Usefulness of High-Sensitivity Troponin I in Risk Stratification and Final Disposition of Patients with Acute Heart Failure in the Emergency Department: Comparison between HFpEF vs. HFrEF
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical Information
2.3. Biomarker Assay Measurements
2.4. Imaging
2.5. Outcome
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
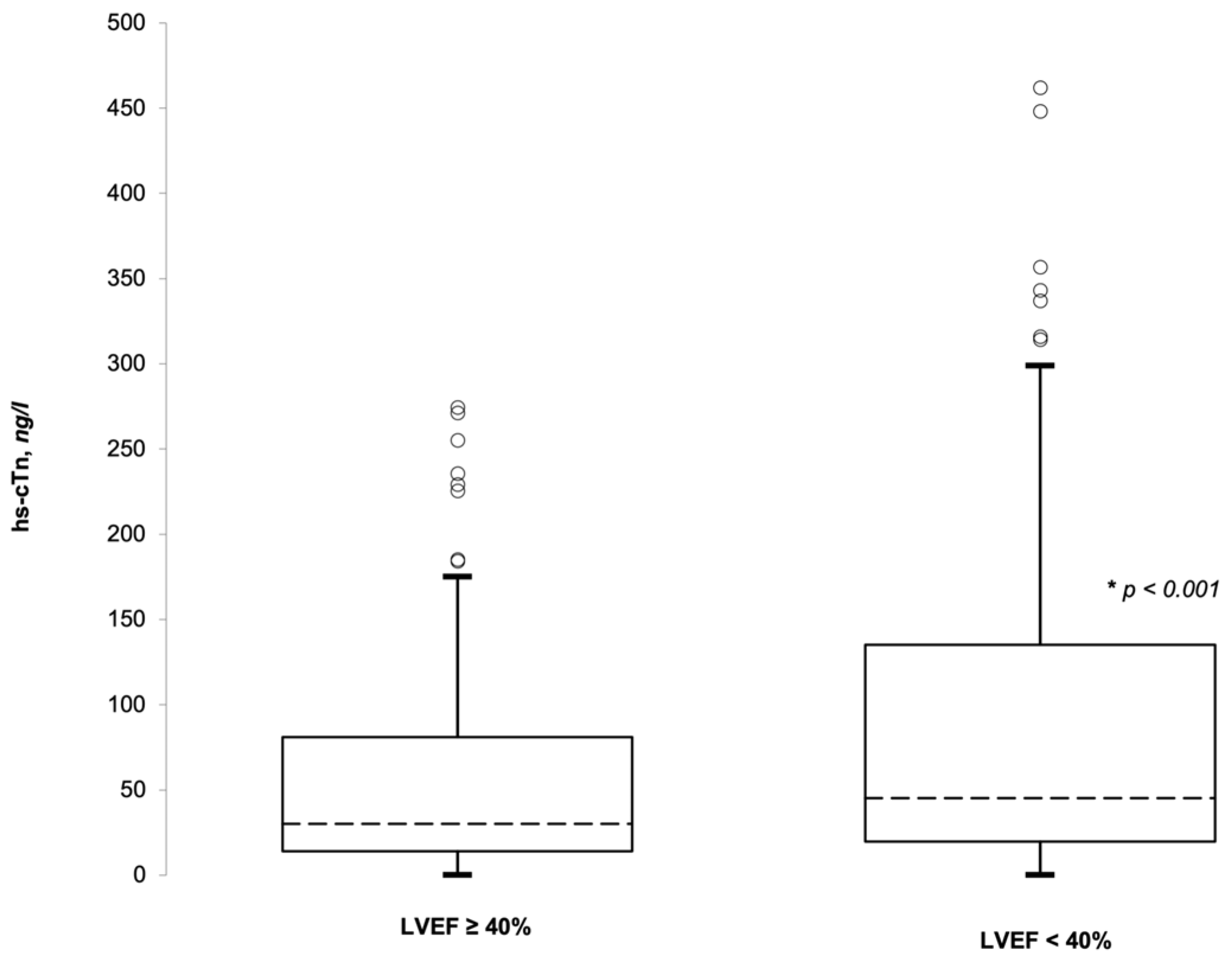
3.2. hs-cTnI and LVEF
3.3. Final Disposition
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788, Erratum in Lancet 2018, 392, 2170; Erratum in Lancet 2019, 393, e44. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Minhas, A.M.K.; Khan, M.S.; Pandey, A.; Michos, E.D.; Mentz, R.J.; Fudim, M. Causes of hospitalization in the USA between 2005 and 2018. Eur. Heart J. Open 2021, 1, oeab001. [Google Scholar] [CrossRef] [PubMed]
- Llorens, P.; the ICA-SEMES Research Group; Javaloyes, P.; Martín-Sánchez, F.J.; Jacob, J.; Herrero-Puente, P.; Gil, V.; Garrido, J.M.; Salvo, E.; Fuentes, M.; et al. Time trends in characteristics, clinical course, and outcomes of 13,791 patients with acute heart failure. Clin. Res. Cardiol. 2018, 107, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Iv, W.F.P.; Fonarow, G.C.; Emerman, C.L.; Mills, R.M.; Wynne, J. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology 2007, 107, 44–51. [Google Scholar] [CrossRef]
- Valli, G.; Galati, E.; Marco, F.D.; Bucci, C.; Fratini, P.; Cennamo, E.; Ancona, C.; Volpe, N.; Ruggieri, M.P. In-hospital mortality in the emergency department: Clinical and etiological differences between early and late deaths among patients awaiting admission. Clin. Exp. Emerg. Med. 2021, 8, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Masip, J.; Peacok, W.F.; Arrigo, M.; Rossello, X.; Platz, E.; Cullen, L.; Mebazaa, A.; Price, S.; Bueno, H.; Di Somma, S.; et al. Acute Heart Failure in the 2021 ESC Heart Failure Guidelines: A scientific statement from the Association for Acute CardioVascular Care (ACVC) of the European Society of Cardiology. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Miró, Ò.; Rossello, X.; Platz, E.; Masip, J.; Gualandro, D.M.; Peacock, W.F.; Price, S.; Cullen, L.; DiSomma, S.; Oliveira, M.T.D., Jr.; et al. Risk stratification scores for patients with acute heart failure in the Emergency Department: A systematic review. