayefi, H.; Kiray, M.; Bagriyanik, H.A.; Pekcetin, C.; Ergur, B.U.; Ozogul, C. The effects of exposure to electromagnetic field on rat myocardium. Toxicol. Ind. Health 2012, 29, 418–425. [Google Scholar] [CrossRef]
- Afolabi, O.B.; Obajuluwa, A.O.; Obajuluwa, T.; Okiki, P.; Oloyede, O.I.; Fadaka, O.A.; Ojo, O.A. Exposure to a 2.5 GHz Non-ionizing Electromagnetic Field Alters Hematological Profiles, Biochemical Parameters, and Induces Oxidative Stress in Male Albino Rats. Biomed Environ. Sci. 2019, 32, 860–863. [Google Scholar]
- Shahin, N.N.; El-Nabarawy, N.A.; Gouda, A.S.; Mégarbane, B. The protective role of spermine against male reproductive aberrations induced by exposure to electromagnetic field—An experimental investigation in the rat. Toxicol. Appl. Pharmacol. 2019, 370, 117–130. [Google Scholar] [CrossRef]
- Zeng, L.; Ji, X.; Zhang, Y.; Miao, X.; Zou, C.; Lang, H.; Zhang, J.; Li, Y.; Wang, X.; Qi, H.; et al. MnSOD expression inhibited by electromagnetic pulse radiation in the rat testis. Electromagn. Biol. Med. 2011, 30, 205–218. [Google Scholar] [CrossRef]
- Al-Damegh, M.A. Rat testicular impairment induced by electromagnetic radiation from a conventional cellular telephone and the protective effects of the antioxidants vitamins C and E. Clinics 2012, 67, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Özorak, A.; Nazıroğlu, M.; Çelik, Ö.; Yüksel, M.; Özçelik, D.; Özkaya, M.O.; Çetin, H.; Kahya, M.C.; Kose, S.A. Wi-Fi (2.45 GHz)- and Mobile Phone (900 and 1800 MHz)-Induced Risks on Oxidative Stress and Elements in Kidney and Testis of Rats During Pregnancy and the Development of Offspring. Biol. Trace Element Res. 2013, 156, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Odacı, E.; Hancı, H.; Yuluğ, E.; Türedi, S.; Aliyazıcıoğlu, Y.; Kaya, H.; Çolakoğlu, S. Effects of prenatal exposure to a 900 MHz electromagnetic field on 60-day-old rat testis and epididymal sperm quality. Biotech. Histochem. 2015, 91, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Górski, R.; Kotwicka, M.; Skibińska, I.; Jendraszak, M.; Wosiński, S. Effect of low-frequency electric field screening on motility of human sperm. Ann. Agric. Environ. Med. 2020, 27, 427–434. [Google Scholar] [CrossRef]
- Okechukwu, C.E. Does the use of mobile phone affect male fertility? A mini-review. J. Hum. Reprod. Sci. 2020, 13, 174–183. [Google Scholar] [CrossRef]
- Tenorio, B.M.; Jimenez, G.C.; de Morais, R.N.; Peixoto, C.A.; Nogueira, R.D.A.; Silva, V.A. Evaluation of testicular degeneration induced by low-frequency electromagnetic fields. J. Appl. Toxicol. 2011, 32, 210–218. [Google Scholar] [CrossRef]
- Hajhosseini, L.; Khaki, A.; Merat, E.; Ainehchi, N. Effect of Rosmarinic Acid on Sertoli Cells Apoptosis and Serum Antioxidant Levels in Rats After Exposure to Electromagnetic Fields. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 477–480. [Google Scholar] [CrossRef]
- Almášiová, V.; Holovská, K.; Andrašková, S.; Cigánková, V.; Ševčíková, Z.; Raček, A.; Račeková, E. Potential influence of prenatal 2.45 GHz radiofrequency electromagnetic field exposure on Wistar albino rat testis. Histol. Histopathol. 2021, 36, 685–696. [Google Scholar] [CrossRef]
- Holdcraft, R.W.; Braun, R.E. Hormonal regulation of spermatogenesis. Int. J. Androl. 2004, 27, 335–342. [Google Scholar] [CrossRef]