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, O.; Wernbom, M.; Mills, N.L.; Mueller, C.; Giannitsis, E.; Jaffe, A.S.; Huber, K.; Mair, J.; Cullen, L.; Möckel, M.; et al. How is cardiac troponin released from cardiomyocytes? Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cardelli, P.; Marino, R.; Lim, S.H.; Somma, S.D. Advantage of Using of High-Sensitivity Troponin I Compared to Conventional Troponin I in Shortening Time to Rule out/in Acute Coronary Syndrome in Chest Pain Patients Presenting to the Emergency Department. Medicina 2022, 58, 1391. [Google Scholar] [CrossRef] [PubMed]
- Valli, G.; Fratini, P.; Volpe, N.; De Marco, F.; Pandolfi, C.; Ancona, C.; Ruggieri, M.P. Analysis of the costs of emergency room management of critically ill patients. Ital. J. Emerg. Med. 2020, 9, 20–28. [Google Scholar] [CrossRef]
- Peacock, W.F.; De Marco, T.; Fonarow, G.C.; Diercks, D.; Wynne, J.; Apple, F.S.; Wu, A.H. Cardiac Troponin and Outcome in Acute Heart Failure. N. Engl. J. Med. 2008, 358, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.S.; Teerlink, J.R.; Voors, A.A.; Ponikowski, P.; Greenberg, B.H.; Filippatos, G.; Felker, G.M.; Davison, B.A.; Cotter, G.; Kriger, J.; et al. Use of High-Sensitivity Troponin T to Identify Patients With Acute Heart Failure at Lower Risk for Adverse Outcomes: An Exploratory Analysis From the RELAX-AHF Trial. JACC Heart Fail. 2016, 4, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Hasselblad, V.; Tang, W.W.; Hernandez, A.F.; Armstrong, P.W.; Fonarow, G.C.; Voors, A.A.; Metra, M.; McMurray, J.J.; Butler, J.; et al. Troponin I in acute decompensated heart failure: Insights from the ASCEND-HF study. Eur. J. Heart Fail. 2012, 14, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Januzzi, J.L.; Vergaro, G.; Ripoli, A.; Latini, R.; Masson, S.; Magnoli, M.; Anand, I.S.; Cohn, J.N.; Tavazzi, L.; et al. Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: An Individual Patient Data Meta-Analysis. Circulation 2018, 137, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Myhre, P.L.; Claggett, B.; Ballantyne, C.M.; Selvin, E.; Røsjø, H.; Omland, T.; Solomon, S.D.; Skali, H.; Shah, A.M. Association Between Circulating Troponin Concentrations, Left Ventricular Systolic and Diastolic Functions, and Incident Heart Failure in Older Adults. JAMA Cardiol. 2019, 4, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Manuale Regionale Triage Intra-Ospedaliero Modello Lazio a Cinque Codici (Numerici/Colore). 2019. Available online: https://www.regione.lazio.it/sites/default/files/2021-04/Manuale-Triage-tml-rev-03-ottobre.pdf (accessed on 28 October 2022).