- Hayes, F.J.; DeCruz, S.; Seminara, S.B.; Boepple, P.A.; Crowley, W.F. Differential Regulation of Gonadotropin Secretion by Testosterone in the Human Male: Absence of a Negative Feedback Effect of Testosterone on Follicle-Stimulating Hormone Secretion1. J. Clin. Endocrinol. Metab. 2001, 86, 53–58. [Google Scholar] [CrossRef]
- Sepehrimanesh, M.; Saeb, M.; Nazifi, S.; Kazemipour, N.; Jelodar, G.; Saeb, S. Impact of 900 MHz electromagnetic field exposure on main male reproductive hormone levels: A Rattus norvegicus model. Int. J. Biometeorol. 2013, 58, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Recchia, K.; Jorge, A.S.; Pessôa, L.V.D.F.; Botigelli, R.C.; Zugaib, V.C.; de Souza, A.F.; Martins, D.D.S.; Ambrósio, C.E.; Bressan, F.F.; Pieri, N.C.G. Actions and Roles of FSH in Germinative Cells. Int. J. Mol. Sci. 2021, 22, 10110. [Google Scholar] [CrossRef] [PubMed]
- Dees, W.L.; Hiney, J.K.; Srivastava, V.K. IGF-1 Influences Gonadotropin-Releasing Hormone Regulation of Puberty. Neuroendocrinology 2021, 111, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Neirijnck, Y.; Papaioannou, M.D.; Nef, S. The Insulin/IGF System in Mammalian Sexual Development and Reproduction. Int. J. Mol. Sci. 2019, 20, 4440. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Hardy, M.P.; Zhou, J.; Bondy, C.; Lupu, F.; Bellvé, A.R.; Efstratiadis, A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996, 10, 903–918. [Google Scholar] [CrossRef][Green Version]
- Colón, E.; Zaman, F.; Axelson, M.; Larsson, O.; Carlsson-Skwirut, C.; Svechnikov, K.V.; Söder, O. Insulin-Like Growth Factor-I Is an Important Antiapoptotic Factor for Rat Leydig Cells during Postnatal Development. Endocrinology 2007, 148, 128–139. [Google Scholar] [CrossRef]
- Puche, J.E.; Castilla-Cortázar, I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J. Transl. Med. 2012, 10, 224. [Google Scholar] [CrossRef]
- Xu, Y.; Han, C.Y.; Park, M.J.; Gye, M.C. Increased testicular insulin-like growth factor 1 is associated with gonadal activation by recombinant growth hormone in immature rats. Reprod. Biol. Endocrinol. 2022, 20, 72. [Google Scholar] [CrossRef]
- Sargent, K.M.; Clopton, D.T.; Lu, N.; Pohlmeier, W.E.; Cupp, A.S. VEGFA splicing: Divergent isoforms regulate spermatogonial stem cell maintenance. Cell Tissue Res. 2015, 363, 31–45. [Google Scholar] [CrossRef]
- Del Vento, F.; Poels, J.; Vermeulen, M.; Ucakar, B.; Giudice, M.; Kanbar, M.; Rieux, A.D.; Wyns, C. Accelerated and Improved Vascular Maturity after Transplantation of Testicular Tissue in Hydrogels Supplemented with VEGF- and PDGF-Loaded Nanoparticles. Int. J. Mol. Sci. 2021, 22, 5779. [Google Scholar] [CrossRef]
- Solek, P.; Mytych, J.; Sujkowska, E.; Grzegorczyk, M.; Jasiewicz, P.; Sowa-Kucma, M.; Stachowicz, K.; Koziorowski, M.; Tabecka-Lonczynska, A. Trade-offs between male fertility reduction and selected growth factors or the klotho response in a lipopolysaccharide-dependent mouse model. Toxicol. Res. 2021, 38, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Ehlers, M.D. Modeling Autism by SHANK Gene Mutations in Mice. Neuron 2013, 78, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Arons, M.H.; Thynne, C.J.; Grabrucker, A.; Li, D.; Schoen, M.; Cheyne, J.; Boeckers, T.M.; Montgomery, J.M.; Garner, C. Autism-Associated Mutations in ProSAP2/Shank3 Impair Synaptic Transmission and Neurexin-Neuroligin-Mediated Transsynaptic Signaling. J. Neurosci. 2012, 32, 14966–14978. [Google Scholar] [CrossRef] [PubMed]