| Baseline Characteristics | n (%) | |
|---|---|---|
| Epidemiological data | ||
| Age (years) | 79.9 | ±10.7 SD |
| Male | 208 (54) | |
| Triage | ||
| Code 1 (red) | 115 (29.8%) | |
| Code 2 (orange) | 190 (49.2%) | |
| Code 3 (blue) | 77 (19.9%) | |
| Code 4 (green) | 4 (1.0%) | |
| Code 5 (white) | 0 (0%) | χ2 < 0.001 |
| Final Disposition | ||
| Discharged | 29 (7.5%) | |
| Low-intensity ward | 286 (74.1%) | |
| High-dependency unit | 44 (11.4%) | |
| Intensive care unit | 22 (5.7%) | |
| Deceased | 5 (1.3%) | χ2 < 0.001 |
| Phenotype at presentation | ||
| Acute decompensated heart failure | 324 (83.9%) | |
| Acute pulmonary oedema | 53 (13.7%) | |
| Isolated right ventricular failure | 8 (2.1%) | |
| Cardiogenic shock | 1 (0.3%) | χ2 < 0.001 |
| Left ventricular ejection fraction | ||
| EF < 40% | 160 (41.5%) | |
| EF between 41 and 49 | 107 (27.7%) | |
| EF > 50% | 119 (30.8%) | χ2 < 0.05 |
| High sensitivity troponin I | ||
| Positive test | 236 (61.1%) |
| LVEF < 40% | LVEF > 40% | ||||
|---|---|---|---|---|---|
| n | 160 (42%) | 226 (59%) | |||
| Median | IQR | Median | IQR | p-Value | |
| Age | 81 | 73–85 | 82 | 76–88 | <0.002 |
| Systolic pressure | 136 | 110–150 | 145 | 130–160 | <0.01 |
| Diastolic pressure | 76 | 70–87 | 80 | 70–90 | n.s. |
| Heart rate | 85 | 75–99 | 85 | 75–98 | n.s. |
| Respiratory rate | 18 | 17–22 | 20 | 18–22 | n.s. |
| Creatinine | 1.19 | 0.92–1.7 | 1.11 | 0.88–1.55 | n.s. |
| Hemoglobin | 12.5 | 10.9–14.0 | 11.8 | 10.1–13.2 | <0.01 |
| La- | 1,7 | 1.0–2.5 | 1.30 | 1.0–1.8 | <0.05 |
| BNP | 2358 | 1060–8770 | 1200 | 587–2503 | <0.001 |
| hs-cTnI | 42.3 | 18.1–116.1 | 29.3 | 13.9–73.0 | <0.001 |
| Deceased, n (%) | 3 (2%) | 2 (1%) | n.s. | ||
| Adverse outcome, n (%) | 40 (25%) | 31 (14%) | <0.01 | ||
| hs-cTnI Negative | hs-cTnI Positive | ||||
|---|---|---|---|---|---|
| n | 150 (39%) | 236 (61%) | |||
| Median | IQR | Median | IQR | p-Value | |
| Age | 81 | 73–85 | 83 | 76–89 | <0.001 |
| Systolic pressure | 140 | 120–150 | 140 | 125–160 | n.s. |