- Vyas, Y.; Cheyne, J.E.; Lee, K.; Jung, Y.; Cheung, P.Y.; Montgomery, J.M. Shankopathies in the Developing Brain in Autism Spectrum Disorders. Front. Neurosci. 2021, 15, 775431. [Google Scholar] [CrossRef]
- Redecker, P.; Gundelfinger, E.D.; Boeckers, T.M. The Cortactin-binding Postsynaptic Density Protein ProSAP1 in Non-neuronal Cells. J. Histochem. Cytochem. 2001, 49, 639–648. [Google Scholar] [CrossRef]
- Tenorio, B.M.; Jimenez, G.C.; Morais, R.N.; Torres, S.M.; Nogueira, R.A.; Junior, V.A.S. Testicular development evaluation in rats exposed to 60 Hz and 1 mT electromagnetic field. J. Appl. Toxicol. 2010, 31, 223–230. [Google Scholar] [CrossRef]
- Burcu, A.; Nevin, E.; Ilkay, A.; Amac, K.; Alper, B.H.; Muge, K. The effects of prenatal and postnatal exposure to electromagnetic field on rat ovarian tissue. Toxicol. Ind. Health 2020, 36, 1010–1018. [Google Scholar] [CrossRef]
- Acikgoz, B.; Ersoy, N.; Aksu, I.; Kiray, A.; Bagriyanik, H.A.; Kiray, M. Gender differences in effects of prenatal and postnatal exposure to electromagnetic field and prenatal zinc on behaviour and synaptic proteins in rats. J. Chem. Neuroanat. 2022, 122, 102092. [Google Scholar] [CrossRef]
- Pekcetin, C.; Ergur, B.U.; Kiray, M.; Bagriyanik, A.; Tugyan, K.; Erbil, G.; Ozogul, C. The protective effects of trimetazidine on testicular ischemia and reperfusion injury in rats. Pediatr. Surg. Int. 2007, 23, 1113–1118. [Google Scholar] [CrossRef]
- Chatterjee, S.; Malhotra, R.; Varghese, F.; Bukhari, A.B.; Patil, A.; Budrukkar, A.; Parmar, V.; Gupta, S.; De, A. Quantitative Immunohistochemical Analysis Reveals Association between Sodium Iodide Symporter and Estrogen Receptor Expression in Breast Cancer. PLoS ONE 2013, 8, e54055. [Google Scholar] [CrossRef]
- Kuzay, D.; Ozer, C.; Sirav, B.; Canseven, A.G.; Seyhan, N. Oxidative effects of extremely low frequency magnetic field and radio frequency radiation on testes tissues of diabetic and healthy rats. Bratisl. Med. J. 2017, 118, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Baltaci, A.K.; Mogulkoc, R.; Oztekin, E. Zinc Prevention of Electromagnetically Induced Damage to Rat Testicle and Kidney Tissues. Biol. Trace Element Res. 2003, 96, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Akdag, M.Z.; Dasdag, S.; Aksen, F.; Isik, B.; Yilmaz, F. Effect of ELF magnetic fields on lipid peroxidation, sperm count, p53, and trace elements. Med. Sci. Monit. 2006, 12, BR366–BR371. [Google Scholar]
- Mailankot, M.; Kunnath, A.P.; Jayalekshmi, H.; Koduru, B.; Valsalan, R. Radio Frequency Electromagnetic Radiation (RF-EMR) from GSM (0.9/1.8GHZ) Mobile Phones Induces Oxidative Stress and Reduces Sperm Motility in Rats. Clinics 2009, 64, 561–565. [Google Scholar] [CrossRef]
- Hancı, H.; Odacı, E.; Kaya, H.; Aliyazıcıoğlu, Y.; Turan, I.; Demir, S.; Çolakoğlu, S. The effect of prenatal exposure to 900-MHz electromagnetic field on the 21-old-day rat testicle. Reprod. Toxicol. 2013, 42, 203–209. [Google Scholar] [CrossRef]
- Jonwal, C.; Sisodia, R.; Saxena, V.K.; Kesari, K.K. Effect of 2.45 GHz microwave radiation on the fertility pattern in male mice. Gen. Physiol. Biophys. 2018, 37, 453–460. [Google Scholar] [CrossRef]
- Di, G.; Xiang, J.; Dong, L.; Wu, J. Testosterone synthesis in testicular Leydig cells after long-term exposure to a static electric field (SEF). Toxicology 2021, 458, 152836. [Google Scholar] [CrossRef] [PubMed]
- Khoshbakht, S.; Motejaded, F.; Karimi, S.; Jalilvand, N.; Ebrahimzadeh-Bideskan, A. Protective effects of selenium on electromagnetic field-induced apoptosis, aromatase P450 activity, and leptin receptor expression in rat testis. Iran. J. Basic Med. Sci. 2021, 24, 322–330. [Google Scholar] [CrossRef]
- Çetkin, M.; Kızılkan, N.; Demirel, C.; Bozdağ, Z.; Erkılıç, S.; Erbağcı, H. Quantitative changes in testicular structure and function in rat exposed to mobile phone radiation. Andrologia 2017, 49, e12761. [Google Scholar] [CrossRef]
- Khaki, A.A.; Tubbs, R.S.; Shoja, M.M.; Rad, J.S.; Farahani, R.; Zarrintan, S.; Nag, T. The effects of an electromagnetic field on the boundary tissue of the seminiferous tubules of the rat: A light and transmission electron microscope study. Folia Morphol. 2006, 65, 188–194. [Google Scholar]
- Kumar, P.; Shukla, V. Ultrastructural changes in rat testicular tissue after whole body exposure to electromagnetic radiation emitted from mobile phones. J. Int. Acad. Res. Multidiscip. 2014, 2, 518–526. [Google Scholar]
- Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Effects of the insulin-like growth factor system on testicular differentiation and function: A review of the literature. Andrology 2017, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Griffeth, R.J.; Bianda, V.; Nef, S. The emerging role of insulin-like growth factors in testis development and function. Basic Clin. Androl. 2014, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Dundar, B.; Cesur, G.; Comlekci, S.; Songur, A.; Gokcimen, A.; Sahin, O.; Ulukut, O.; Yilmaz, H.R.; Sutcu, R.; Calıskan, S. The effect of the prenatal and post-natal long-term exposure to 50 Hz electric field on growth, pubertal development and IGF-1 levels in female Wistar rats. Toxicol. Ind. Health 2009, 25, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Sangun, O.; Dundar, B.; Darici, H.; Comlekci, S.; Doguc, D.K.; Celik, S. The effects of long-term exposure to a 2450 MHz electromagnetic field on growth and pubertal development in female Wistar rats. Electromagn. Biol. Med. 2013, 34, 63–71. [Google Scholar] [CrossRef]
- Potter, S.J.; DeFalco, T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction 2017, 153, R151–R162. [Google Scholar] [CrossRef]
- Tian, R.; Yang, S.; Zhu, Y.; Zou, S.; Li, P.; Wang, J.; Zhu, Z.; Huang, Y.; He, Z.; Li, Z. VEGF/VEGFR2 Signaling Regulates Germ Cell Proliferation in vitro and Promotes Mouse Testicular Regeneration in vivo. Cells Tissues Organs 2016, 201, 1–13. [Google Scholar] [CrossRef]
- Monache, S.D.; Angelucci, A.; Sanità, P.; Iorio, R.; Bennato, F.; Mancini, F.; Gualtieri, G.; Colonna, R.C. Inhibition of Angiogenesis Mediated by Extremely Low-Frequency Magnetic Fields (ELF-MFs). PLoS ONE 2013, 8, e79309. [Google Scholar] [CrossRef]
- Sisman, A.R.; Kiray, M.; Camsari, U.M.; Evren, M.; Ates, M.; Baykara, B.; Aksu, I.; Güvendi, G.; Uysal, N. Potential Novel Biomarkers for Diabetic Testicular Damage in Streptozotocin-Induced Diabetic Rats: Nerve Growth Factor Beta and Vascular Endothelial Growth Factor. Dis. Markers 2014, 2014, 108106. [Google Scholar] [CrossRef]
- Li, F.; Lei, T.; Xie, K.; Wu, X.; Tang, C.; Jiang, M.; Liu, J.; Luo, E.; Shen, G. Effects of extremely low frequency pulsed magnetic fields on diabetic nephropathy in streptozotocin-treated rats. Biomed. Eng. Online 2016, 15, 8. [Google Scholar] [CrossRef]