| Diastolic pressure | 80 | 70–86 | 80 | 70–90 | n.s. |
| Heart rate | 81 | 74–95 | 86 | 76–100 | 0.05 |
| Respiratory rate | 18 | 16–22 | 20 | 18–22 | <0.05 |
| Creatinine | 1.08 | 0.88–1.38 | 1.23 | 0.95–1.71 | <0.001 |
| Hemoglobin | 11.9 | 10.4–13.4 | 12.1 | 10.7–13.4 | n.s. |
| La- | 1.2 | 1.0–1.7 | 1.5 | 1.0–2.2 | 0.05 |
| BNP | 1192 | 595–2460 | 1797 | 752–4700 | <0.001 |
| hs-cTnI | 12 | 9–18 | 66 | 38–143 | <0.001 |
| Deceased, n (%) | 0 (0%) | 5 (2%) | 0.07 | ||
| Adverse outcome, n (%) | 19 (13%) | 51 (22%) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisanti, L.; Valli, G.; Cennamo, E.; Capolino, A.; Fratini, P.; Cesaro, C.; Adducchio, G.; De Magistris, A.; Terlizzi, F.; Ruggieri, M.P.; et al. Usefulness of High-Sensitivity Troponin I in Risk Stratification and Final Disposition of Patients with Acute Heart Failure in the Emergency Department: Comparison between HFpEF vs. HFrEF. Medicina 2023, 59, 7. https://doi.org/10.3390/medicina59010007
Crisanti L, Valli G, Cennamo E, Capolino A, Fratini P, Cesaro C, Adducchio G, De Magistris A, Terlizzi F, Ruggieri MP, et al. Usefulness of High-Sensitivity Troponin I in Risk Stratification and Final Disposition of Patients with Acute Heart Failure in the Emergency Department: Comparison between HFpEF vs. HFrEF. Medicina. 2023; 59(1):7. https://doi.org/10.3390/medicina59010007
Chicago/Turabian StyleCrisanti, Luca, Gabriele Valli, Elisa Cennamo, Alessandro Capolino, Paolo Fratini, Claudio Cesaro, Gloria Adducchio, Antonio De Magistris, Ferdinando Terlizzi, Maria Pia Ruggieri, and et al. 2023. "Usefulness of High-Sensitivity Troponin I in Risk Stratification and Final Disposition of Patients with Acute Heart Failure in the Emergency Department: Comparison between HFpEF vs. HFrEF" Medicina 59, no. 1: 7. https://doi.org/10.3390/medicina59010007
APA StyleCrisanti, L., Valli, G., Cennamo, E., Capolino, A., Fratini, P., Cesaro, C., Adducchio, G., De Magistris, A., Terlizzi, F., Ruggieri, M. P., Mirante, E., Savoriti, C., Sukruang, K., Valeriano, V., Pugliese, F. R., Travaglino, F., & Di Somma, S. (2023). Usefulness of High-Sensitivity Troponin I in Risk Stratification and Final Disposition of Patients with Acute Heart Failure in the Emergency Department: Comparison between HFpEF vs. HFrEF. Medicina, 59(1), 7. https://doi.org/10.3390/medicina59010007