- Saygin, M.; Asci, H.; Ozmen, O.; Cankara, F.N.; Dincoglu, D.; Ilhan, I. Impact of 2.45 GHz microwave radiation on the testicular inflammatory pathway biomarkers in young rats: The role of gallic acid. Environ. Toxicol. 2015, 31, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Redecker, P.; Kreutz, M.R.; Bockmann, J.; Gundelfinger, E.D.; Boeckers, T.M. Brain Synaptic Junctional Proteins at the Acrosome of Rat Testicular Germ Cells. J. Histochem. Cytochem. 2003, 51, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, E.; Dorr, N.P.; Elder, G.A.; Buxbaum, J.D. Absence of strong strain effects in behavioral analyses of Shank3-deficient mice. DMM Dis. Model. Mech. 2014, 7, 667–681. [Google Scholar] [CrossRef] [PubMed]

| Groups | n | Body Weight (g) | Testis Weight (mg) |
|---|---|---|---|
| Sham | 15 | 144.14 ± 2.25 | 90.00 ± 7.02 |
| EMF-28 | 10 | 140.32 ± 11.15 | 70.00 ± 12.39 |
| EMF-42 | 10 | 115.44 ± 4.81 * | 39.00 ± 3.77 * |
| p (Sham vs. EMF-42) | 0.002 | <0.001 | |
| p (EMF-28 vs. EMF-42) | 0.013 | 0.020 |
| Hormones (Mean ± SEM) | ||||
|---|---|---|---|---|
| Groups | n | FSH (IU/L) | LH (mIU/mL) | Testosterone (ng/dL) |
| Sham | 10 | 2.10 ± 0.22 | 5.52 ± 0.35 | 17.41 ± 3.25 |
| EMF-28 | 10 | 2.25 ± 0.35 | 5.76 ± 0.12 | 25.81 ± 4.69 |
| EMF-42 | 10 | 1.31 ± 0.15 * | 4.73 ± 0.10 * | 17.66 ± 3.38 |
| p (Sham vs. EMF-42) | 0.037 | 0.019 | NS | |
| p (EMF-28 vs. EMF-42) | 0.015 | 0.003 | NS | |
| Group | n | MDA (µM/mg pr) | GSH (µM/mg pr) |
|---|---|---|---|
| Sham | 15 | 0.55 ± 0.037 | 989.97 ± 66.98 |
| EMF-28 | 10 | 0.69 ± 0.038 * | 511.12 ± 75.53 # |
| EMF-42 | 10 | 0.71 ± 0.038 * | 959.89 ± 98.97 |
| p (Sham vs. EMF-28) | 0.014 | <0.001 | |
| p (Sham vs. EMF-42) | 0.006 | NS | |
| p (EMF-28 vs. EMF-42) | NS | 0.001 |
| Group | n | SHANK3 (ng/mL) | IGF1 (pg/mL) | VEGF (pg/mL) |
|---|---|---|---|---|
| Sham | 15 | 11.64 ± 1.45 | 985.54 ± 41.17 | 331.66 ± 46.18 |
| EMF-28 | 10 | 7.15 ± 0.87 # | 859.50 ± 72.23 | 238.55 ± 36.81 |
| EMF-42 | 10 | 13.38 ± 0.96 | 737.51 ± 59.38 * | 165.92 ± 34.08 * |
| p (Sham vs. EMF-28) | 0.013 | NS | NS | |
| p (Sham vs. EMF-42) | NS | 0.003 | 0.009 | |
| p (EMF-28 vs. EMF-42) | 0.001 | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersoy, N.; Acikgoz, B.; Aksu, I.; Kiray, A.; Bagriyanik, H.A.; Kiray, M. The Effects of Prenatal and Postnatal Exposure to 50-Hz and 3 mT Electromagnetic Field on Rat Testicular Development. Medicina 2023, 59, 71. https://doi.org/10.3390/medicina59010071
Ersoy N, Acikgoz B, Aksu I, Kiray A, Bagriyanik HA, Kiray M. The Effects of Prenatal and Postnatal Exposure to 50-Hz and 3 mT Electromagnetic Field on Rat Testicular Development. Medicina. 2023; 59(1):71. https://doi.org/10.3390/medicina59010071
Chicago/Turabian StyleErsoy, Nevin, Burcu Acikgoz, Ilkay Aksu, Amac Kiray, Husnu Alper Bagriyanik, and Muge Kiray. 2023. "The Effects of Prenatal and Postnatal Exposure to 50-Hz and 3 mT Electromagnetic Field on Rat Testicular Development" Medicina 59, no. 1: 71. https://doi.org/10.3390/medicina59010071
APA StyleErsoy, N., Acikgoz, B., Aksu, I., Kiray, A., Bagriyanik, H. A., & Kiray, M. (2023). The Effects of Prenatal and Postnatal Exposure to 50-Hz and 3 mT Electromagnetic Field on Rat Testicular Development. Medicina, 59(1), 71. https://doi.org/10.3390/medicina59010071






